Introduction
Ever since the insights of David Lack (Reference Lack1954, Reference Lack1968), it has been broadly accepted that clutches laid by birds are close to the size that maximizes, over the parent’s lifetime, the number of offspring that will recruit to the next generation. This postulate does not preclude the possibility that there will be year-to-year variation in clutch size reflecting, for example, population size (smaller clutches when population density is greater; Perrins Reference Perrins1965), food supply (larger clutches when food is more abundant; Perrins Reference Perrins1965, Grant et al. Reference Grant, Grant, Keller and Petren2000) or timing (possibly smaller clutches when breeding is later; Barba et al. Reference Barba, Gil-Delgado and Monrós1995 ). This variation is perhaps most famously evident in predatory birds of the Arctic tundra which may lay large clutches when lemmings are abundant and forgo breeding altogether when lemmings are scarce (Pitelka et al. Reference Pitelka, Tomich and Treichel1955). However, food-related variation in reproductive metrics is also known from passerine birds.
The evidence for such variation may be derived from observation. For example Darwin’s finches Geospiza spp. lay larger clutches, averaging four instead of three eggs, and make more breeding attempts in wet El Niño years when food is more abundant than in dry non-El Niño years (Grant et al. Reference Grant, Grant, Keller and Petren2000). Evidence may also come from experiments. Given supplementary food, Magpies Pica pica lay earlier and produce larger clutches, about 0.5 more eggs on average, than controls (Hogstedt Reference Hogstedt1981).
Afrotropical lark species may be either sedentary or nomadic. In the latter case, birds settle in an area following rain (Willoughby Reference Willoughby1971). Species in both groups frequently breed during or shortly after the period of rainfall (Brown et al. Reference Brown, Bridgeford, Braine, Paxton and Versfeld2015), although this is not universally true (Ndithia et al. Reference Ndithia, Matson, Versteegh, Muchai and Tieleman2017). Limited evidence suggests that increased rainfall may be followed by an increase in clutch size of approximately one egg in Spike-heeled Lark Chersomanes albofasciata but by no change in the clutches of Sclater’s Lark Spizocorys sclateri (Lloyd Reference Lloyd1999; see also Suárez et al. Reference Suárez, Herranz, Yanes, Sánchez, García and Manrique2005). More generally in South Africa, ground-feeding insectivores, including some larks, lay larger clutches in mesic regions than in arid zones (Lepage and Lloyd Reference Lepage and Lloyd2004).
The population of the Raso Lark Alauda razae, confined to the 7-km2 island of Raso (16°37′N 24°35′W) in the Cape Verdes, has been monitored annually since 2001. The first monitoring visit in October 2001 was reported by Donald et al. (Reference Donald, De Ponte, Pitta Groz and Taylor2003). Subsequent visits in 2002–2017 by the author and colleagues have taken place in November and/or December. During this period, the lark has shown dramatic variation in population size, from a low of about 57 individuals in 2004, to a high of 1,500–1,550 individuals in 2011, 2012 and 2017 (Brooke et al. Reference Brooke, Flower, Campbell, Mainwaring, Davies and Welbergen2012, unpubl. data). Although there is some annual variation in adult survival, these considerable population increases appear primarily driven by enhanced reproductive output (Dierickx Reference Dierickx2018).
Although rainfall in the Cape Verdes is erratic and averages about 200 mm/yr (http://www.holiday-weather.com/cape_verde/averages), it is often concentrated in the months August–October. Therefore our visits take place at or just after the period when Raso Lark breeding activity is most likely. This allows the question of whether Raso Lark clutch size is influenced by rainfall to be asked, with population size as a possible confounding factor.
Methods
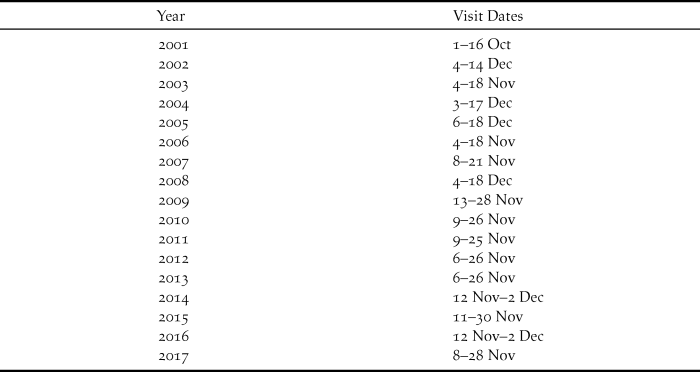
The present study commenced in 2002 and has continued annually to 2017 with single visits each year lasting 11–20 days. In general the visits, involving the author and a single co-worker, have become longer as the lark population has expanded. More recent years have witnessed greater constancy in timing of visits, notably since 2009 (Table 1). Fieldwork has concentrated on catching and colour-ringing birds to obtain survival estimates and also to permit population estimates. In brief, after sustained reading of colour-rings attached in previous years, that consistently generates re-sighting rates greater than 90% (Dierickx Reference Dierickx2018), combined with ringing of new birds, the number of colour-ringed birds on the island is fairly accurately known by the end of a visit. Transects across the island then allow an estimate of the proportion of birds that are colour-ringed, and hence a population estimate.
