Introduction
Forest-nesting raptor populations can act as valuable bioindicators of changes and stresses in ecosystems, as they are sensitive to land use and habitat structure alterations and are highly susceptible to local extinctions (Sergio et al. Reference Sergio, Scandolara, Marchesi, Pedrini and Penteriani2005, Bednarz Reference Bednarz, Bird and Bildstein2007). For a variety of forest-nesting raptors, breeding success is influenced by habitat quality (Newton Reference Newton1989), food resources (Korpimaki Reference Korpimaki1987), and competition (Hakkarainen and Korpimaki Reference Hakkarainen and Korpimaki1996, Krüger Reference Krüger2004), among other drivers. Medium- and large-sized forest raptors select for nest-building large wide-trunk trees in mature forests with open space around the trunks (Suárez et al. Reference Suárez, Balbontin and Ferrer2000, Barrientos and Arroyo Reference Barrientos and Arroyo2014), because they provide both a greater accessibility to nests and leaf cover above the nesting site (Martínez et al. Reference Martínez, Cremades, Pagán, Calvo, Chancellor and Meyburg2004). Nest predation for these species is relatively low, and protection from thermal extremes may be one of the most important factors in nest-site selection (Newton Reference Newton1979, Janes Reference Janes and Cody1985). In small raptors, leaf cover provided by the treetop above nests, reduces the rate of detection by aerial predators and protects nests from wind, rain, and heating from solar radiation (Walsberg Reference Walsberg and Cody1985, Lohmus Reference Lohmus2006). In addition, high quality trees for nesting can be occupied alternatively by different species through different breeding seasons when they last for several years (Jiménez-Franco et al. Reference Jiménez-Franco, Martínez and Calvo2014).
When several raptor species occupy the same habitat, this could cause conspecific and interspecific competition for available prime nesting habitats and also food resources, and may promote intraguild predation (Hakkarainen et al. Reference Hakkarainen, Mykra, Kurki, Tornberg and Jungell2004). The effect of competition might be stronger in populations near carrying capacity, a situation that would conduct raptors to occupy territories of lower quality, particularly in years of a higher density of breeding pairs (Newton Reference Newton1979, Krüger and Lindström Reference Krüger and Lindström2001). Nevertheless Martínez et al. (2006) found that medium-sized raptors seem to select nesting patches close to conspecifics. Interspecific competition would also benefit larger raptors over smaller ones, because they compete more successfully (Schoener Reference Schoener1983, Basset Reference Basset1996).
Multispecies studies of raptor nest-site habitat selection in temperate forest are abundant, but they are not as common for Mediterranean regions (BirdLife International 2004, Barrientos and Arroyo Reference Barrientos and Arroyo2014, Poirazidis Reference Poirazidis2017, Bouchahdane et al. Reference Bouchahdane, Boukhemza, Souttou and Derridj2019). Maamora Cork Oak forest in northern Morocco is the largest single stand of Cork Oak in the world (Emberger Reference Emberger1939). Forestry practices, mainly the periodical harvesting of tree bark and the planting of non-autochthonous species (Fennane and Rejdali Reference Fennane and Rejdali2015), make it particularly vulnerable to disturbances of its forest raptors (Margalida et al. Reference Margalida, Moreno-Opo, Arroyo and Arredondo2010). Such practices include for example, the reduction of areas of mature Cork Oak trees, their replacement by young and dense plantations of Eucalyptus globulus and Acacia saligna, or the inadequate bark harvesting of the Cork Oak trees not respecting the age, height, and health stage of each tree (Lahssini et al. Reference Lahssini, Lahlaoi, Alaoui, Hlal, Bagaram and Ponette2015). In addition, those practices favour the increase of the road network, which can conflict with raptor conservation and can modify habitat selection patterns (Bielanski Reference Bielanski2006). In addition, human disturbances, i.e. the weekly presence of 5,000 cars and 30,000 visitors on sunny days (Fennane and Rejdali Reference Fennane and Rejdali2015), indicate the possibility of disturbance for raptor nesting. However, these disruptive actions are regulated in some protected reserves within Maamora and are hotspot areas for conservation (Segura and Acevedo Reference Segura and Acevedo2019).
