Introduction
The island of São Tomé (Gulf of Guinea, Central Africa) is an important centre of endemism (Jones Reference Jones1994). The number of endemic birds it holds is particularly remarkable for a small island (Stattersfield et al. Reference Stattersfield, Crosby, Long and Wege1998, Kier et al. Reference Kier, Kreft, Lee, Jetz, Ibisch, Nowicki, Mutke and Barthlott2009, Buchanan et al. Reference Buchanan, Donald and Butchart2011, Le Saout et al. Reference Le Saout, Hoffmann, Shi, Hughes, Bernard, Brooks, Bertzky, Butchart, Stuart, Badman and Rodrigues2013). It has 50 resident bird species, of which 17 are single-island endemic species, three are Gulf of Guinea endemic species and eight are endemic subspecies, ranging across eight orders and 19 families (Jones and Tye Reference Jones and Tye2006, Melo Reference Melo2007). The island is also unusual among oceanic islands with isolated and unique avifaunas in that there are no recorded anthropogenic extinctions of birds (Jones and Tye Reference Jones and Tye2006).
The endemic avifauna of São Tomé is clearly associated with the persistence of the island’s forest-dominated landscape (de Lima et al. Reference de Lima, Dallimer, Atkinson and Barlow2013a). Preserving the remaining native forests and restoring degraded habitat are top conservation priorities, namely within and around the island’s only protected area, the São Tomé Obô Natural Park (ONP), where most of the endemic species are found (de Lima Reference de Lima2012, Ndang’ang’a et al. Reference Ndang’ang’a, Ward-Francis, Costa, de Lima, Palmeirim, Tavares, Buchanan, Carvalho, Melo, Dallimer and Valle2014). These forests are under high level anthropogenic pressure (Salgueiro and Carvalho Reference Salgueiro and Carvalho2007). Threats such as land-use intensification, overexploitation and invasive species are likely to continue to have a strong impact on forest ecosystems and on the endemics in the nearby future (Jones et al. Reference Jones, Burlison and Tye1991).
Nine of São Tomé’s endemic bird species are currently classified as threatened, including three which are ‘Critically Endangered’; the Dwarf Olive Ibis Bostrychia bocagei, São Tomé Fiscal Lanius newtoni and São Tomé Grosbeak Neospiza concolor (IUCN 2013, Ndang’ang’a et al. Reference Ndang’ang’a, Ward-Francis, Costa, de Lima, Palmeirim, Tavares, Buchanan, Carvalho, Melo, Dallimer and Valle2014). The ibis is a lowland species found in old-growth or mature secondary rainforest in the south and centre of the island, and it breeds from September to February (Jones and Tye Reference Jones and Tye2006, Maia et al. Reference Maia, Gascoigne, de Deus and de Lima2014, Azevedo Reference Azevedo2015, Margarido Reference Margarido2015). The fiscal is known only from well-preserved forest, with low understorey density and in areas of high rainfall (Jones and Tye Reference Jones and Tye2006), occurring from the lowlands up to 1,395 m above sea level (Maia and Alberto Reference Maia and Alberto2009). The grosbeak was thought to be a lowland old-growth forest specialist, but recent observations in secondary forest at 1,400 m suggest that it might be more widespread than previously thought (Solé et al. Reference Solé, Alberto, Samba, Santana and de Lima2012).
The implementation of effective conservation measures, requires basic ecological knowledge, which is currently lacking. A better knowledge of distribution and habitat associations is needed in order to identify target areas for intervention and protection, monitor population trends, manage the habitat and tackle threats. Here we describe an intensive survey of this island’s core forest ecosystems that, together with ad hoc observations, has been used to produce maps of potential species distribution and to identify the areas of the island which are most important for all three species, applying reserve selection algorithms. We also describe broad habitat associations for the species. Finally we assess the implications of this new information for reviewing the conservation status of the species and to guide conservation activities.