Table 1. Dates of annual visits to Raso, 2001–2017, to study larks. 2001 visit reported by Donald et al. (Reference Donald, De Ponte, Pitta Groz and Taylor2003), other visits this study.
In the course of this work, all nests found were recorded and their fates followed until failure, successful fledging, or our departure. Nests were found at all stages (building, incubation, chick-rearing) but clutch size data are presented only for nests where the clutch was believed complete, or for nests containing young (and unhatched eggs) when first found.
The number of nests found is probably not a very robust index of the extent of breeding activity. Obviously a higher lark population creates the possibility that more nests are built. However a larger population means more working time was devoted to catching and ring-reading, potentially diverting attention from finding nests.
Rainfall in the Cape Verdes can be very localised. For example, in 2015 it was evident that before our arrival, significant rain had fallen on one half of Raso. On that half, all 17 nests we found were located and the vegetation was clearly greener than elsewhere. Consequently, in the absence of any continuous run of rainfall data for the study period either from Raso or adjacent islands, rainfall data were downloaded from the website http://climexp.knmi.nl which provided a satellite-based monthly Tropical Rainfall Measuring Mission (TRMM) precipitation index centred on Raso at 14°–19° N, 22°–27 W. Units are mm/day. The dataset ends in early 2015 and therefore the late 2014 visit is the last used in rainfall-related analyses. In every study year, rainfall was greater in August than July, and therefore the analyses presented in Results explore relationships between clutch size as recorded November/December and rainfall in the preceding months of August–October, and also between clutch size and rainfall in the entire year (November-October) prior to a visit. The rainfall data are shown in Table S1 in the online supplementary material.
Results
Table 2 shows that average clutch size, taken across nests found at both egg and chick stages, varies greatly from year to year, from 1.00 to 3.57, and that does not include three years (2002, 2005, 2014) where no nests whatsoever were found. It is impossible to assert that there were no nests in these years but it seems likely since the island was very dry. Generally there was minimal difference in the mean clutch size of nests found at egg and chick stages, and therefore the mean clutch regardless of stage found was used in the rainfall analysis. Only in 2016 did the difference exceed 0.5 eggs, and it was then significant (t-test, P = 0.028). However in the absence of rainfall data for 2016 (Methods), this year was not used in the rainfall analysis.
Table 2. Mean clutch size of Raso Lark nests found at egg and chick stages, and both combined, in 17 study years. Also given is the estimated lark population in each year. Dashes indicate no nests found at that stage during the year’s visit.
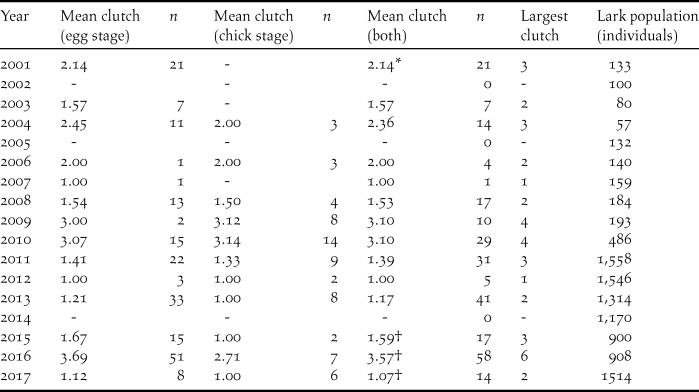
* Data excluded from rainfall analysis since timing of visit differed by over one month from all other visits.
† In absence of rainfall data, year not used in clutch size v rainfall analysis.
Correlation coefficients between clutch size and various measures of rainfall preceding the year’s fieldwork visit were universally positive and achieved their highest value when the correlation was between clutch size and rainfall averaged over three months, from the start of the rainy season (August) until October (Table 3). This highest correlation is shown in Figure 1. It remains positive and significant (r = 0.737, n = 10, P < 0.02) even when the three years with no nests recorded are excluded.
Table 3. Correlation coefficients (r) between mean Raso Lark clutch size in the 13 years, 2002–2014, and rainfall in single months, two months, three months and the entire 12 months before that year’s fieldwork visit. The three years with no nests found (Table 2) were treated as clutch size zero. * P < 0.05, ** P < 0.01.