Mediterranean forest raptors might be considered as indicators of the health of threatened Mediterranean ecosystems because of their environmental sensitivity and their role at the top of the food chain (Sergio et al. Reference Sergio, Scandolara, Marchesi, Pedrini and Penteriani2005). For instance, medium-sized raptors such as Booted Eagle Hieraaetus pennatus – strictly a forest species – nest in tall trees in mature forests characterised by low disturbance (Suárez et al. Reference Suárez, Balbontin and Ferrer2000), and the opportunistic Long-legged Buzzard Buteo rufinus and Black Kite Milvus migrans reuse nests previously built by medium-sized raptors such as Booted Eagle (Sergio et al. Reference Sergio, Pedrini and Marchesi2003, Messabhia et al. Reference Messabhia, Bensaci, Telailia, Rebbah and Saheb2019). The three species have been documented foraging wild European Rabbits Oryctolagus cuniculus as a main prey, when it is highly available (Veiga Reference Veiga1986, Viñuela and Veiga Reference Viñuela and Veiga1992, Alivizatos and Goutner Reference Alivizatos and Goutner1997). The small-sized Common Kestrel Falco tinnunculus does not build nests and takes advantage of both medium-sized raptor and corvid nests and selects more open habitats (Fargallo et al. Reference Fargallo, Martínez-Padilla, Viñuela, Blanco, Torre, Vergara and de Neve2009). The four raptor species are protected in Morocco by Law nº 29 05 for the protection of species of flora and fauna and the control of their trade. Internationally, they are red listed as “Least Concern” by the International Union for Conservation of Nature (IUCN) (Garrido et al. Reference Garrido, Numa, Barrios, Qninba, Riad, Haitham and Hasnaoui2021). Their populations in North Africa are stable or not believed to be decreasing rapidly enough to approach the thresholds of a threatened category, even though the Long-legged Buzzard population in Morocco has decreased (Garrido et al. Reference Garrido, Numa, Barrios, Qninba, Riad, Haitham and Hasnaoui2021).
Our study aimed to identify common targets of nest-site selection for Mediterranean populations of these raptor species, since an important overlap between forest traits and habitat use could be expected. To address this goal within a protected reserve characterised by a lack of forestry practices and human disturbances we aimed (1) to build four species-specific models to identify the drivers (i.e. habitat, food availability, and competition) that might determine nest-site selection and (2) analyse the spatial environmental overlap of raptor nest sites. This study provides ecological information that can be used to identify conservation priorities for these forest raptors in Mediterranean habitats in North Africa and support management actions for effective conservation plans.
Methods
Study area
The study was conducted in an area of low elevation (72–185 m a.s.l.) and sandy soil within Maamora forest in north-west Morocco (34°02’ 54.19’N, 6°27’ 19.24’W), in which the closest village (Sidi bou Kalkal, 7,200 inhabitants) is 5 km away. The climate is Mediterranean, with hot, dry summers, and the annual rainfall range is between 300 mm and 500 mm. Maamora forest is dominated by Cork Oak Quercus suber trees, with scattered endemic wild Pear Pyrus mamorensis, wild Olive Olea europaea, Green Olive Phillyrea latifolia, and Mastic Pistacia lentiscus, with a sparse understorey comprising scrub species such as Mediterranean Brooms Genista linifolia, Cytisus arboreus, Stauracanthus genistoides, Dwarf Palm Chamaerops humilis, French Lavender Lavandula stoechas, Sage-leaved Rockrose Cistus salviifolius, Halimium halimifolium, and Thymelaea lythroides. Dense Cork Oak forest (more than 200 trees/ha) covers only 4,110 ha, whereas the majority of the forest (54,000 ha) is considered an open forest with a medium density of Cork Oak trees (Aafi Reference Aafi2007).
The study was conducted on a private protected reserve (2,500 ha) dominated by 80–198 Cork Oak trees/ha (see Figure 1). It is characterised by considerable and varied undergrowth (i.e. high species richness and cover) compared with other unprotected sites in Maamora (Segura and Acevedo Reference Segura and Acevedo2019). It holds a high diversity of diurnal raptors, both breeders (seven species, see Table 1) and winter residents (seven species, see Supplementary material Table S1), and nocturnal breeder raptors (four species, Table S1). There are high densities and diversity of small/medium-sized birds (14 species, e.g. Great Spotted Woodpecker, wild pigeon, Chaffinch; Segura Reference Segura2017), amphibians (five species, e.g. Moroccan Spadefoot Toad Pelobates varaldii; de Pous et al. Reference de Pous, Beukema, Dingemans, Donaire, Geniez and El Mouden2011), reptiles (18 species, e.g. Spiny Footed Lizard), and small mammals (11 species, e.g. wild rabbit). Carnivores are in low numbers due to previous population regulation and are represented by Red Fox Vulpes vulpes and Common Weasel Mustela nivalis.