Methods
Study area
São Tomé (857 km2) is located just north of the Equator, 255 km west of continental Africa and belongs to the small island nation of São Tomé and Príncipe. The island is rugged, especially in the centre and south-west, with several peaks above 1,500 m and the highest peak, Pico de São Tomé, at 2,024 m. The mountainous topography creates strong climatic gradients, with the annual rainfall ranging from less than 600 mm in the north-east to more than 7,000 mm in the south-west, and the mean annual temperatures ranging from around 30°C at sea level to 18° at higher altitudes. Humidity and cloud cover are high throughout the year for most of the island, but there is a well-marked seasonality. The main dry season, locally known as gravana, extends from mid-May to early September and is characterised by low rainfall and lower temperatures. The rainy season extends through the rest of the year, with a small dry season, the gravanito, occurring between December and February (Silva Reference Silva1958, Tenreiro Reference Tenreiro1961).
The native forest can be separated in four main types, differentiated by climatic conditions and plant species composition; mangrove, lowland, montane and mist (Monod Reference Monod1960). The mangrove is restricted to small coastal areas. Lowland forest goes from sea level up to 800 m and is characterised by a sparse understorey, and a high and dense canopy. Montane forest spans 800–1400 m and has a high tree density and species richness, with medium understorey and epiphyte density. Mist forest occupies the summit of the island and is typically much lower, with sparse tree cover and very high epiphyte densities.
The island was first described as being entirely covered by dense tropical forest, but since then, humans have extensively changed its ecosystems (Eyzaguirre Reference Eyzaguirre1986). At least 10% of the island is now covered by non-forest land-use types, such as oil palm monoculture, horticulture and open savanna. The remaining area is covered by similar extents (c.30 %) of shade plantation, secondary forest and native forest (Salgueiro and Carvalho Reference Salgueiro and Carvalho2007). Native forest persists only in mountainous portions of the island, where human presence remains scarce. Most of these best preserved forests are now classified as ONP (DGA 2006). The park extends through most of the centre and south-west of the island, covering nearly one third of it, but enforcement is weak and protection is not very effective (de Lima et al. Reference de Lima, Sampaio and Buchanan2013b).
This study focuses on the three Critically Endangered bird species endemic to São Tomé; the Dwarf Olive Ibis, the São Tomé Fiscal and the São Tomé Grosbeak. The ibis is presumed to occupy an area of 213 km2, with a declining population of 70–400 individuals (IUCN 2013). It is a lowland species found in old-growth or mature secondary rainforest in the south of the island. It is usually found feeding on the ground and it breeds from September to February (Jones and Tye Reference Jones and Tye2006, Maia et al. Reference Maia, Gascoigne, de Deus and de Lima2014, Azevedo Reference Azevedo2015, Margarido Reference Margarido2015). It is threatened by habitat loss and degradation, hunting and human disturbance, with climate change and predation by exotic species being considered as potential serious threats (Ndang’ang’a et al. Reference Ndang’ang’a, Ward-Francis, Costa, de Lima, Palmeirim, Tavares, Buchanan, Carvalho, Melo, Dallimer and Valle2014).
The fiscal is thought to occupy 260 km2 and, as a precautionary measure, it is assumed to have a population smaller than 50 adult individuals (IUCN 2013). It is known only from well-preserved forest, with low understorey density and in areas of high rainfall (Jones and Tye Reference Jones and Tye2006). It has been found from the lowlands up to 1,395 m (Maia and Alberto Reference Maia and Alberto2009, Lewis Reference Lewis2015). It is usually heard in the distance or found perching on low-lying branches, from where it flies to hunt small invertebrates (Jones and Tye Reference Jones and Tye2006, Lewis Reference Lewis2015). There are some indications that it breeds from November to February. Habitat degradation by exotic plant species is considered a potential threat (Ndang’ang’a 2014).
The grosbeak is presumed to occur in an area of 88 km2 and, as a precautionary measure, it is assumed to have a population smaller than 50 adult individuals (IUCN 2013). Thought to be a lowland old-growth forest specialist, restricted to the south of the island, the species was recently found using an area of secondary forest in the central mountain range, at an altitude of 1,400 m It has also been found feeding on fruits of relatively abundant and widespread species, some of which are typical of disturbed areas. These new observations suggest that the scarcity of records for this species might be linked to its shy behaviour, and that it might be more abundant and widespread than previously thought (Solé et al. Reference Solé, Alberto, Samba, Santana and de Lima2012). Habitat degradation due to human disturbance and spread of exotic species have been identified as the major threats to the survival of this species (Ndang’ang’a et al. Reference Ndang’ang’a, Ward-Francis, Costa, de Lima, Palmeirim, Tavares, Buchanan, Carvalho, Melo, Dallimer and Valle2014).
