Introduction
As seabirds are experiencing worldwide decreases in population (Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012, Paleczny et al. Reference Paleczny, Hammill, Karpouzi and Pauly2015, Dias et al. Reference Dias, Martin, Pearmain, Burfield, Small, Phillips, Yates, Lascelles, Borboroglu and Croxall2019), current information on breeding distributions is needed to better estimate population trends and to focus conservation actions (Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012). In particular, gadfly petrels of the genus Pterodroma are experiencing some of the most rapid declines of any seabird species (Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012, Dias et al. Reference Dias, Martin, Pearmain, Burfield, Small, Phillips, Yates, Lascelles, Borboroglu and Croxall2019) but remain among the least studied groups of seabirds (Ramos et al. Reference Ramos, Carlile, Madeiros, Ramírez, Paiva, Dinis, Zino, Biscoito, Leal, Bugoni and Jodice2017). Although they range widely over tropical, subtropical, temperate and subantarctic pelagic waters of each ocean basin, their breeding populations are limited to a comparatively small number of oceanic archipelagoes, to which they are mostly endemic (Warham Reference Warham1990). Gadfly petrels are predominantly nocturnal on land and often breed in inaccessible places in small numbers, thus impeding the collection of basic data on breeding distributions that would allow effective conservation actions.
The Black-capped Petrel Pterodroma hasitata (known regionally as Diablotin) is a medium-size gadfly petrel endemic to the Caribbean. The species has a declining population and is considered ‘Endangered’ throughout its range (BirdLife International 2016). Population estimates based on at-sea observations range from 2,000 to 4,000 individuals, with a fragmented breeding population estimated at 500 to 1,000 pairs (Goetz et al. Reference Goetz, Norris and Wheeler2012, Simons et al. Reference Simons, Lee and Haney2013, BirdLife International 2018). Once widespread in the Caribbean (Collar et al. Reference Collar, Gonzaga, Krabbe, Madroño Nieto, Naranjo, Parker and Wege1992), currently the only confirmed breeding areas are located on the island of Hispaniola (Simons et al. Reference Simons, Lee and Haney2013), although recent surveys suggest possible nesting populations in Dominica (Brown Reference Brown2015), Jamaica (Brown Reference Brown2016) and Cuba (Yasit Segovia, BioEco and Nicasio Viña Davila, Corridor Biologico del Caribe pers. comm., 2020). All known nesting sites are in mountainous areas 1,500–2,000 m above sea level. There, Black-capped Petrels nest in the thick and mesic understorey of steep montane forests and excavate burrows in soil or karstic crevasses.
Our current understanding of Black-capped Petrel conservation and ecology suggest that, unlike most other species of petrels (Rodríguez et al. Reference Rodríguez, Arcos, Bretagnolle, Dias, Holmes, Louzao, Provencher, Raine, Ramírez, Rodríguez and Ronconi2019), the Black-capped Petrel is predominantly affected by changes to and threats within its breeding habitat (Goetz et al. Reference Goetz, Norris and Wheeler2012, U.S. Fish and Wildlife Service Reference Fish2018). Threats include a mix of direct and indirect habitat disruption driven by anthropogenic activities. Habitat loss is occurring via deforestation, forest fires, agricultural encroachment (including illegal agriculture within the boundaries of national parks) and, particularly in Haiti, firewood collection and charcoal production (Simons et al. Reference Simons, Lee and Haney2013). Deterioration of habitat quality is occurring via predation of adults and juveniles at nest sites by introduced mammalian predators (Goetz et al. Reference Goetz, Norris and Wheeler2012, Rupp pers. obs.) and collisions of adult petrels with lighted telecommunication towers erected on mountaintops (Goetz et al. Reference Goetz, Norris and Wheeler2012, Simons et al. Reference Simons, Lee and Haney2013). Due in large part to these threats, the U.S. Fish and Wildlife Service (Reference Fish2018) predicted that the two major breeding areas on Hispaniola are likely to face extirpation by 2070 and, if no additional nest sites are found, this would represent a potential loss of up to 85–95% of the currently known breeding population. Although surveys are underway to locate unidentified nesting areas, such efforts are complex and labour-intensive, involving terrestrial radar surveys near suspected breeding areas, acoustic surveys using automated recording units to confirm petrel activity, and intensive nest searches at active locations. To date, these methods have resulted in the discovery of two additional nesting areas.
Therefore, to aid conservation efforts for the Black-capped Petrel, we developed a predictive habitat suitability model for nesting Black-capped Petrels on the island of Hispaniola. Our goal is that the model be used to support the identification of target areas for in situ nest-search efforts per recommendations of the International Black-capped Petrel Working Group (IBCPWG 2018). Species distribution models such as the one developed herein can use the relationship between a species’s observed occurrence and environmental predictor variables to quantify its habitat requirements, which can then be extrapolated over wide geographical extents (Elith and Leathwick Reference Elith and Leathwick2009). Our objectives were to 1) describe important habitat characteristics for breeding Black-capped Petrels on Hispaniola, 2) identify unknown areas where nesting could occur, 3) quantify the amount of remaining available habitat predicted by the model, and 4) quantify the amount of predicted habitat impacted by forest loss. We specifically chose to use openly available environmental datasets to ensure that all stakeholders could access the datasets and further develop the model once new nesting sites have been located.
Methods
Study extent and data collection
To date, all known nests (n = 81) of Black-capped Petrels found since the rediscovery of the species in the 1960s (Wingate Reference Wingate1964) have been located on the island of Hispaniola, in four distinct areas in three mountain ranges (Figure 1): 29 nests were found in the La Visite nesting area, La Visite mountain range, Haiti, during the 2018 nesting period (Jean et al. Reference Jean, Louis, Jeune, Raymond and Brown2018); 43 nests were found in the Morne Vincent (n =3) and Loma del Toro (n = 40) nesting areas, western Sierra de Bahoruco mountain range, in Haiti and the Dominican Republic, between the 2011 and 2018 nesting periods; five nests were found in the Loma Quemada nesting area, eastern Sierra de Bahoruco, Dominican Republic, during the 2015 nesting period; and four nests were found in the Valle Nuevo nesting area, Cordillera Central mountain range, Dominican Republic, during the 2018 and 2019 nesting periods. Most nesting areas are located within the boundaries of the La Visite, Sierra de Bahoruco or Valle Nuevo national parks. For the purposes of this study, we used all 81 Black-capped Petrel nest locations. Except for the three nests in the Morne Vincent nesting area and three in the Loma del Toro nesting area, all nests were monitored during the 2019 nesting season, and > 90% were found to be active.