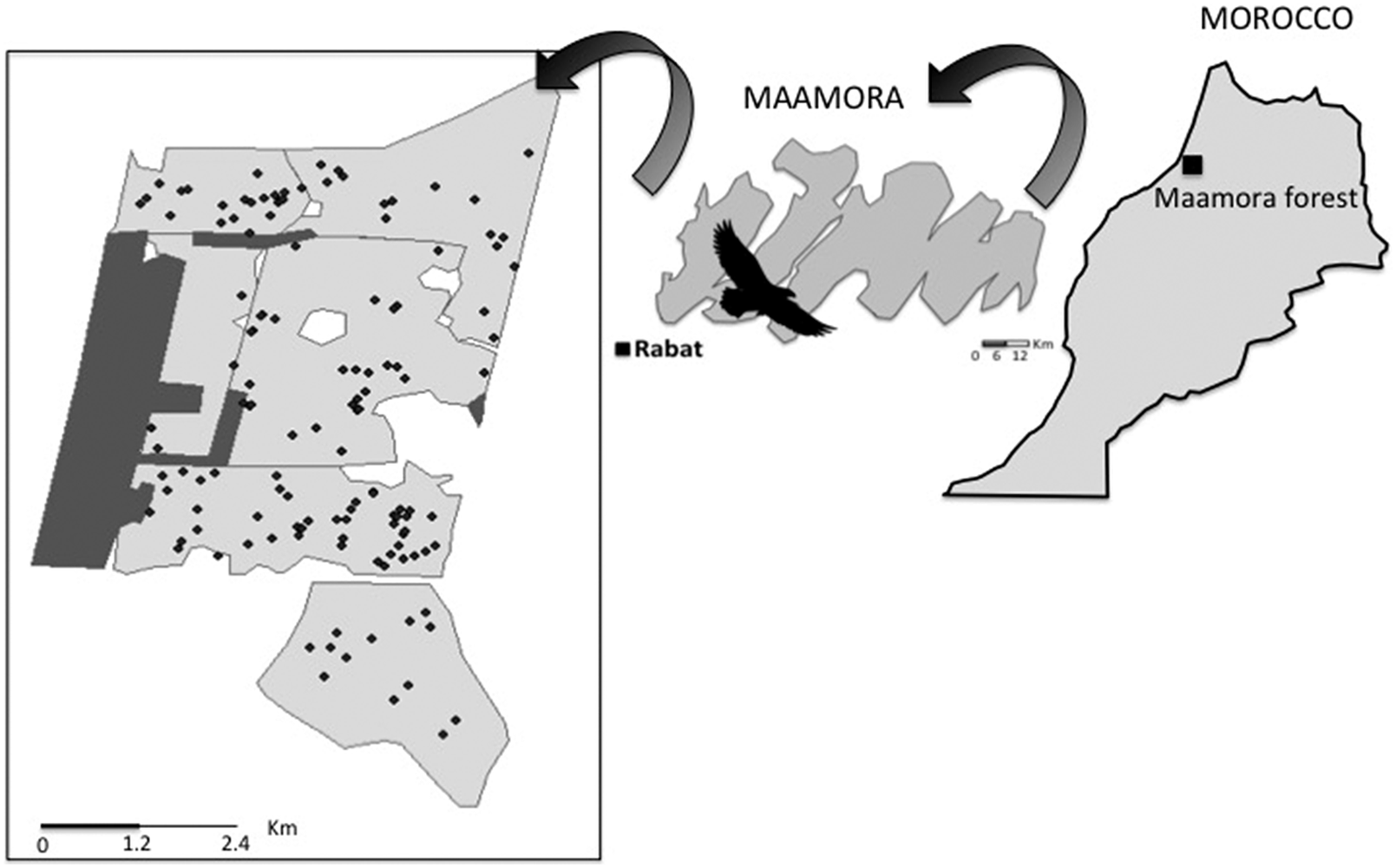
Figure 1. The location of the study area (represented by the eagle) in Maamora forest, north-western Morocco, close to Rabat city. The study site is dominated by Cork Oak trees (in light grey) and to a lesser extent by plantations of Eucalyptus globulus and Acacia saligna (in dark grey). Nest locations are represented by black dots.