Survey design
We gathered occasional and systematic observations of the Critically Endangered bird species of São Tomé. Occasional observations included our own sporadic records and those collected by other ornithological researchers over the last 15 years. We contacted those authors and compiled all available information in a database with a GIS component, based on locations recorded on GPS.
Systematic surveys of São Tomé’s main forest block took place between 2013 and 2015. The study area was divided into 99 square tetrads of 4 km2 (Figure 1). We surveyed a randomly selected quarter of each tetrad throughout the study area and more intensively in some areas, such as the south-east (de Lima et al. Reference de Lima, Sampaio and Buchanan2013b). In each 1-km2 quarter we undertook five 10-minute point counts, separated by at least 200 m. When feasible, point counts were scattered across the 1-km2 quarter so as to represent environmental variability roughly in proportion to its availability (e.g. habitat type, altitudinal gradient, and distance to rivers and roads). The number of individuals of each Critically Endangered bird species detected during each point count was recorded. The location and altitude of each point were registered using a GPS. Habitat at each point was assessed in terms of broad land-use type, slope, number of trees and understorey density (Table 1). To assess seasonality, sampling took place during the main dry season (gravana) of 2013 and 2014, and during the gravanito of 2014 and 2015, which corresponds to a small dry season at the end of the breeding season for most bird species in São Tomé (Jones and Tye Reference Jones and Tye2006).
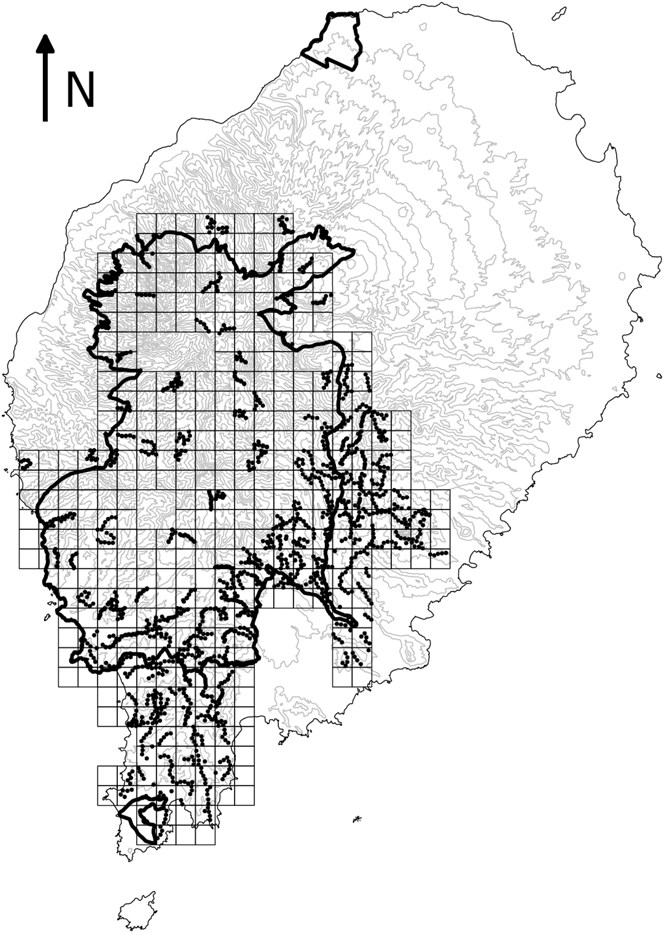
Figure 1. Map of São Tomé Island showing study area. The squares represent 1-km2 quadrats in the 4-km2 tetrads that were sampled at least once. The dots indicate systematic point counts. The boundaries of the São Tomé Obô Natural Park are shown by the bold black lines. The 100-m contour lines are shown in grey and island outline in black.
Table 1. Point count habitat variables. Each variable was assessed within a 20-m radius around each point count.