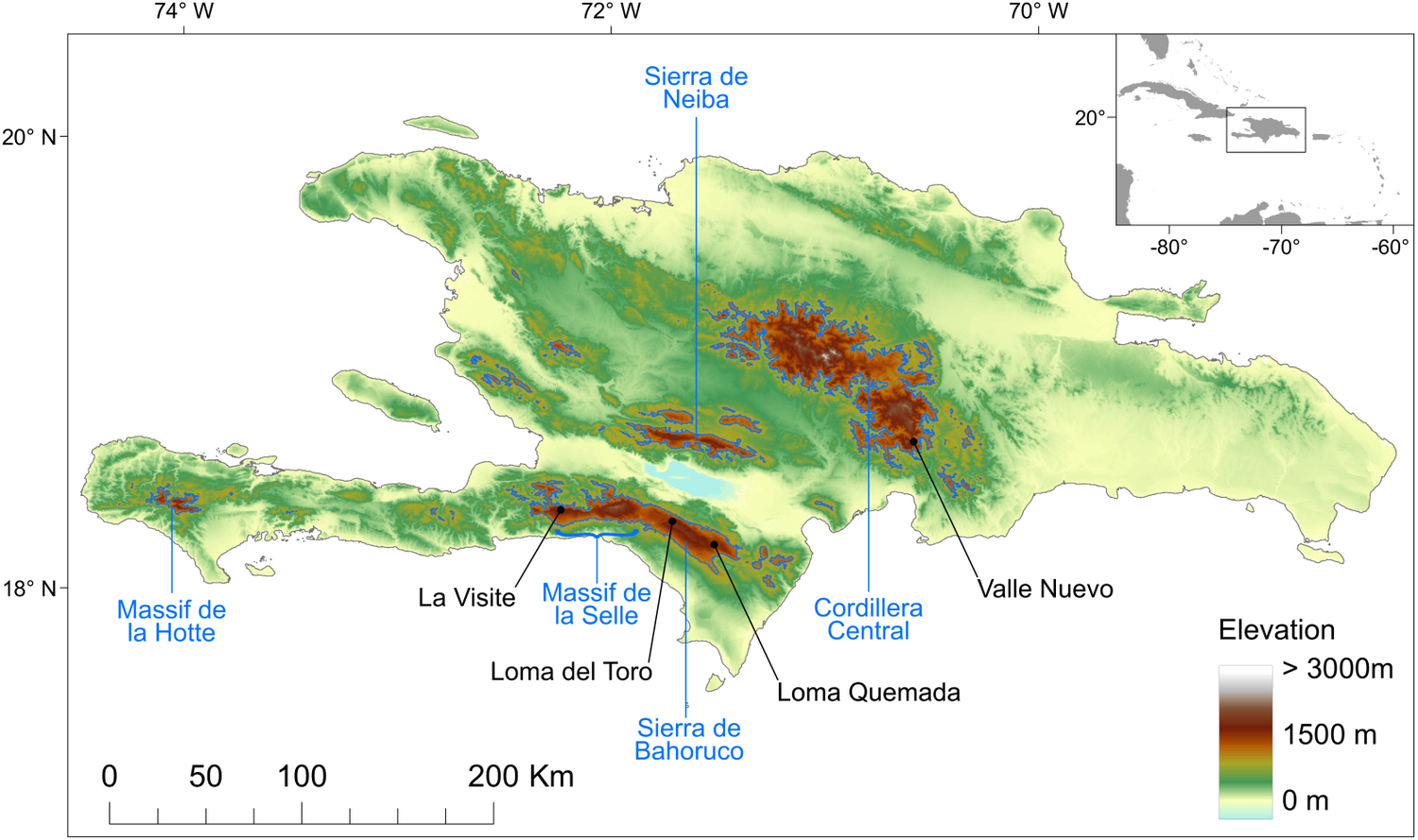
Figure 1. Topography of the island of Hispaniola and location of known Black-capped Petrel nesting areas. Black dots show the locations of Black-capped Petrel nest sites used in this study. Blue polygons delineate the areas above 1,200 m above sea level used to randomly select pseudo-absence locations. The major mountain ranges are indicated in blue. Insert locates the island of Hispaniola within the Caribbean. A color version of this figure may be found in the electronic version of this article.
Modelling overview
Detailed methods may be found in Appendix S1 in the online supplementary materials. Unless mentioned otherwise, we performed all statistical and geographic computing in program R. Because we could not confirm if observed absences at search areas were true absences or failures to detect presence, we could not use a presence-absence framework to estimate and locate suitable nesting habitat. Instead, we followed methods in Troy et al. (Reference Troy, Holmes, Veech, Raine and Green2014) and used a univariate generalized linear model (i.e. logistic regression) to compare the habitat characteristics of known Black-capped Petrel nests sites with those of potentially available sites (i.e. presence and pseudo-absence sites, respectively). We located 500 pseudo-absence sites at random in all mountain ranges on Hispaniola above 1,200 m (Figure 1), an apparent threshold for use as nest sites by the species (Simons et al. Reference Simons, Lee and Haney2013). This included random pseudo-absence locations that were more likely to be true absences, in areas not sampled for petrels but where radar surveys showed that there was no breeding activity (Cordillera Central and Sierra de Neiba; Brown Reference Brown2017). All pseudo-absence sites were located ≥ 500 m from presence sites. We partitioned presence and pseudo-absence sites into two groups for subsequent analysis: a training dataset containing 80% of both groups, which was used to train the statistical models, and a validation dataset containing the remaining 20% of sites, which was used to assess the fit of the models. Black-capped Petrels often nest in clusters of 5–10 burrows within 100–200 m; therefore, to avoid risks of pseudo-replication and to better quantify environmental data at nest sites, we created buffers of 50 m radius around each presence site and combined any overlapping buffers into presence polygons. Since more than one nest can occur in a polygon, adjacent nest sites were combined and the number of polygons is less than the number of nests. We used the same methodology to create and combine any overlapping pseudo-absence 50-m buffers into pseudo-absence polygons. Finally, we reduced the effect of differences in sample size between the presence and pseudo-absence groups by weighting them to simulate an equal number of presence and pseudo-absence sites, such that the total weight of the presence data was the same as the total weight of the pseudo-absence data (Barbet-Massin et al. Reference Barbet‐Massin, Jiguet, Albert and Thuiller2012).
To allow for future reproducibility in the greater Caribbean by local stakeholders, we estimated habitat characteristics from open-access environmental datasets (rasters) readily available for the region (Table 1). Variables were broadly classified as static (elevation, distance to coast, distance to ridgeline, slope, aspect, flow accumulation) and dynamic (Enhanced Vegetation Index, EVI; Leaf-area Index, LAI; evapotranspiration; primary productivity; percentage tree cover, hereafter tree cover; aboveground live woody biomass density, hereafter woody biomass; mean wind speeds; and monthly average radiance). Satellite-derived vegetation datasets such as EVI and LAI may overestimate areas with low or no vegetation (such as bare soil, rocks or snow): indeed, these areas sometimes reflect light in the wavelengths recorded by satellites and may appear in raster datasets as if vegetation is present (i.e. false positives; Satgé pers. obs.). Therefore, we created a new dataset as a composite of tree cover for the year 2000 (Global Forest Watch 2019a) and 16-day Enhanced Vegetation Index, rescaled between 0 and 1 (tree cover-EVI). Finally, we resampled all environmental rasters to match the resolution of the digital elevation model (DEM: 90 m x 90 m; bilinear interpolation).
Table 1. Environmental variables used to estimate the characteristics of Black-capped Petrel nesting habitat on Hispaniola.

* Tree cover was not used as a stand-alone predictor in analyses but was used to compute the variable Tree cover-Enhanced Vegetation Index.
We calculated the habitat characteristics of presence and pseudo-absence sites by averaging each environmental variable within a buffer of 50-m radius located at each site (Troy et al. Reference Troy, Holmes, Veech, Raine and Green2014). We first tested environmental variables individually using univariate logistic regressions and retained the variables that (1) showed a significant relationship with the presence of active petrel sites at P ≤ 0.01 (Table 2); and (2) were not collinear with other environmental predictors. We checked for collinearity using a generalized variance-inflation factor. Vegetation variables showed strong collinearity among themselves and with the composite dataset tree cover-EVI. Therefore, we only retained tree cover-EVI because it best represented actual field conditions. Elevation and mean wind speeds also showed collinearity: we omitted the wind variable because it had the least significant regression coefficient (PMean Wind = 0.05 vs. PElevation < 0.001). Variance-inflation factors for the remaining variables were < 1.8, which suggested that these variables could be included in the generalized linear model without large risk of collinearity (Quinn and Keough Reference Quinn and Keough2002). The retained environmental predictors were: elevation, distance to coast, and tree cover-EVI.
