Introduction
The Yellow Bunting Emberiza sulphurata is an endangered migratory passerine found in the East Asian flyway (Yong et al. Reference Yong, Liu, Low, Espanola, Choi and Kawakami2015). Although it has a few wintering sites across East Asia (e.g. Philippines, Taiwan, and southern China), the breeding sites are exclusively restricted to the northern part of Honshu Island, Japan (Ministry of the Environment 2004, Byers et al. Reference Byers, Ofsson and Curson2013). The total number of Yellow Buntings has been estimated at approximately 3,500–15,000 (IUCN 2014). Owing to significant habitat loss, it is listed as globally ‘Vulnerable’ on the IUCN Red List (IUCN 2014) and ‘Near-Threatened’ at the national level in Japan (Ministry of the Environment 2014). Although it is known to be associated with forest edge, shrubs, and thickets (del Hoyo et al. Reference del Hoyo, Elliott and Christie2011), the detailed habitat requirements have not been elucidated (IUCN 2014, Japan Ministry of the Environment 2014). Niigata Prefecture, which is located in the northern part of Honshu Island, is one of the regions where Yellow Buntings breed at a high density (Ministry of the Environment 2004). Within the Niigata Prefecture, they breed in hilly agricultural landscapes (Kaneko Reference Kaneko1979), akin to the traditional “yatsu” landscape in Japan, which is characterized by hills, with paddyfields in the valley bottom and secondary forests on the valley slopes (Takeuchi et al. Reference Takeuchi, Brown, Washitani, Tsunekawa and Yokohari2002).
In Japan, the abandonment of paddyfields has increased since the 1980s, owing to socioeconomic changes (Katayama et al. Reference Katayama, Yuki, Kusumoto and Tanaka2015a). Abandonment has occurred primarily in the hilly and mountainous areas (Katayama et al. Reference Katayama, Yuki, Kusumoto and Tanaka2015a) owing to factors such as depopulation, aging farmers and lower productivity compared with plains areas (Katayama et al. Reference Katayama, Osawa, Amano and Kusumoto2015b). Field abandonment accompanied by vegetation succession alters the habitats for birds, consequently affecting diversity within bird communities in the agricultural landscape (Suárez-Seoane et al. Reference Suárez-Seoane, Osborne and Baudry2002, Sirami et al. Reference Sirami, Brotons and Martin2007, Reference Sirami, Brotons, Burfield, Fonderflick and Martin2008, Nikolov Reference Nikolov2010, Ambarlı and Bilgin Reference Ambarlı and Bilgin2014, Zakkak et al. Reference Zakkak, Kakalis, Radović, Halley and Kati2014). Threatened bird species in Europe, such as the Corn Bunting Emberiza calandra (Zakkak et al. Reference Zakkak, Kakalis, Radović, Halley and Kati2014) and Golden Eagle Aquila chrysaetos (Pedrini and Sergio Reference Pedrini and Sergio2001) have lost areas of habitat due to similar instances of abandonment. Negative impacts of paddy abandonment on Grey-faced Buzzard Butastur indicus have been found in Japan (Ueta et al. Reference Ueta, Kurosawa and Matsuno2006, Kadowaki et al. Reference Kadowaki, Murayama and Kojima2007). However, field abandonment could also serve as an opportunity for birds to recover their native habitats (Queiroz et al. Reference Queiroz, Beilin, Folke and Lindborg2014).
Yellow Buntings, which inhabit hilly rural areas, might be affected by paddyfield abandonment in these regions. Furthermore, the growing tendency towards field abandonment means the trend is likely to continue in such areas (Ichinose Reference Ichinose2007, Katayama et al. Reference Katayama, Yuki, Kusumoto and Tanaka2015a). Therefore, to conserve the Yellow Bunting, understanding its habitat requirements and the effects of changes in its habitat, caused by paddy abandonment, is extremely important. The goal of this study was to document the habitat selection by Yellow Buntings and to determine the effects of paddyfield abandonment on habitat suitability.