Table 1. Protection and population stage in Africa (Garrido et al. Reference Garrido, Numa, Barrios, Qninba, Riad, Haitham and Hasnaoui2021) and characteristics of diurnal forest raptors that breed in trees in Maamora forest. Despite Booted Eagle creating the nests, Long-legged Buzzard and Black Kite repair or decorate with ropes and plastics previous Booted Eagle nests. Common Kestrel occupies nests created in wild Pear trees by Magpies. Lanner Falcon and Long-legged Buzzard are the first nesters in Maamora and most of their fledglings leave the nest at the end of April; Eurasian Hobby is the latest nester because it breeds in nests earlier occupied by Common Raven.

* Species with fewer than 10 nests in the two-year period.
On the study species
We considered the four most widespread species: Booted Eagle, which is an effective hunter particularly sensitive to the management of forest ecosystems (García Dios and Viñuela Reference García Dios and Viñuela2000, Suárez et al. Reference Suárez, Balbontin and Ferrer2000, Bosch et al. Reference Bosch, Borras and Freixas2005); Long-legged Buzzard, an adaptable predator with a broad habitat niche; Black Kite, which is an opportunistic hunter and scavenger (Sergio et al. Reference Sergio, Pedrini and Marchesi2003, Messabhia et al. Reference Messabhia, Bensaci, Telailia, Rebbah and Saheb2019); Common Kestrel, a small generalist falcon (Fargallo et al. Reference Fargallo, Martínez-Padilla, Viñuela, Blanco, Torre, Vergara and de Neve2009). All these species occur together in some other Mediterranean areas (Poirazidis Reference Poirazidis2017). The nests of the studied species represented 89% of the total raptor nests found in the area. We excluded five nests of Lanner Falcons Falco biarmicus (first occurrence documented in Maamora till date), four nests of Black-winged Kite Elanus caeruleus, and 10 nests of Eurasian Hobby Falco subbuteo due to the small populations in the study area.
The four studied species are an example of plasticity in foraging behaviour and food consumption. They feed on small mammals (lagomorphs and rodents), small/medium-sized birds, reptiles, and amphibians. Among mammals, wild rabbits seem to represent a high biomass in Booted Eagle (27% biomass; Martínez et al. Reference Martínez, Cremades, Pagán, Calvo, Chancellor and Meyburg2004; see also Veiga Reference Veiga1986, Moreno and Villafuerte Reference Moreno and Villafuerte1995), a high percentage of the diet of Long-legged Buzzard (59%; Alivizatos and Goutner Reference Alivizatos and Goutner1997), and a staple prey for Black Kites; whenever rabbits are abundant, they can even determine nesting density and distribution in Mediterranean areas such as the Doñana National Park in southern Spain (Viñuela and Veiga Reference Viñuela and Veiga1992, Viñuela et al. Reference Viñuela, Villafuerte and le Court1994, Villafuerte and Viñuela Reference Villafuerte and Viñuela1999). The major threats to these species in North Africa are habitat destruction and disturbances in the forests, leading them to abandon their breeding territories with a potential association with an increase in powerline electrocutions (Godino et al. Reference Godino, Garrido, El Khamlichi, Buron, Machado, Ameziam and Irizi2016, Garrido et al. Reference Garrido, Numa, Barrios, Qninba, Riad, Haitham and Hasnaoui2021).
Sampling forest raptors
The forest raptor species nests were sought out across the study area each spring (March–June) in 2019 and 2020. Nests were visited approximately twice a month after an adult was observed incubating or young could be seen in the nest. The location of the active nests and the number of breeding pairs were recorded, along with their reproductive success, quantified as the percentage of nests with fledglings that left their nest between May and early June (Table S3). The COVID pandemic did not interfere in the nesting of the raptors in 2020 due to the non-disturbance characteristics of the study area.