Table 2. Characteristics of environmental variables at Black-capped Petrel nesting sites and pseudo-absence sites on Hispaniola. Mean, standard deviation (SD), and range (Min. = minimum values, Max. = maximum values) and p-values of individual univariate logistic regressions are provided. Variables that showed a significant difference between presence and pseudo-absence sites are shown in italics. Variables that were retained to compute the habitat suitability model are shown in bold (others removed due to collinearity; see Methods).
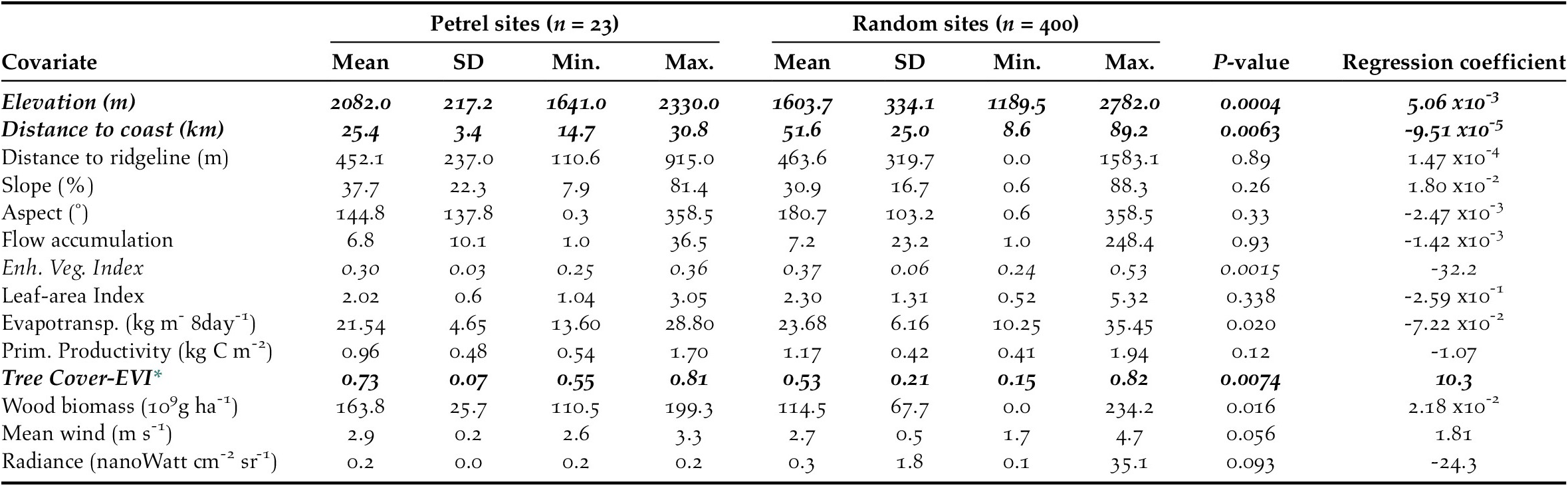
* Tree Cover - Enhanced Vegetation Index
We then ran generalized linear models which included all possible single-level combinations of the three significant predictors, with a binomial logistic structure with presence vs pseudo-absence as the response variable. Limiting the number of model predictors to three environmental variables helped avoid overly complex candidate models that could impair functional accuracy (Warren et al. Reference Warren, Matzke and Iglesias2020). We compared candidate models using Akaike’s Information Criterion for small sample sizes (AICc): a single model had a ΔAICc value < 2 and an Akaike weight > 0.9; therefore, we considered it the top performing model (Table 3). We used a continuous Boyce Index (Hirzel et al. Reference Hirzel, Le Lay, Helfer, Randin and Guisan2006) to estimate how much the model predictions differed from random expectation. Moreover, species distribution models with large spatial extent and high spatial autocorrelation may incorrectly appear to have good discrimination capabilities (Hijmans Reference Hijmans2012). To address this issue, we calculated the amount of spatial sorting bias, which ranges from zero (highly correlated dataset) to one (uncorrelated dataset; Hijmans Reference Hijmans2012). As advised by Lobo et al. (Reference Lobo, Jiménez‐Valverde and Real2008), we also calculated the sensitivity (i.e. true positive rate, or proportion of instances of presence correctly predicted as presence), specificity (i.e. ‘true’ negative rate, which, in our study, refers to the proportion of instances of pseudo-absence predicted as absence), and the threshold of specificity-sensitivity (maximum of the sum of the sensitivity and the specificity; Hijmans Reference Hijmans2012).
Table 3. Characteristics of the candidate models, and Intercept-only model, for Black-capped Petrel nesting habitat suitability on Hispaniola. The best performing model (ΔAICc < 2) is shown in bold.
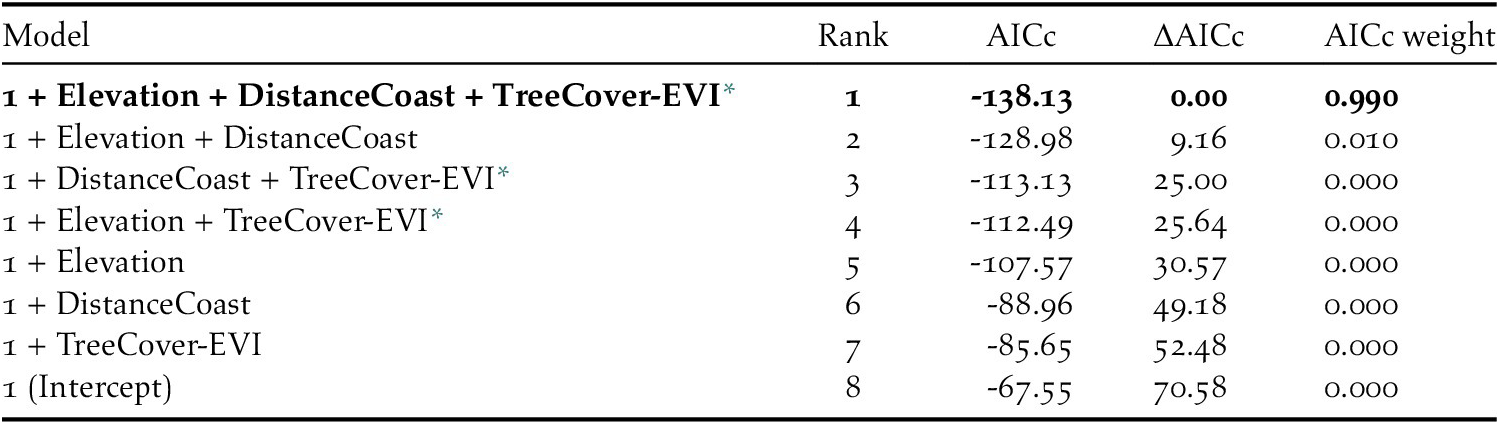
* Tree Cover - Enhanced Vegetation Index
Finally, we applied the regression equation for the final habitat suitability model to the raster layers of the retained environmental variables to produce a map of predicted habitat suitability for the island of Hispaniola, with cell values ranging from zero (habitat not suitable for Black-capped Petrel nesting) to one (habitat highly suitable for Black-capped Petrel nesting). We assessed the validity of the model by calculating predicted suitability values for the presence and pseudo-absence sites in the validation dataset and compared the distribution of suitability values between both groups using an F-test. In addition, we used an independent dataset of locations where petrel activity was recorded by acoustic monitors (McKown et al. Reference McKown, Fleishman and Earl2016, Fleishman and McKown Reference Fleishman and McKown2017), and a dataset of locations where radar surveys performed between 2012 and 2017 suggested possible breeding activity (Brown Reference Brown2017). We then calculated the mean suitability values at survey locations in each dataset.