Methods
Study area
The study was conducted in hilly rural areas akin to the “yatsu” landscape, in the Koshiji (37°23ʹN, 138°47ʹE) and Oguni (37°18ʹN, 138°42ʹE) regions (c.14.4 ha) of Nagaoka City, within the Niigata Prefecture in northern Japan (Figure 1). The study area comprised hills of sedimentary rocks and numerous mountain streams flowing into the Shibumi River. The mean annual temperature in Nagaoka City (37°27ʹN, 138°49ʹE) is 12.9°C, with a minimum monthly average of 1.3°C in January and a maximum of 26.0°C in August, and the mean annual precipitation is 2,324.8 mm. These mean values were based on observed values over 30 years, 1981–2010 (Japan Meteorological Agency 2015). In addition, the study area is located in a heavy snow region, where small avalanches occur every year (Kaneko Reference Kaneko1979, Reference Kaneko2012). The snow starts falling in late December and often accumulates to over 100 cm every year. Small-scale avalanches and landslides often lead to the development of ‘avalanche vegetation’, which is unique to heavy snow regions and consists mostly of dwarf trees or tall grasses such as Alnus pendula, Weigela hortensis, and Miscanthus sinensis (Kaneko Reference Kaneko1979, Kikuchi Reference Kikuchi2001). We use a few terms associated with vegetation types in this paper with the following meanings, bush: middle stages of succession, such as shrubland and tall grassland, including forest edge; woodland: small range, such as abandoned paddyfields covered with tall trees; forest: wide range, covered with tall trees on hill and valley slopes. The vegetation in our study area mainly comprised plantations of Cryptomeria japonica and secondary forests dominated by Quercus serrata, Acer amoenum var. matsumurae, and Magnolia obovata (Kaneko Reference Kaneko2012). Furthermore, there were grasslands dominated by M. sinensis at the avalanche sites (Kaneko Reference Kaneko2012). The valley bottoms were mainly covered with rice paddy, as well as croplands, ginkgo Ginkgo biloba orchards, and ponds for breeding coloured carp cyprinus carpio or irrigation of smaller areas. Paddyfield abandonment occurred in the 1970s in this area (Kaneko Reference Kaneko1979). Vegetation in the abandoned paddyfields is mentioned below in the section ‘Landscape characteristics’. We randomly selected 30 points across the valleys in the study area (58–75 m asl). At each of these points, we established a transect (321–683 m long, c.100 m wide), to quantify the abundance and landscape characteristics. In this region, Yellow Buntings are found only from breeding to the autumn migration period (April-October), and are absent during the winter (Kaneko Reference Kaneko1981, Deguchi et al. unpublished data). Yellow Bunting is one of the dominant bird species in this region during their breeding season (Kaneko Reference Kaneko1981).