Species distribution models
Species distribution models (SDMs) were created using MaxEnt in the “dismo” R package (Hijmans et al. Reference Hijmans, Phillips, Leathwick and Elith2015, R Development Core Team 2015). MaxEnt is a machine learning method that produces niche models from environmental data, and has been found to perform well in comparison to other SDM methods (Elith and Graham Reference Elith and Graham2009).
We created an SDM for the annual gravana (long dry season) and gravanito (short dry season) distribution of each species. Given that the exact dates for the seasons can vary, the gravana was truncated to June, July and August, and the gravanito to January and February. We identified 33 potential predictor variables for inclusion in the SDMs: 19 bioclimate layers (Hijmans and Cameron Reference Hijmans and Cameron2005; www.worldclim.org/bioclim), 12 normalised difference vegetation index (NDVI) layers (Spot-vegetation sensor through VITO; www.spot-vegetation.com), elevation (Jarvis et al. Reference Jarvis, Reuter, Nelson and Guevara2008) and slope (created from the elevation layer). The NDVI layers for a 13-year period (1999–2012) were combined to create a set of monthly averages, and used as a continuous summary of land cover. All predictor variables were standardised to a 1-km2 spatial scale and processed using ArcMap version 10.2. We created a set of uncorrelated predictor variables and a set of predictor variables that had the greatest average percentage contribution to the maximal model (Table S1 in the online supplementary material). For the seasonal models, only the corresponding NDVI layers were retained.
The SDMs were built using the systematic surveys as training data and tested using the occasional observations (Table S2), except for the grosbeak in the gravana. In this case, because there were too few systematic observations, both sets of observations were combined as training data and the resulting model was not tested. We used 886 pseudo-absences in MaxEnt, the number of non-duplicated training records, unique records for each 1-km2 raster cell. The optimal feature function combination was identified based on the number of unique sample points (Phillips and Dudík Reference Phillips and Dudík2008), the “ENMeval” R package (Muscarella et al. Reference Muscarella, Galante, Soley-Guardia, Boria, Kass, Uriarte and Anderson2014, R Development Core Team 2015) was used to run a series of models with varying regularisation values (from 0.5 to 5 at intervals of 0.5), and the final model was chosen based on AICc (Warren and Seifert Reference Warren and Seifert2011).
The final models were created with a raw output for calculating the model AICc. Ten cross validations were undertaken to generate folds of randomly selected presence data, allowing us to run each model 10 times, exclude each fold in turn and use the fold to validate the data (Phillips and Dudík Reference Phillips and Dudík2008). This enabled us to assess whether the response curves were smooth and biologically sensible. To aid interpretation, these models were repeated with a logistic output, being partitioned using the mean equal training sensitivity and specificity threshold values to identify the minimum area of land required for each species. This threshold was chosen because it minimises the rate of false positives and negatives. We calculated the spatial overlap between the SDM of each species for the predicted annual, gravana and gravanito distribution, using the “calc.niche.overlap” function of the “ENMeval” R package (Schoener’s D and Warren’s I statistics; R Development Core Team 2015) and Map Comparison Kit 3.2.3. (Cohen’s Kappa statistic; Visser and De Nijs Reference Visser and De Nijs2006).
Spatial conservation planning
We used the spatial conservation planning software ‘Zonation’ to identify important areas for the study species in the three periods considered and giving equal weight to each species. This software produces a hierarchical prioritisation based on the conservation value of sites (Moilanen et al. Reference Moilanen, Franco, Early, Fox, Wintle and Thomas2005, Moilanen Reference Moilanen2007) using a complementarity-based algorithm that iteratively removes the cells whose loss causes the smallest decrease in conservation value in the remaining network. The resulting hierarchy of nested outputs corresponds to different degrees of conservation value within the landscape and may be used as a guide to determine the level of protection needed. It differs from previous target based planning or maximum coverage approaches that provide a single optimal output (Moilanen Reference Moilanen2007). One grid cell was removed in each iteration step (warp factor). The spatial overlap between the three Zonation outputs was calculated using the “calc.niche.overlap” function from the “ENMeval” R package (Schoener’s D and Warren’s I statistics; R Development Core Team 2015).