Habitat availability and habitat loss
Because the habitat suitability model was built using tree cover data for 2000, we calculated the total area of predicted habitat suitable for nesting Black-capped Petrels in Hispaniola in 2018 by subtracting areas where forest loss occurred during 2000–2018 from the Black-capped Petrel habitat suitability raster. We used Google Earth Engine to obtain spatial datasets of total forest loss (Hansen Global Forest Change v1.6: 2000–2018; Hansen et al. Reference Hansen, Potapov, Moore, Hancher, Turubanova, Tyukavina, Thau, Stehman, Goetz, Loveland and Kommareddy2013). Similarly, we also estimated changes in the availability of predicted habitat due to forest loss during the periods 2000–2015 and 2015–2018. We chose 2015 as a cut-off because it is the year when most nest sites were located, and the median year for all nest searches. We estimated the total area and proportion of predicted suitable nesting habitat lost to forest loss during 2000–2015 and 2015–2018, for predicted suitability levels above the specificity-sensitivity threshold and > 0.9. Areas calculated directly from the habitat suitability raster (i.e. two-dimensional areas) would fail to account for the topography of the landscape; therefore, we calculated landscape surface areas on the basis of the DEM (Jenness Reference Jenness2004), using the surfaceArea function in R (package sp) for both suitability levels.
Results
Combining overlapping presence polygons and combining overlapping pseudo-absence polygons resulted in sample sizes of n = 23 presence polygons, with a mean area of 11.1 x103 m2 (range: 7.8 x103 – 28.1 x103 m2), and n = 500 pseudo-absence polygons, each with an area of 7.8 x103 m2 (i.e. no pseudo-absence buffers overlapped). Of the 14 environmental covariates that we compared individually between presence and pseudo-absence sites, six differed significantly and, of those, three were retained for use in generalized linear models (P ≤ 0.01 for each; Table 2).
The predicted suitability of a site for breeding Black-capped Petrels increased with elevation and tree cover-EVI. In contrast, sites were less suitable as distance to coast increased and as EVI increased. Of the eight models assessed, one clearly performed as the top-ranked model. The model that included each of the three retained variables (elevation, distance from site to coast, and tree cover-EVI) carried 99.9% of the model weights (Table 3). Elevation was the strongest contributor to the model (null model: residual deviance = 61.00; elevation: residual deviance = 39.15, deviance = 21.85, pr(>Chi) < 0.005), followed by distance to coast (residual deviance = 27.95, deviance = 11.20, pr(>Chi) < 0.005), and tree cover-EVI (residual deviance = 21.68, deviance = 6.26, pr(>Chi) < 0.05). The top model had a continuous Boyce Index of 0.63, suggesting that model predictions were consistent with the distribution of presence locations in our validation dataset. The dataset had a spatial sorting bias of 0.43, indicating a moderate spatial correlation. The top model had a specificity of 0.63, a sensitivity of 0.71, and the threshold of specificity-sensitivity had a value of 0.65 (Figure 2).
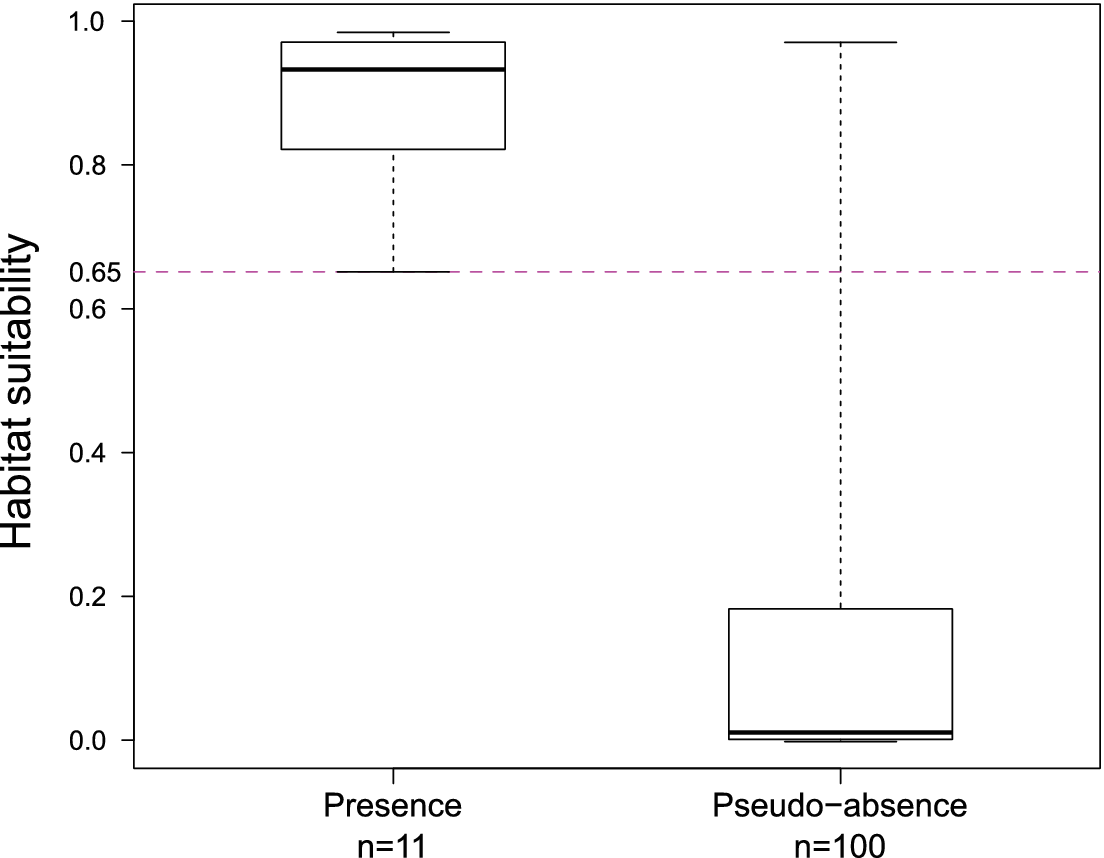
Figure 2. Distribution of predicted habitat suitability values for presence and pseudo-absence sites for Black-capped Petrel on Hispaniola. Solid lines within boxes represent the median, edge of boxes represent quartiles, and whiskers extend to 5th and 95th percentiles. The threshold of specificity-sensitivity is shown with a dashed line.
The presence and pseudo-absence datasets retained for validation had significantly different distributions (F11,100 = 0.12, P < 0.005). The presence dataset had a mean predicted suitability value of 0.88 (range: 0.65–0.98; Figure 2) while the pseudo-absence dataset had a mean predicted suitability value of 0.15 (range: 0.00–0.97; Figure 2). Results suggest that known Black-capped Petrel nesting sites were classified correctly by our model (i.e. predicted suitability values > 0.65). Most sites where petrel activity had been recorded based on other surveys were also classified correctly (Table 4).