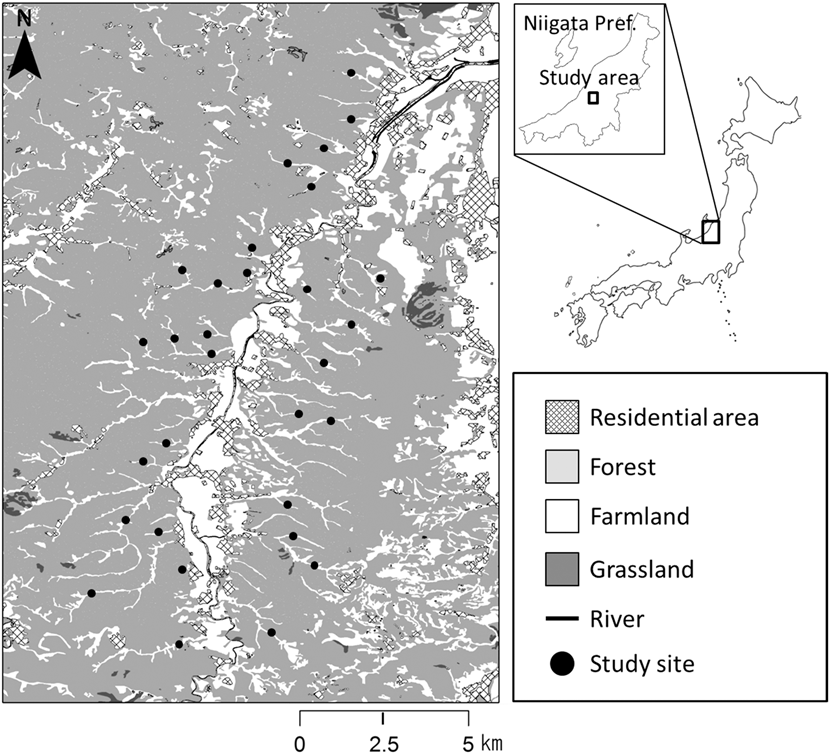
Figure 1. Map of the study area. Closed circles indicate the 30 transects (321–683 m long, ca. 50 m wide).
Bird data
We counted the number of singing males as an estimate of abundance along the line transects. One observer walked along the transect and counted the number of birds within a 50-m band on either side of the line. The duration of surveys was 10 min and they were conducted twice each in May and June 2014 during the four hours following dawn on calm days, along all transects. To avoid recording the same bird more than once, efforts were made to record synchronously singing males. We used the maximum number of birds detected at a given transect as an estimate of abundance.
Landscape characteristics
In order to examine the habitat requirements of Yellow Buntings, edge density (EDG_D) for each transect was calculated by dividing the sum of perimeters of valley bottoms, that is, the total length of edge between open land and forest, by the area of valley bottom (m/m2). Furthermore, distances in metres from the central points of each transect to the nearest residential area (DNR) were measured. DNR indicated whether a transect was in the upper or the lower reach of the valley as residential areas are usually found in the lowest reaches of the valley. We also measured the area affected by landslides adjoining each transect because it and small avalanches together can establish avalanche vegetation which could be associated with the distribution of Yellow Buntings (Kaneko Reference Kaneko1979, Nakamura Reference Nakamura and Torinokai1994). In comparison to vegetation on abandoned paddy, avalanche vegetation tends to persist because regular disturbance such as small avalanches and landslides prevents vegetation succession. We used the values of landslide area per length of transect (m2/m, LSLIDE) in the analysis. To evaluate the effects of paddyfield abandonment on Yellow Buntings, we measured the proportion of land cover (%) of cultivated (CU) and abandoned paddies on each transect. We categorised the abandoned paddyfields into three types according to their successional stages: (1) the early stage, with short grasses (< 50 cm), where Equisetum arvense and Polygonum thunbergii were predominant (AB_E); (2) the middle stage, covered primarily with Phragmites australis, Miscanthus sacchariflorus, or M. sinensis, and some shrubs such as Weigela hortensis or Lespedeza bicolor (AB_M), and (3) the late stage, where trees such as Alnus fauriei or Salix sp. were found (AB_L). We also quantified the land cover heterogeneity (L_HTR) using Shannon’s Diversity Index, Hʹ = -Σ p i × ln p i , where p i is the proportion of land cover i (Katayama et al. Reference Katayama, Osawa, Amano and Kusumoto2015b) because some species use different habitat types (Dunning et al. Reference Dunning, Danielson and Pulliam1992, Brotons et al. Reference Brotons, Wolff, Paulus and Martin2005). However, land cover heterogeneity was not applicable to our analysis because of high inter-correlation with the area of abandoned paddyfields at the middle stage (r = 0.7; Table 1). We used Arc Map 10.2 (ESRI 2013) along with geographically referenced topographical maps at 1:2,500 scale and aerial photographs taken in 2014 by Digital Globe (www.digitalglobe.com) and ZENRIN (www.zenrin.co.jp) for all measurements of landscape characteristics, except the landslide areas. The landslide areas were determined using landslide GIS data (National Research Institute for Earth Science and Disaster Prevention 2000).