Ecological, biotic, and anthropogenic characteristics
Each forest raptor nest was georeferenced and the height (H) (m) and diameter at breast height (DBH) (m) of the tree in which it was located (focal tree) were measured. We also recorded and estimated: (1) the DBH average of the three closest trees; (2) their average distance from the focal tree; (3) the density of young and mature trees (<30 cm or >30 cm DBH, respectively); (4) the scrub cover (percentage) in a 20-m radius buffer around each nest tree (James and Shugart Reference James and Shugart1970); these are variables previously reported as relevant in forest raptor habitat selection studies and representative of the nesting microhabitat (Selas Reference Selas1997, Poirazidis et al. Reference Poirazidis, Goutner, Tsachalidis and Kati2007, Barrientos and Arroyo Reference Barrientos and Arroyo2014). These measurements were made in the year in which each nest was monitored but after the young had fledged. To measure anthropogenic influence on raptors’ behaviour, we estimated the distance from the nest to the nearest road (Selas Reference Selas1997, Poirazidis et al. Reference Poirazidis, Goutner, Tsachalidis and Kati2007, Barrientos and Arroyo Reference Barrientos and Arroyo2014). Finally, the shortest distance to the nearest conspecific and interspecific nest was estimated to characterise the spatial distribution of the nests and infer the potential effects of competition (Segura and Acevedo Reference Segura and Acevedo2021). All spatial measures were carried out using ArcGIS 10.8.1 (ESRI 2011).
The influence of prey abundance on raptors’ nest selection in our study area was inferred by including the rabbit population relative abundance as predictor (Selas Reference Selas1997, Marti et al. Reference Marti, Bechard, Jaksic, Bird and Bildstein2007). The rabbit population was surveyed at night using spotlights while driving at speeds of 20–30 km/hour along 11 tracks of 3–5-km length and 30-m wide on non-brightest nights (full moon) in March 2019 and 2020. These transects were regularly distributed by chance over the study area (see Figure S2). Therefore, the relative abundances of the rabbit population were estimated as kilometric abundance indices (Barrio et al. Reference Barrio, Acevedo and Tortosa2009), averaging the two-year period due to the similarities of the rabbit densities.
Modelling nest-site selection
To determine nest-site selection, we randomly sampled 80 trees without a raptor nest. They were separated by at least 400 m (Bakaloudis et al. Reference Bakaloudis, Vlachos, Pagageorgiou and Holloway2001, Barrientos and Arroyo Reference Barrientos and Arroyo2014), and the same environmental and anthropogenic variables ascertained for those trees with nests were estimated. The singularities of the trees with a nest were identified using generalised linear mixed models, with a binomial distribution and logit link function (response variable: presence or absence of nest). Prior to modelling, we assessed the collinearity among predictors using the variation inflation factor (VIF). The most parsimonious model was then selected using a forward stepwise procedure based on Akaike Information Criteria (AIC) (Akaike Reference Akaike1974). Nest ID and area (territorial unit used to characterise rabbit population abundance, n = 11) were considered as random effect factors since the same nest was used in more than one year and several nests were considered within the same area to account for prey-related effects. Tree height, DBH, density of young and mature trees, DBH of the three closest trees and their distance from the nest, scrub cover, distance to road, conspecific and interspecific distance, and rabbit density were considered covariates.
Environmental overlap test
The environmental principal component analysis (PCA) method was used to characterise and compare the environmental characteristics selected between the four forest raptor species (Broennimann et al. Reference Broennimann, Fitzpatrick, Pearman, Petitpierre, Pellissier, coz and Thuiller2012). PCA was performed with the eight environmental variables estimated for forest raptor modelling and a kernel density function was used to create occurrence density plots for each forest raptor species. PCA analysis, occurrence density plots, and environmental overlap were estimated using the “ecospat” R package (Broennimann et al. Reference Broennimann, Fitzpatrick, Pearman, Petitpierre, Pellissier, coz and Thuiller2012). Schoener’s overlap (D) (Warren et al. Reference Warren, Glor and Turelli2008) was selected as overlap index in nest preferences between pairs of species (0 no overlap to 1 identical environmental selection). In order to compare statistically the observed overlap between pairs of species and the overlap between their environmental envelopes, which were created with random reallocations of observed occurrences, a similarity test was performed using the “ecospat.niche.similarity.test” function in the “ecospat” package.