Table 4. Proportion of sites from a validating dataset of existing nest sites, and from datasets of acoustic monitoring and radar surveys correctly classified as suitable by the Black-capped Petrel nesting habitat suitability model on Hispaniola. Proportions are calculated as the percentage of sites with predicted suitability values above the suitability threshold.
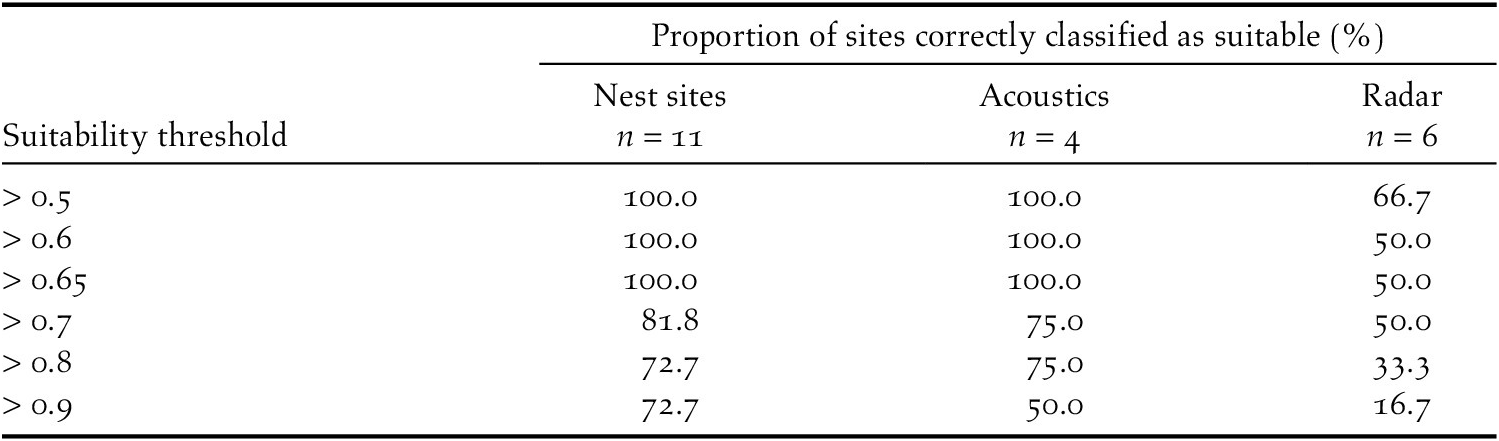
Our model suggested that the higher elevations of Massif de la Hotte, Massif de la Selle, Sierra de Bahoruco, and the south-east Cordillera Central are suitable for nesting Black-capped Petrels (Figure 3, Figure S1; Table 5). The higher elevations of the lower foothills to the southeast of Sierra de Bahoruco also appear highly suitable (Figures 3 and S1). In contrast, Sierra de Neiba and the entirety of the occidental Cordillera Central do not appear suitable for nesting Black-capped Petrels.
Table 5. Total area of predicted suitable habitat currently available for nesting Black-capped Petrel, and amount and proportion of habitat lost to forest loss during 2000–2018 at the four main nesting areas on Hispaniola.
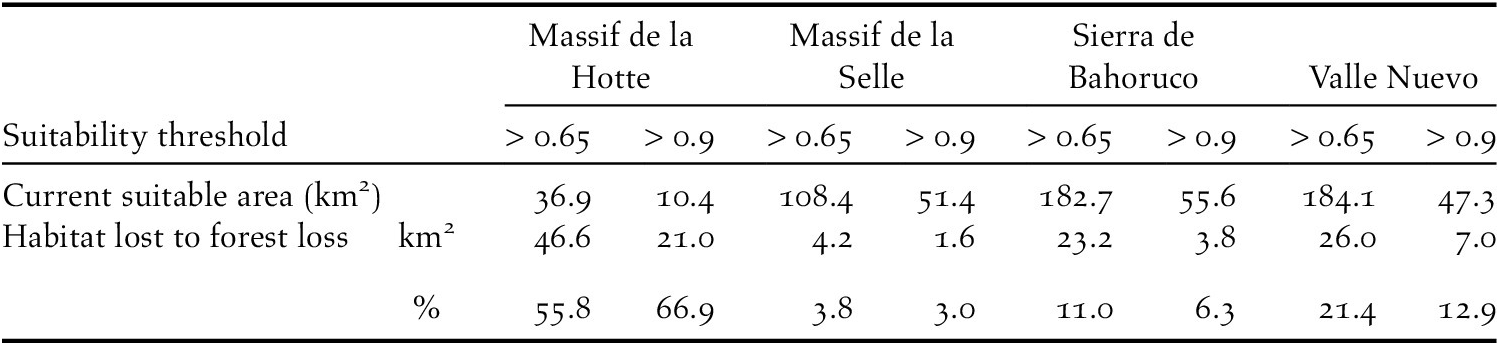
Our models also identified the extent of predicted nesting habitat lost since 2000. In 2018, 563 km2 (suitability > 0.65) and 167 km2 (predicted suitability > 0.9) were available to nesting Black-capped Petrels in Hispaniola (Figures 3 and S2). The majority of predicted suitable areas were located in the Dominican Republic (c.75%; Table 5). Between 2000 and 2015, there was a decline of 6.3% (> 0.65; 41.9 km2) and 3.9% (> 0.9; 7.8 km2) of the predicted habitat originally available in 2000 due to forest loss (see Hansen et al. Reference Hansen, Potapov, Moore, Hancher, Turubanova, Tyukavina, Thau, Stehman, Goetz, Loveland and Kommareddy2013 for definition of forest loss); between 2015 and 2018, there was a decline of 9.9% (> 0.65; 61.6 km2) and 13.3% (> 0.9; 25.6 km2) of the predicted habitat available in 2015 due to forest loss. Overall, between 2000 and 2018, there was a decline of 15.5% (> 0.65; 103.5 km2) and 16.7% (> 0.9; 33.4 km2) of the predicted habitat available in 2000 on Hispaniola due to forest loss (Figure 4). Forest loss primarily affected the Massif de la Hotte, Cordillera Central, and Sierra de Bahoruco with 66.9%, 12.9%, and 11.0% of predicted suitable habitat (> 0.9) lost in each area between 2000–2018, respectively (Table 5).