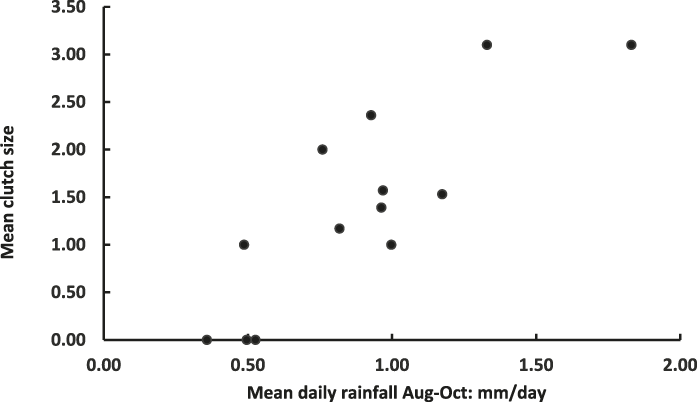
Figure 1. Plot of mean Raso Lark clutch size (Table 2) and mean daily rainfall August-October in 13 study years (2002–2014).
No rainfall data were available for 2016, which therefore cannot be plotted on Figure 1. Unfortunately that was the year of the largest mean clutch size and also the year when the largest clutches were recorded, nine of five and one of six. Previously the largest clutch recorded had been four, in 2009 and 2010 (Table 2). Despite the 25-fold population expansion during the study period, the highly significant multiple regression of clutch size on population size and daily Aug-Oct rainfall (Mean clutch size = -0.368-0.000425 population size + 2.24 rainfall; F 2,10 = 16.86, P = 0.001) yielded no support for an effect of population on clutch (P = 0.138) whereas the effect of rainfall was strong (P < 0.001).
Discussion
The Raso Lark shows exceptional variation in clutch size, and the largest clutches of five or six exceed those recorded from 18 lark species in Namibia (Brown et al. Reference Brown, Bridgeford, Braine, Paxton and Versfeld2015). This variation is strongly related to rainfall but apparently not to population size. It contributes to the rapid population growth rates that the species has achieved after rain, rates that are as high as any achieved by ‘Critically Endangered’ species (Brooke et al. Reference Brooke, Flower, Campbell, Mainwaring, Davies and Welbergen2012). On the other hand, in the driest years Raso Larks may forgo breeding altogether. Given the species’ consistently high annual survival (Brooke et al. Reference Brooke, Flower, Campbell, Mainwaring, Davies and Welbergen2012), the long-term population impact of barren years may not be great.
Whether the impact of rainfall on clutch size is via its effect on the lark’s food supply, which includes more invertebrates in wetter years (pers. obs.), or via the birds’ better hydration is unknown. While the latter chain of causation has been suggested for Black-throated Sparrow Amphispiza bilineata in the Mojave Desert of southern California (Coe and Rotenberry Reference Coe and Rotenberry2003), it seems less likely in the lark. In the driest years, Raso Larks visit littoral rock pools to drink seawater (Donald and Brooke Reference Donald and Brooke2006).
It is possible that wetter conditions on Raso are associated not only with larger clutches but also with more breeding attempts per individual female (Grant et al. Reference Grant, Grant, Keller and Petren2000). However the nature of this study’s fieldwork protocol, a single relatively short visit each year, means there are no data on the number of breeding attempts made by individual birds. For the same reason, there is no information on the age at which individuals starting breeding. Given that the population has grown 2–4 fold from one year to the next on several occasions (Table 2), it certainly seems likely that Raso Larks can start breeding when a few months old, as is known for other passerines of arid regions (Zann Reference Zann1994). Presumably such early breeding is more likely when rainfall has been plentiful.
In arid regions, passerine birds can adjust their breeding effort to rainfall to two ways that are not mutually exclusive. They can lay more eggs in wetter years, as reported here. Alternatively they can adopt a nomadic lifestyle, arriving in areas shortly after rain and starting to breed, at least if rainfall has passed some threshold. Species following the latter strategy may not show the range of clutch sizes seen in the Raso Lark since they either do not breed at all or lay a clutch that varies only slightly according to local rainfall (Lloyd Reference Lloyd1999). Actually documenting this variation over many years is likely to be difficult because of the practical problems of monitoring the breeding effort of a nomadic species. Fortunately, the Raso Lark, a single island endemic, is not nomadic; that accessibility has allowed the accumulation of the data on clutch size variation described in this paper.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270918000163
Acknowledgements
The Raso Lark study has been generously supported by Julian Francis, RSPB, CEPF, BirdLife International’s Preventing Extinctions Initiative, the Sir Peter Scott Studentship of Trinity College, Cambridge, the Desertas Fund of Sociedade Caboverdiana de Zoologia, a component of the GEF-SGP project (CPV/SGP/OP5Y1/CORE/BD/11/13), and the MAVA Foundation with a grant to SPEA and Biosfera. Immense thanks also to Multa and his boat Andorinha do Mar for providing reliable transport to Raso and to the long-suffering contributors to this study, Mark Bolton, Ewan Campbell, Simon Davies, Elisa Dierickx, Mike Finnie, Tom Flower, Lee Gregory, Sabine Hille, Mark Mainwaring, Jason Moss, and Justin Welbergen. Nick Davies, Paul Donald and Nick Horrocks kindly commented on a draft of the paper.