Results
The total abundance of nests of the four species was similar in both years (75 and 74, for 2019 and 2020, respectively). The density of breeding pairs between years was 3.3–3.0 pairs/km2: Booted Eagle (1.2–1.1); Long-legged Buzzard (0.4–0.5); Black Kite (0.7–0.8); Common Kestrel (0.7–0.6). Breeding success was over 82% in the four species in the two-year period (see Table S3). All the nests of Booted Eagle, Long-legged Buzzard, and Black Kite in the two years were located in Cork Oak trees and those of Common Kestrel in both Cork Oak trees and wild Pear trees (n = 129; see Table 2 for differences between years). Some nests were reused between years (13%) by the same species (Black Kite n = 5, Booted Eagle n = 7, Common Kestrel n = 3) or different species (Black Kite used previous Booted Eagle nest n = 3, Common Kestrel used previous Long-legged Buzzard nest n = 1), but new ones were also built each year, mostly by Booted Eagles.
Table 2. Characteristics of trees with and without (random tree) forest raptor nests: height (H) and diameter at breast height (DBH), distance and DBH of the three closest trees, density of mature and immature trees (>30 and <30 cm DBH within a radius of 15 m, respectively), scrub cover in 400 m, nearest road distance, nearest conspecific and interspecific nest distance in the study area, and both number of nests and nests that succeeded for the two seasons included in this study. When possible, standard deviations for each parameter are included.

Interestingly, there were certain areas where nests were located within less than 200 m of each other in 2019 (20, 6, 11, and 12 in Booted Eagle, Long-legged Buzzard, Black Kite, and Common Kestrel, respectively) and 2020 (17, 7, 12, and 10 in Booted Eagle, Black Kite, Common Kestrel, and Long-legged Buzzard, respectively) (see Figure 2). All the species showed a higher conspecific than interspecific distance between nests (see Table 2 and Figure 2). Long-legged Buzzard had the highest average conspecific distance and Black Kite the lowest (see Table 2).

Figure 2. Distribution of nests in 2019 (triangles) and 2020 (circles): white = Booted Eagle; brown = Long-legged Buzzard; black = Black Kite; orange = Common Kestrel. The nests with a distance of <200 m are highlighted in grey.
VIF analyses did not exclude any predictor (VIF <3) and therefore all were considered in the models. The final models for Booted Eagle (76% explained deviance), Long-legged Buzzard (95% explained deviance), and Black Kite (55% explained deviance) included the conspecific distance (see Table 3), i.e. the probability of locating the nests of those species was significantly and positively related with greater conspecific distance. In addition, the final models for Booted Eagle, Black Kite, and Common Kestrel (32% explained deviance) included the rabbit population abundance as nests of the two former species were positively associated with areas of higher rabbit abundance (see Table 3). The Booted Eagle model also included the height of the nest tree, the DBH of the three closest trees (higher tree diameters), and the percentage of scrub cover of the area, as nest selection is related to high trees surrounded by big size trees in areas of low scrub cover. The model for Long-legged Buzzard also included height and DBH of the nest tree, the distance between the three closest trees, and the percentage of scrub cover, as nest selection is related to shorter and taller trees surrounded by distant trees in areas of low scrub cover.
Table 3. Statistical parameters of the generalised linear mixed model used to explain Booted Eagle, Long-legged Buzzard, Black Kite, and Common Kestrel nest-site selection. H = height; DBH = diameter at breast height. See Table S4 for model selection.

The highest environmental overlap (D) was found between Common Kestrel and both Long-legged Buzzard and Booted Eagle (0.71) (Table 4). Significant moderate overlap was also found between Black Kite and both Long legged Buzzard and Booted Eagle (0.43) (Table 4), but no significant overlap was obtained for Black Kites and Common Kestrels or Booted Eagle and Long-legged Buzzards.
Table 4. Environmental overlap in nest selection quantified by Schoener’s D.

* Significant values.