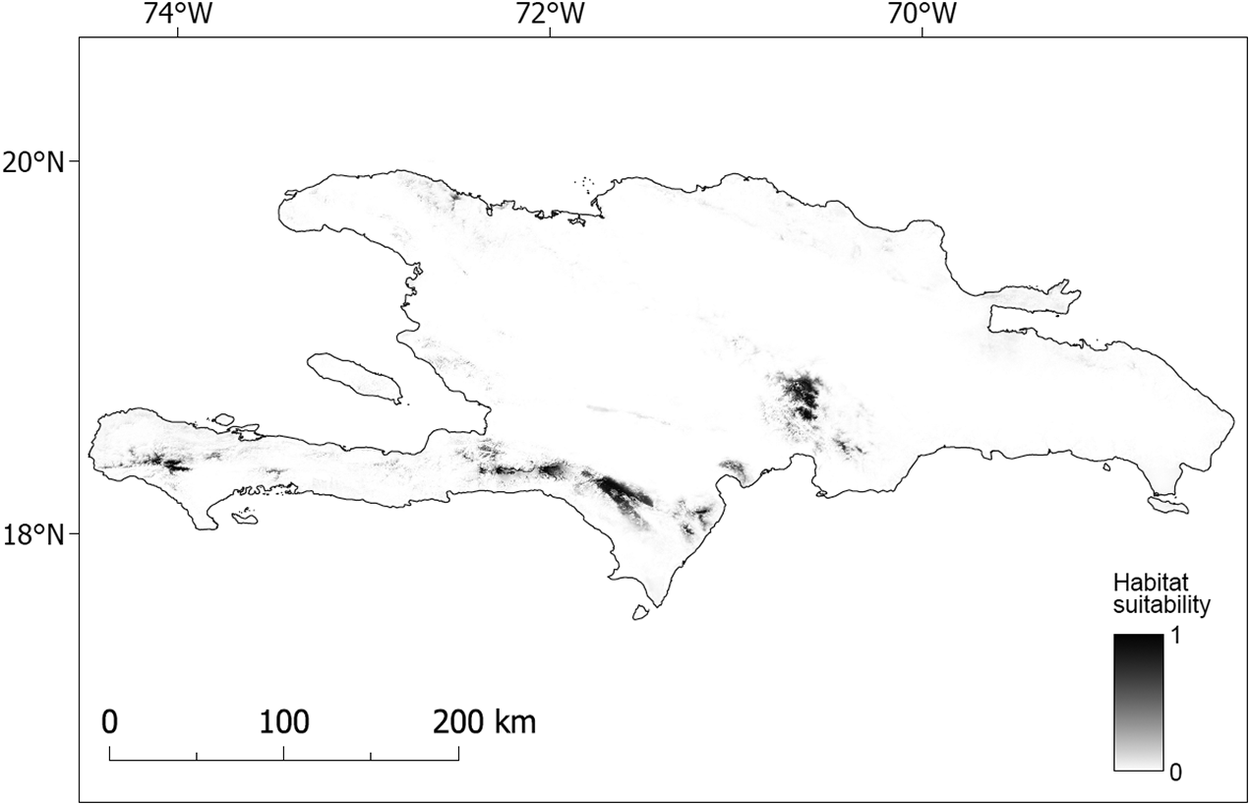
Figure 3. Map of predicted nesting habitat suitability for Black-capped Petrel on Hispaniola.
Areas most suitable for nesting Black-capped Petrels are shown in black. A larger version of this figure is provided as a georeferenced file of 90-m pixel resolution in Figure S2 (https://doi.org/10.5066/P9FWJPBD). A version of this figure showing only the suitable (predicted suitability > 0.65) and most highly suitable habitats (predicted suitability > 0.9) are available in Figure S3 (https://doi.org/10.5066/P9FWJPBD).
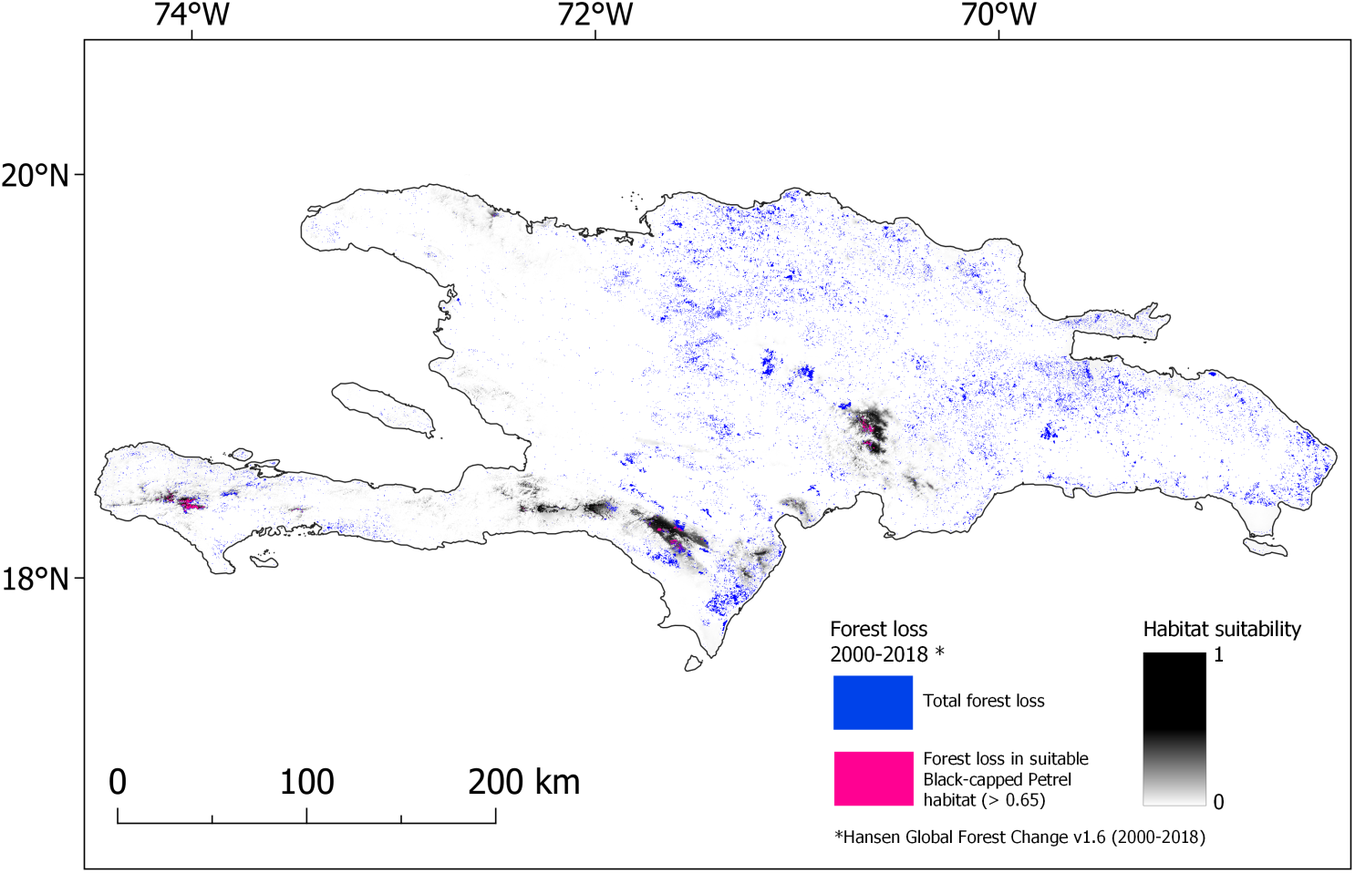
Figure 4. Map of forest lost during 2000-2018 overlaid on nesting habitat suitability for Black-capped Petrel on Hispaniola. Forest loss that occurred in habitat suitable for Black-capped Petrel (> 0.65) is shown in pink; forest loss that occurred elsewhere in Hispaniola is shown in blue. A color version of this figure may be found in the electronic version of this article.
Discussion
Our study provides details on the large-scale habitat characteristics of Black-capped Petrel nesting areas, locates the main suitable areas for the species in Hispaniola, and estimates the extent of the remaining suitable habitat. We documented significant selection for abiotic (elevation and distance to coast) and biotic (EVI and tree cover) habitat features. Our detailed predictive map of Black-capped Petrel habitat suitability located highly suitable habitat in all four elevated areas of Hispaniola where the species is currently known to nest, and in nearby surrounding areas at lower elevations. We also found that predicted suitable habitat is absent from the occidental Cordillera Central. Finally, we calculated the decline in predicted suitable nesting habitat of Black-capped Petrel due to forest loss throughout the last decade.