Table 1. Pearson’s correlation coefficients among the candidate explanatory variables.
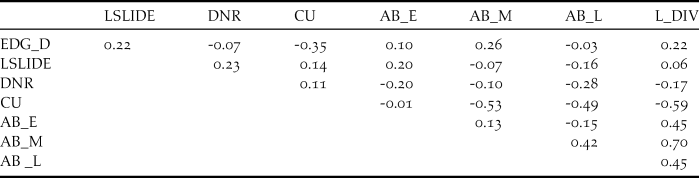
Abbreviations: EDG_D: edge density calculated by dividing the sum of perimeters of valley bottoms and forest edges by the area of valley bottom. Land cover heterogeneity was calculated using the Shannon’s Diversity Index (H’). LSLIDE: landslide area per transect (m/m2), DNR: Distance from nearest residence area (m), L_HTR: land cover heterogeneity calculated with Shannon’s Diversity Index (Hʹ). Following variables indicate land cover proportions: CU: cultivated paddy (%), AB_E: abandoned paddy at the early succession stage (%), AB_M: abandoned paddy at the middle succession stage (%), AB_L: abandoned paddy at the late succession stage (%). L_HTR was removed from the analysis due to its high correlation with variable AB_M (-0.59).
Statistical analyses
We analysed the relationships between bird abundance and landscape variables using the generalized linear mixed model (GLMM) with a Poisson distribution and a log link function, using the R package ‘lme4’. Valley bottom identities (ID) were fitted as random effects and the areas of valley bottoms (ha) were used as offset terms because transects were of different sizes (1.22–6.39 ha). We used abundance of singing birds as the response variable and landscape characteristics as the explanatory variables. All of the explanatory variables were standardized to make the regression coefficients comparable. Since we obtained multiple candidate models, including the null model, with ΔAIC < 2.0 (Burnham and Anderson Reference Burnham and Anderson2002), model selection using AIC was not performed. We evaluated the significance of variables using 95% confidence intervals. To evaluate goodness-of-fit of the full model, we used the coefficient of determination (R2) for GLMM using the ‘MuMIn’ package in R, following Nakagawa and Schielzeth (Reference Nakagawa and Schielzeth2013). We calculated the marginal R2 (R2 m), which examines the variance explained by only the fixed effects, and conditional R2 (R2 c), which examines the variance explained by both fixed and random effects (Nakagawa and Schielzeth Reference Nakagawa and Schielzeth2013). These analyses were conducted using R ver. 3.2.0 (R Development Core Team 2015).
Results
Occurrence patterns of Yellow Buntings
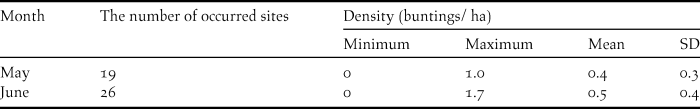
Of the 30 transects investigated, Yellow Buntings occurred in 19 and 26 transects in May and June, respectively (Table 2). There was negligible difference in the mean densities of Yellow Bunting between May (0.4 ha-1) and June (0.5 ha-1) (Table 2).
Table 2. The number of transects where Yellow Buntings occurred and their density (buntings ha-1) in May and June 2014.