Discussion
This study attempted to assess the overlaps and differences among environmental traits for four different forest raptor species selecting nest sites in the Mediterranean basin. In this two-year study, Booted Eagle, Long-legged Buzzard, and Black Kite selected for nesting in those areas where conspecific distance was higher, likely related to the greater competition between individuals of the same species. This fact highlights the importance of conspecific competition (Hakkarainen and Korpimaki Reference Hakkarainen and Korpimaki1996, Krüger Reference Krüger2004) and the absence of effect of interspecific competition in the studied Mediterranean populations. The abundance of prey as food might reduce interspecific competition. Indeed, Booted Eagle and Black Kite nest-site selection was influenced by the relative abundance of rabbits around the nests. Accordingly, in Doñana National Park, which has a landscape similar in soil and forest terms to Maamora forest, Booted Eagle actively selected nesting habitats where rabbits were abundant (Moreno and Villafuerte Reference Moreno and Villafuerte1995, Suárez et al. Reference Suárez, Balbontin and Ferrer2000), and Black Kites tended to nest close to open areas with many rabbit warrens, which favour optimal hunting (Viñuela et al. Reference Viñuela, Villafuerte and le Court1994). Even more, rabbit availability might affect breeding success as has occurred in other areas and species, where the lower percentage of rabbits in the diet was important in the brood reduction of Booted Eagle and Spanish Imperial Eagle Aquila adalberti (Viñuela and Veiga Reference Viñuela and Veiga1992, Margalida et al. Reference Margalida, González, Sánchez, Oria, Prada, Caldera and Aranda2007, Casado et al. Reference Casado, Ferrer and Suárez-Seoane2008).
Territory size and breeding density are strongly connected with food availability and competition, i.e. where food resources are abundant the species occupies smaller territories (Newton Reference Newton1979). In Maamora, the average distance between occupied territories was short and the densities of forest raptor breeding pairs and their breeding success were high (3.1 pairs/km2), especially Booted Eagles (1.2 pairs/km2, 92% breeding success) compared with Doñana National Park (0.21 pairs/km2; Suárez et al. Reference Suárez, Balbontin and Ferrer2000; 64% breeding success; Casado et al. Reference Casado, Ferrer and Suárez-Seoane2008). Indeed, the territories of some breeding pairs of different species overlapped both spatially (<200 m) and environmentally. The nests of Common Kestrel overlapped highly with those of Booted Eagle and Long-legged Buzzard; Common Kestrels in their second year of nesting were found in previous Long-legged Buzzard nests. This might be associated with their preference for larger nests, when compared with the smaller ones repaired by Black Kites (Sergio et al. Reference Sergio, Pedrini and Marchesi2003). The fact that nests of Black Kite moderately overlapped with Booted Eagle and Long-legged Buzzard might be associated with the generalist character and less selective behaviour of the former when selecting nest sites (Messabhia et al. Reference Messabhia, Bensaci, Telailia, Rebbah and Saheb2019). Nevertheless, there was no overlap between the nests of Booted Eagle and Long-legged Buzzard, which was reflected in the different characteristics of both trees suitable for nesting and the surroundings of the nest sites. In fact, Booted Eagle selected tall trees providing more stable nest support (Newton Reference Newton1979, Suárez et al. Reference Suárez, Balbontin and Ferrer2000, Barrientos and Arroyo Reference Barrientos and Arroyo2014) and reducing exposure to thermal extremes (Walsberg Reference Walsberg and Cody1985). Selected nest trees were surrounded by older trees creating a dense stand. Long-legged Buzzard selected shorter trees with a large diameter, as occurs in other Mediterranean areas with other forest raptors including Black Kite (Sergio et al. Reference Sergio, Pedrini and Marchesi2003, Barrientos and Arroyo Reference Barrientos and Arroyo2014), surrounded by distant trees, which might be related to more open areas. Interestingly, both Booted Eagle and Long-legged Buzzard selected areas dominated by low scrub cover, where the higher detectability of prey might favour hunting. This has been documented in Doñana National Park for Black Kites in areas with a high density of continuous warrens with many openings and very low scrub cover due to excavation and foraging by the rabbits (Viñuela et al. Reference Viñuela, Villafuerte and le Court1994).
Overall, in our study area forest, raptors did not randomly occupy the forest. In addition to the availability of food resources, previous occupancy and reproductive outputs might have a strong influence on subsequent territory occupation (Krüger Reference Krüger2002, Martínez et al. Reference Martínez, Pagán and Calvo2006). Booted Eagle has been documented in long-term studies as a species with a high fidelity to breeding sites, consecutive occupancies in the same territory (over six years), and by a high reuse of their nests (Jiménez-Franco et al. Reference Jiménez-Franco, Martínez and Calvo2014). Although the reuse of nests was low (13%) in our study when compared with 85% reuse in other Mediterranean areas (Jiménez-Franco et al. Reference Jiménez-Franco, Martínez and Calvo2014), long-term studies of the reuse of available nests during different breeding periods throughout the years could determine their importance in the selection of areas for nesting. Finally, in the acquisition of a better nest territory, temperature and rainfall might indirectly affect food resources, the timing of nesting, and the predator effect, which will also play their part in nest-site selection (Krüger Reference Krüger2002, Tapia and Zuberogoitia Reference Tapia, Zuberogoitia, Sarasola, Grande and Negro2018).