Environmental variables
Elevation was the strongest predictor in our generalized linear model, with nests occurring c. 2,100 m above sea level. A preference for higher elevation nest sites is consistent with other species of gadfly petrels (Zino et al. Reference Zino, Oliveira, King, Buckle, Biscoito, Neves and Vasconcelos2001, Rayner et al. Reference Rayner, Clout, Stamp, Imber, Brunton and Hauber2007, Pinet et al. Reference Pinet, Salamolard, Probst, Russell, Jaquemet and Le Corre2009, Scott et al. Reference Scott, Moller, FletcHer, Newman, Aryal, Bragg and Charleton2009, Troy et al. Reference Troy, Holmes, Veech, Raine and Green2017). Black-capped Petrels may nest at higher elevations to avoid predation by introduced mammalian predators (Simons et al. Reference Simons, Lee and Haney2013), which appear to be more common at lower elevations, or to avoid anthropogenic disturbances including deforestation. However, unlike other tropical and subtropical petrel species (Rayner et al. Reference Rayner, Clout, Stamp, Imber, Brunton and Hauber2007, Pinet et al. Reference Pinet, Salamolard, Probst, Russell, Jaquemet and Le Corre2009, Scott et al. Reference Scott, Moller, FletcHer, Newman, Aryal, Bragg and Charleton2009, Troy et al. Reference Troy, Holmes, Veech, Raine and Green2017, Krüger et al. Reference Krüger, Paiva, Petry, Montone and Ramos2018), nesting sites of Black-capped Petrels did not appear to be located more on areas with steep slopes than on less steep areas. In their study of Cory’s shearwaters Calonectris borealis, Oppel et al. (Reference Oppel, Hervias, Oliveira, Pipa, Silva, Geraldes, Goh, Immler and McKown2014) found that nests tended to occur either on vertical cliffs or less steep areas, but not at sites with intermediate slopes. Similarly, known nest sites for Black-capped Petrels have either been located on extremely steep slopes in Haiti, or in varied mountainous terrain (varied slopes, flat ridges, bottom of thalwegs) in the Dominican Republic. As with elevation, the occurrence of nesting sites on steep slopes in Haiti may be more a consequence of anthropogenic disturbances than intentional selection, where extremely steep terrain creates a refuge from agriculture. Thus, habitat availability due to regional variation in mountainous terrain may outweigh any potential selectivity based on slope.
Known nesting sites of Black-capped Petrels were associated with increased tree cover-EVI, and decreased EVI. An ad hoc composite variable, tree cover-EVI provides information on the forest structure, while the stand-alone EVI provides information on the type and richness of the vegetation. The intermediate EVI values associated with nesting areas, while representative of mature forested areas, are typically associated with sparser green vegetation and intermediate tree richness (in comparison, dense rainforests and diverse forests have higher EVI values; Waring et al. Reference Waring, Coops, Fan and Nightingale2006). Therefore, our results suggest that, at the macro-habitat level, Black-capped Petrels nest in productive forests with undergrowth and greater canopy cover but appear to prefer drier pine forests rather than dense cloud-forests. Camera trapping and lost feathers suggest that petrels sometimes take off directly from the ground after selecting a suitable location near an opening in the canopy. Thus, the thin canopy cover of pine forests may ease access to and from nesting sites. However, our model may be influenced by the habitat types associated with the highest frequency of nests. For example, a larger proportion of nest sites have been located in the Sierra de Bahoruco, a karstic mountain range characterized by forests of Hispaniolan Pine Pinus occidentalis in uniform stands (i.e. drier pine forest) or mixed with evergreen broadleaf species (Darrow and Zanoni Reference Darrow and Zanoni1990, ESA Climate Change Initiative 2019). Areas with differing but potentially suitable vegetation communities (e.g. broadleaf forests interspersed with Hispaniolan Pines in Valle Nuevo, and shrub-like broadleaf vegetation with few large trees on steep slopes in La Visite) have not yet been searched as extensively. Therefore, as searches for additional nesting sites are ongoing, it would be valuable to improve this habitat suitability model with the inclusion of new nesting locations in differing habitats.
Nest sites of Black-capped Petrels were associated with areas closer to the coastline (mean = 25.4 km). Since all known nest sites have been located within c.30 km of the nearest coastline, our model was strongly influenced by nest sites within close range of the coast. This may partly explain why two central mountainous areas on Hispaniola suspected to host Black-capped Petrels (Sierra de Neiba and occidental Cordillera Central) are not categorized as suitable by our model. Black-capped Petrels typically fly at sustained speeds of 15–49 km/h at sea (Jodice et al. Reference Jodice, Ronconi, Rupp, Wallace and Satgé2015, Satgé et al. Reference Satgé, Rupp and Jodice2019) and above 50 km/h over land (Brown Reference Brown2016) and may rapidly reach any location in Hispaniola. Other seabirds do nest far inland: recently, a nesting area of the Hornby’s Storm-petrel Oceanodroma hornbyi has been discovered c.75 km from the coastline (Barros et al. Reference Barros, Medrano, Silva and Groote2018); Marbled murrelets Brachyramphus marmoratus may nest up to 90 km away from the coast (Hamer Reference Hamer and Ralph1995); and inland colonies of Antarctic petrels Thalassoica antarctica can be located > 200 km from the nearest open water (van Franeker et al. Reference Franeker, Gavrilo, Mehlum, Veit and Woehler1999). However, radar surveys of the central mountain ranges on Hispaniola suggest that, although petrels may reach the areas, they do not breed there (Brown Reference Brown2014). Furthermore, distance to coast was not the strongest predictor in our model, which was most strongly influenced by elevation. In fact, distance to coast and tree cover-EVI had a smaller deviance between each other than distance to coast had with elevation. This suggests that it is the combined influence of distance and vegetation type, more so than higher detection of nest sites in more coastal areas, that identified the central mountain ranges as not suitable for nesting Black-capped Petrels.
Location of suitable habitat
The only existing assessment of potential nesting habitat for the Black-capped Petrel (Leon, cited in U.S. Fish and Wildlife Service Reference Fish2018) calculated a maximum potential area of 2,343 km2 based solely on elevation (all habitat on Hispaniola ≥1,500 m above sea level). By using environmental predictors, we refined the estimate in Leon (U.S. Fish and Wildlife Service Reference Fish2018) to include suitable habitat below and above 1,500 m. Our most liberal estimate of potential nesting habitat is 563.3 km2 (predicted suitability > 0.65), with our most conservative predictions indicating that the most suitable habitat is limited to a total area of 167.1 km2 (predicted suitability > 0.9). Our model correctly located the four known major nesting areas. Among them, areas in Haiti (Massif de la Hotte and Massif de la Selle) are the most limited in size (145.3 km2, predicted suitability > 0.65; c.25% of the predicted suitable habitat on Hispaniola). In the Dominican Republic, 366.8 km2 are predicted to be available (> 0.65). This difference in available habitat between the two nations is due in part to the preponderance of mountainous areas in the Dominican Republic compared to Haiti. Further, most forested areas in Haiti have been cut throughout the 20th century and prior to the baseline year (2000) of our analysis (Hedges et al. Reference Hedges, Cohen, Timyan and Yang2018), thus effectively reducing the extent of mature forests to high mountain tops and steep slopes (Hedges et al. Reference Hedges, Cohen, Timyan and Yang2018).