Habitat associations
We used generalised linear models (Bolker et al. Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009, Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009, R Development Core Team 2015) to assess the influence of environmental variables (altitude, slope, number of trees, understorey density, habitat type and season) on the presence of each species. There were 607 point count locations from the gravanito and 316 point count locations from the gravana, for which we had a complete characterisation of all environmental variables. Since there was a high prevalence of absences of Critically Endangered birds during the point counts, presences were modelled against data from an equal number of randomly selected point counts during which each species had not been recorded. To identify which variables had the greatest support for explaining the presence of the Critically Endangered birds, we used model averaging and relative variable importance based on second-order Akaike information criterion (AICc) automated model selection (Burnham and Anderson Reference Burnham and Anderson2002, Barlow et al. Reference Barlow, Louzada, Parry, Hernandez, Hawes, Peres, Vaz-de-Mello and Gardner2010), from the “MuMIn” R package (Barton Reference Barton2013, R Development Core Team 2015).
Results
We sampled 720 point counts during the gravanito and 960 during the gravana. In total we recorded 33,137 birds, belonging to 39 species, including the 20 endemic species and all endemic subspecies, except Harlequin Quail Coturnix delegorguei histrionica and Barn Owl Tyto alba thomensis. We detected 38 ibises in 21 point counts, 111 fiscals in 86 point counts and 22 grosbeaks in 16 point counts. The larger sampling effort during the gravana was not reflected in the number of records for the Critically Endangered birds, with just 18 ibises in 8 points, 46 fiscals in 35 points and three grosbeaks in three points from this season. We present these figures to indicate broad encounter rates, since we cannot rule out the possibility of counting the same individual on proximal squares or subsequent seasons.
Distribution
We obtained records of new areas of occurrence for all three of the target species. The ibis was registered along the Lembá, Ana Chaves and Iô Grande river valleys, and in the proximity of Maria Fernandes Peak, outside the ONP. The fiscal seems to occur mostly at mid-altitudes south of Pico de São Tomé and around Cabumbé Peak. The distribution of the grosbeak was greatly expanded, with records from Morro de Dentro, Ana Chaves Peak, the Lembá river valley and the southeast slopes of Cabumbé Peak (Figure S2).
The SDMs scored generally high in the AUC test, ranging from 0.80 ± 0.10 SD to 0.95 ± 0.07 SD (Table S3). The models using all significant predictors always performed better than those using the uncorrelated set of predictors, except for the fiscal in the gravana. The variables that best predicted the presence of the three target species for the annual models were those related to: elevation; NDVI, especially in June; and precipitation, namely during the wettest and warmest months (Table S4). The variables related to precipitation were consistently chosen as predictors for the presence of the bird species across the seasons, most notably for the ibis.
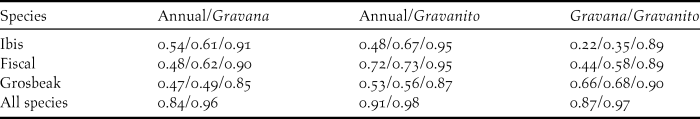
The logistic outputs from the SDMs were turned into binary outputs of potentially occupied and unoccupied 1-km2 cells, based on the equal training selectivity and specificity thresholds. The resulting maps (Figure. 2a, 2b and 2c) indicated that, across the whole year, the potential area of occurrence was 127 km2 for the ibis, 117 km2 for the fiscal and 174 km2 for the grosbeak. During the gravana these areas expand to over 165 km2 for the ibis, 197 km2 for the fiscal and 190 km2 for the grosbeak, while in the gravanito they change to 65, 113 and 201 km2, respectively (Figure S1). There is a strong spatial overlap in the seasonal distribution of each species, with the ibis exhibiting the most accentuated seasonal changes in distribution (Table 2, Figure S1).