Further long-term monitoring studies at the landscape scale, which will allow the effect of climatic variables to be taken into account, are required to reveal additional factors that could potentially explain nest selection and other related patterns in Maamora and other Mediterranean forests. An analysis of the lifespan of nests in the context of population conservation will help to determine whether a given pool of nests is sufficient for species that depend on these resources. The common nesting patterns of this community of forest raptors facilitate an overview of the interaction of food resources, competition, and habitat quality in Maamora forest, which might reflect both the singularity of North Africa and similarities with other Mediterranean areas such as southern Spain. Such a breeding raptor community study is likely to provide important information that could be used for elaborating an integrated long-term management and conservation plan in Mediterranean habitats.
Conservation
Habitat protection is of prime importance for maintaining forest raptor populations (Newton Reference Newton1979, Suárez et al. Reference Suárez, Balbontin and Ferrer2000, Sergio et al. Reference Sergio, Caro, Brown, Clucas, Hunter, Ketchum and MacHugh2008). The protected area of Maamora where the study took place holds 18 species of forest raptors and has a dense spring breeding population of Booted Eagle, Long-legged Buzzard, Black Kite, and Common Kestrel with a high breeding success. This area is characterised by mature forest with a well-represented undergrowth and not limiting food resources, and by the absence of forestry practices, livestock overgrazing, and human disturbance. Nevertheless, most of the Maamora forest is unprotected and forestry practices, intense overgrazing by livestock, and the leisure activities of human inhabitants have caused the loss of 50% of Cork Oak tree cover (from 134,000 ha to 70,000 ha; Lahssini et al. Reference Lahssini, Lahlaoi, Alaoui, Hlal, Bagaram and Ponette2015, Fennane and Rejdali Reference Fennane and Rejdali2015). In particular, a gradient of biodiversity loss of birds – including the forest raptors – has been documented in those areas surrounding both areas of highest grazing intensity and areas situated near large cities associated with habitat transformation (Cherkaoui et al. Reference Cherkaoui, Selmi, Boukhriss, Hamid and Dakki2009). Therefore the abundance, species composition, and breeding success of forest raptor populations in those unprotected areas with fewer available nest sites in terms of feeding and refuge might show differences compared with the protected areas. The disturbance and destruction of forests is considered the main threat to the raptors in North Africa (Garrido et al. Reference Garrido, Numa, Barrios, Qninba, Riad, Haitham and Hasnaoui2021). To guarantee forest raptor nesting in North Africa our results suggest that (1) the protection of dense areas holding old Cork Oak trees, (2) the delimitation of areas close to rural areas to avoid overgrazing, which will favour herb growth to increase rabbit abundance and scrub cover, (3) the replacement of allochthonous plantations with Cork Oak trees, and (4) controlling forestry practices, such as decorking in breeding areas in June, will allow suitable nesting conditions for the community of forest raptors (see management recommendations in Margalida et al. Reference Margalida, Moreno-Opo, Arroyo and Arredondo2010). In species-specific terms, Long-legged Buzzard monitoring might be prioritised due to the documented decline of the population in Morocco. This species might benefit from the absence of Booted Eagle (and competition) in unprotected areas of Maamora, where it has not yet been documented (pers. obs.). The challenge is the protection of the forests of North Africa, particularly Maamora forest, to encourage breeding of a wide variety of forest raptors, including the return of the threatened Spanish Imperial Eagle. Even more so, however, this threatened forest plays a special role for a wide community of wild species that includes key (Segura Reference Segura2017) and threatened species (de Pous et al. Reference de Pous, Beukema, Dingemans, Donaire, Geniez and El Mouden2011, Segura and Acevedo Reference Segura and Acevedo2019, Reference Segura and Acevedo2021).
Acknowledgements
We are very grateful to Oscar Rodríguez, Absallam Belhajjamia, and Bouhali Kaddouri for their field assistance. We truly appreciate their commitment to the forest raptor nest surveys. We would like to thank HCEFLCD services for their guidance. We would also like to thank Greg Trollip and Jacob Mwanzia for their support and interest in wild species conservation. Two anonymous reviewers improved the manuscript.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0959270923000266.