Relationship between Yellow Bunting abundance and landscape characteristics
Values of all landscape characteristics varied among the transects (Table 3). The proportion of cultivated paddyfield was higher than that of the three types of abandoned paddyfield (Table 3). The landscape characteristics used in the GLMM analysis as explanatory variables were not strongly correlated (Table 1). The full model had an R2 m value of 0.55 and an R2 c value of 0.77. Abundance of Yellow Bunting was correlated significantly and positively to edge density and landslide area (Figures 2 and 3). DNR and abandoned paddyfields with progressed succession (middle and late) had a positive effect on Yellow Bunting abundance, although the effects were not significant (Figures 2 and 3). Edge density showed a higher coefficient value (0.73 ± 0.19) compared with the other variables, for which it ranged between -0.21 and 0.48 (Figure 2; Appendix S1 in the online supplementary material). On the other hand, cultivated and early abandoned paddyfields had a negative effect on Yellow Bunting abundance, although not significant (Figures 2 and 3). Paddyfield abandonment showed a hump shaped effect with a peak at the middle succession stage (Figure 2).
Table 3. Summary of landscape characteristics of the 30 transects in the study area. Each value is calculated using the total value of each landscape feature for the 30 transects.
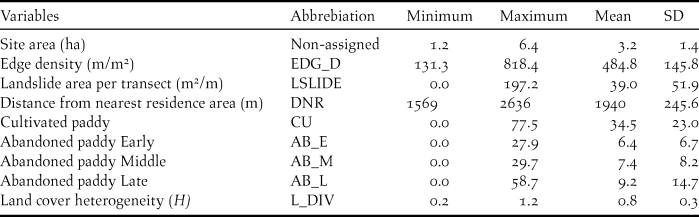
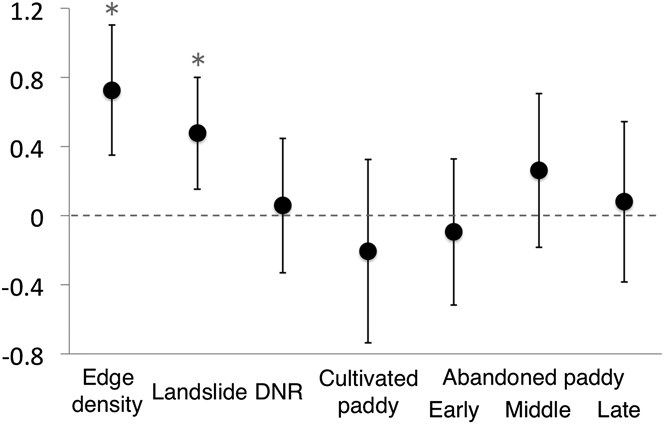
Figure 2. Regression coefficients [mean ± 95% confidence interval (CI)] estimated using generalized linear mixed model (GLMM) for Yellow Bunting abundance. Asterisks signify factors for which the 95% CI did not include zero.

Figure 3. Response curves of Yellow Bunting abundance with respect to the landscape characteristics.
Discussion
Yellow Bunting density was stable through May (0.4 ha-1) and June (0.5 ha-1). Kaneko (Reference Kaneko1981) investigated this study area in 1977 and reported a similar trend (May: 0.3 ha-1, June: 0.28 ha-1). The current density has not changed significantly compared with that recorded about 40 years ago (Kaneko Reference Kaneko1981). We show that edge density, and as Nakamura (Reference Nakamura and Torinokai1994) stated, landslide areas have a positive effect on Yellow Bunting abundance. Greater edge density is associated with the availability of forest-edge habitat. Landslide sites are usually dominated by shrub species with several sprouting shoots (Kikuchi Reference Kikuchi2001). These bushy habitats, which are favoured by the Yellow Buntings (Nakamura and Nakamura Reference Nakamura and Nakamura1995), were consistently found adjacent to forests. Hence, Yellow Buntings need not only bush, but also habitats consisting both bush and forests. DNR was not significant, which means that there was no relationship found between the location of transects (upper or lower reach of the valley) and Yellow Bunting abundance. It is possible that the environment of valleys did not vary drastically between the upper and lower reaches because of the very small scale of the mountain streams.