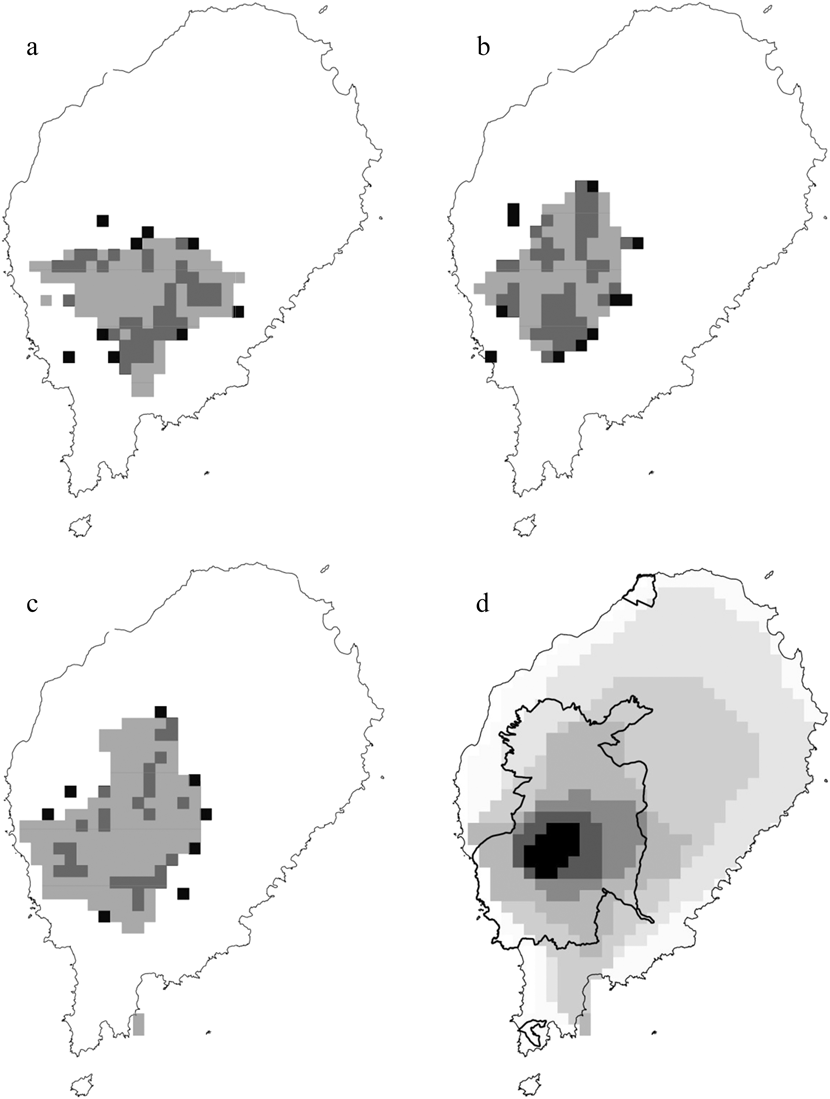
Figure 2. The distribution of São Tomé Dwarf Olive Ibis (a), Fiscal (b) and Grosbeak (c). The black quadrats represent confirmed locations, while the superimposed grey areas represent suitable ranges, according to the categorical annual distribution predicted by logistic MaxEnt modelling. Zonation based on categorical SDM (d) is also shown, with the darkest colours indicating the most important conservation areas and the coolest colours indicating the least important conservation areas (0–19% = almost white, 20–49% = very light grey, 50–74% = light grey, 75–89% = intermediate grey, 90–94% = dark grey, 95–97% = very dark grey and 98–100% = black).
Table 2. Spatial overlap between categorical MaxEnt SDM outputs. The lines show the values for each of the three Critically Endangered bird species and across all species. Each column shows the values for each possible comparison between the three seasonal models considered (annual, gravana and gravanito). Values correspond to Schoener’s D, Warren’s I and Cohen’s K statistics, respectively. For across the species, only D and I statistics values are shown. For D and I statistics, 0 means no overlap and 1 complete overlap. For K 0 means no agreement, values between 0 and 0.20 slight agreement, between 0.21 and 0.40 fair agreement, between 0.41 and 0.60 moderate agreement, between 0.61 and 0.80 substantial agreement and between 0.81 and 1 almost perfect agreement.
The south-west central region of São Tomé has high potential for the occurrence of the three Critically Endangered birds, and was thus identified as the most important area of the island in the output of the zonation analysis (Figure 2d). This key area for conservation is largely coincident with the ONP: 99.1% for the top 10% threshold, and 82.7% for the top 25% threshold.