In Haiti, the predicted suitable areas for nesting Black-capped Petrels are located on the slopes and cirques of massifs de la Hotte and de la Selle. The steep north-facing slopes of the La Visite escarpment and banks of steep valleys descending from Pic de la Selle were also classified as highly suitable. In the Dominican Republic, most of the predicted suitable habitat occurs in the Sierra de Bahoruco and Cordillera Central ranges and covers the higher elevations of both massifs. Predicted suitable habitat is also available on lower foothills of the oriental Sierra de Bahoruco. A further detailed description of the location of predicted suitable habitat in Haiti and the Dominican Republic is available in Appendix S2.
Habitat loss
Our study shows that forest loss was the cause of a significant decline in the amount of predicted suitable habitat for nesting Black-capped Petrels in Hispaniola between 2000 and 2018. Although the reasons for forest loss were not explicit in the dataset we used (Hansen et al. Reference Hansen, Potapov, Moore, Hancher, Turubanova, Tyukavina, Thau, Stehman, Goetz, Loveland and Kommareddy2013), we suggest that three main causes are responsible for the majority of the loss on Hispaniola: hurricanes, forest fires, and deforestation. Habitat loss was not consistent among all years and the source data we used (Hansen et al. Reference Hansen, Potapov, Moore, Hancher, Turubanova, Tyukavina, Thau, Stehman, Goetz, Loveland and Kommareddy2013) indicate that forest loss was nearly double from 2015 to 2018 compared to 2000–2015. Most of the habitat loss during 2015–2018 occurred in the Massif de la Hotte, where 55.8% (46.6 km2) of the predicted suitable nesting habitat (> 0.65) was lost during the last quarter of 2016 (Global Forest Watch 2019b). This swift event was likely caused by Hurricane Matthew, a major hurricane of category 4 which made landfall on the Haitian coast c.50 km east of La Hotte and caused extensive damage (Stewart Reference Stewart2017). Black-capped Petrels are thought to have adapted their phenology to avoid nesting and rearing chicks during the hurricane season (Simons et al. Reference Simons, Lee and Haney2013). However, due to the loss or degradation of forest habitat caused by large storms, hurricanes are causes of concern for the resiliency of the species: the U.S. Fish and Wildlife Service (Reference Fish2018) has listed the increase of hurricane intensity and frequency due to climate change as a major threat to the viability of the Black-capped Petrel population. Since no nesting sites have been located in the Massif de la Hotte, Hurricane Matthew would not have impacted the statistical correlation between nesting sites and environmental predictors, or the resulting equation used in our habitat model. Nevertheless, it could have decreased the amount of predicted habitat that was categorized as suitable by our model in the area, thus more suitable habitat may have been available for nesting Black-capped Petrel in the Massif de la Hotte than predicted in our study.
In the Dominican Republic, the predicted suitable habitats of Sierra de Bahoruco and Cordillera Central appear to have been affected by several forest fires from 2006 to 2016 (Global Forest Watch 2019b, Lloyd and León Reference Lloyd and León2019). Natural forest fires are recurrent events in dry forests of Hispaniola Pine and, to an extent, may be beneficial for Black-capped Petrels in creating the open overstorey needed to easily access and leave nesting sites (Simons et al. Reference Simons, Lee and Haney2013). However, fires strong enough to affect the canopy of Hispaniolan Pines, and thus be recorded as forest loss in satellite imagery, are likely to also cause extensive damage to the forest floor and understorey, hence affecting the mesic microhabitat needed by Black-capped Petrels to nest.
Finally, to a lesser, but more persistent extent than hurricanes or natural forest fires, deforestation for agriculture appears to have impacted predicted Black-capped Petrel habitat in both countries. In particular, illegal deforestation appears to occur regularly in petrel habitat within the boundaries of the La Visite (Brown pers. obs.), Sierra de Bahoruco and Valle Nuevo national parks (Lloyd and León Reference Lloyd and León2019, Rupp pers. obs.). In La Visite, deforestation is limited by the extreme slopes of observed petrel habitat but occurs on the periphery of predicted suitable habitat. In Sierra de Bahoruco, avocado plantations are progressing inside the national park boundaries and reaching the lower fringe of predicted suitable habitat, while in Valle Nuevo, farming of cash crops is occurring within hundreds of meters of the recently discovered nesting sites (Rupp pers. obs.). In Hispaniola, national parks offer some level of protection but are often not entirely effective at protecting against small scale deforestation and accumulated encroachment (Sangermano et al. Reference Sangermano, Bol, Galvis, Gullison, Hardner and Ross2015, Hedges et al. Reference Hedges, Cohen, Timyan and Yang2018, Lloyd and León Reference Lloyd and León2019). Although the areas currently lost to deforestation represent only a small fraction of the predicted suitable habitat for Black-capped Petrels and of the overall protected areas in Hispaniola, anthropogenic activities may create additional disturbances beyond habitat loss (e.g. lighted buildings, noise, fires, collection of firewood in nesting habitat) and subsequently attract mammalian predators (e.g. rats, cats, dogs, mongoose) into nesting areas. Moreover, our estimation of habitat loss only accounts for predicted habitat that was directly changed. Predicted habitat that may have been encroached upon or disturbed and made less suitable for Black-capped Petrels may not have been captured in our analysis. Therefore, we suggest that our results represent a minimum estimate of habitat loss.
Conclusion
Our study characterizes the macro habitat for nesting Black-capped Petrels in Hispaniola and demonstrates that available suitable habitat has been increasingly impacted by natural and anthropogenic forest loss over the last two decades. The results of our study can inform searches for unknown nesting areas in Hispaniola and can be applied to other islands in the region where the species is suspected to breed. Finally, our results demonstrate that conservation of the mountainous habitat of Hispaniola would benefit the species by protecting habitat suitable for nesting. Given that other globally rare and vulnerable species use similar habitat, such as Bicknell’s Thrush Catharus bicknelli (McFarland et al. Reference McFarland, Rimmer, Goetz, Aubry, Wunderle, Sutton, Townsend, Sosa and Kirkconnell2013) and Hispaniolan Solenodon Solenodon paradoxus (Rodríguez Reference Rodríguez2011), conservation actions applied in these regions may have multi-species benefits.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0959270920000490.
Acknowledgements
We thank Theodore Simons and two anonymous reviewers who improved earlier versions of this manuscript. Support for this analysis was provided by the South Carolina Cooperative Fish and Wildlife Research Unit and Clemson University. In addition to the institutions of the authors, nest searches were supported by the American Bird Conservancy, the U.S. Fish and Wildlife Service, Cornell University, Disney Conservation, and the BirdsCaribbean Betty Petersen Fund for Conservation. In addition to the authors, nest sites used in this study were located by James E. Goetz, Esteban Garrido, Anderson Jean, René Jeune, Tinio Louis, Lionel Raymond, Jonel Bazil, Pirrín Jairo Matos, Gerson Feliz, José Luis Castillo, and Ivan Terrero. We thank members of the International Black-capped Petrel Conservation Group for helpful comments at all stages of the analysis. Permits for field research were provided by the Ministerio de Medio Ambiente y Recursos Naturales de la República Dominicana, and the Ministère de l’Environnement de la République d’Haïti. The South Carolina Cooperative Fish and Wildlife Research Unit is supported by the South Carolina Department of Natural Resources, Clemson University, the US Fish and Wildlife Service, and the US Geological Survey. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US government.