Paddyfield abandonment showed a hump-shaped effect. Mid-abandoned paddyfields could be better suited than cultivated ones for Yellow Buntings because the former are similar to edge or landslide habitats, which develop bushy cover. In hilly rural areas of Japan, Yellow Buntings use the abandoned paddyfields occupied by reeds both as nesting sites during the breeding season (Kaneko Reference Kaneko1979) and as stopover sites during their autumn migration (Yoshida Reference Yoshida2007, S. Deguchi unpubl. data), or as shelter from predators (Maeda Reference Maeda2001). Previous studies have revealed that cultivated paddy can have a negative effect on species (Maeda Reference Maeda2001, Amano et al. Reference Amano, Kusumoto, Tokuoka, Yamada, Kim and Yamamoto2008) such as the Meadow Bunting Emberiza cioides (Katayama et al. Reference Katayama, Osawa, Amano and Kusumoto2015b). However, the effect of paddyfield abandonment was not significant in our study. Therefore, it is possible that abandoned paddyfields are just an accidental habitat for the Yellow Buntings, in contrast to the forest edge, and they share no special relationship with paddy cultivation, with respect to habitat selection. Levees surrounding the cultivated paddyfields are favoured by many passerines (Maeda Reference Maeda2001), and we also observed Yellow Bunting forage on a levee once. However, herbicides are often sprayed on the levees in the study area. High levels of chemical agents such as pesticides are also one of the major threats to the Yellow Bunting (del Hoyo et al. Reference del Hoyo, Elliott and Christie2011, IUCN 2014).
Conservation implications
We found that forest edges and landslide sites are the most important habitats for Yellow bunting. These habitats are composed of bush and forest cover. Paddyfield abandonment was found to have no effect on Yellow Buntings, although it could provide them with marginal habitats.
To conserve Yellow Bunting habitats in rural hilly areas, we should first protect the forest edge and landslide sites, which seemed to be original Yellow Bunting habitats, from urbanisation and exploitation. Development should be undertaken with care, as suggested by Kaneko (Reference Kaneko2012), who implied that excessive sand mining in the pits had reduced Yellow Bunting abundance. In addition, we should continue cultivation practices, as open lands such as arable paddyfields contribute toward shaping forest edges in the hilly rural landscape. Habitat supplementation by paddyfield abandonment can be temporary because Yellow buntings rarely use the interior of the forests (Katou Reference Katou1990, Nakamura Reference Nakamura and Torinokai1994). Therefore, dense woodlands caused by the progression of vegetation succession could have negative effects. To maintain the reed beds in abandoned paddyfields, we should maintain a high ground water level (Ikegami et al. Reference Ikegami, Nishihiro and Washitani2011). We should also determine the suitable tree density or vegetation cover for Yellow Buntings, as done for the congeneric Black-faced Bunting Emberiza spodocephala (Fujimaki Reference Fujimaki1986, Deguchi et al. Reference Deguchi, Chiba and Nakata2015), in preparation for succession toward dense forests in the abandoned fields. In addition, we must pay attention to not only habitat loss but also the threat of trapping (IUCN 2014) as another species, Yellow-breasted Bunting Emberiza aureolai, has recently seen a major decline in its populations due to illegal trapping (Kamp et al. Reference Kamp, Oppel, Ananin, Durnev, Gashev, Hölzel, Mishchenko, Pessa, Smirenski, Strelnikov, Timonen, Wolanska, Timonen and Chan2015).
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270916000435
Acknowledgements
We thank Dr. Y. Kaneko for his helpful comments and discussion on our study. We also thank K. Ohira, R. Ogawa, and C. Tamura for their assistance in the field surveys. We thank Dr. S. Nakagawa, Dr. S. Mochizuki and Y. Nakamura for their helpful advice concerning data analysis, and anonymous reviewers for their helpful comments on this manuscript. Nagaoka City provided the geographical maps of the study area. This research was supported by The 13th Koshiji Nature Foundation.