Habitat associations
We recorded the ibis in 13 point counts with complete habitat characterisation, the fiscal in 75 and the grosbeak in 12. Comparing the habitat characteristics of these locations with that of an equal number of unoccupied locations, indicated that the three species showed a preference for native forest. Additionally, the Dwarf Olive Ibis selected areas with higher number of trees and was detected mostly during the gravanito, while the fiscal preferred a lower number of trees and intermediate altitudes (Table 3, Figures 3 and S3).
Table 3. Relative importance (Imp.) and averaged coefficients (Coef.) of variables obtained from generalised linear models on the presence of São Tomé’s Critically Endangered bird species. The grey shading highlights variables with the highest relative importance values (larger than 0.3) and the asterisks indicate variables that on their own perform better than the null model. A relative importance value of 1 means that the variable is included in all best models (Figure S3). Habitat and Season are factorial variables with positive values corresponding to a preference for secondary forest and gravanito, respectively.
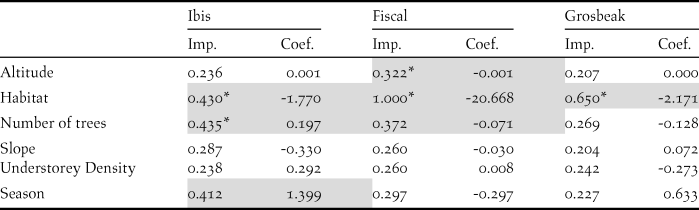
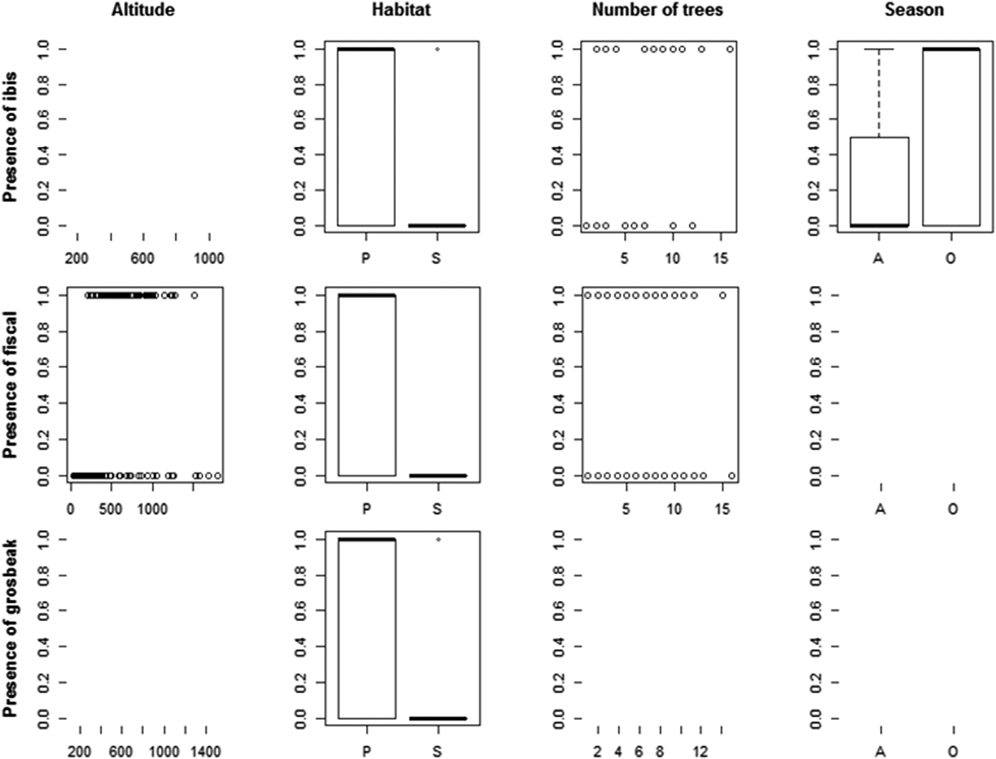
Figure 3. Relationship between the presence of São Tomé’s Critically Endangered bird species and environmental variables. Only the variables with the highest relative importance values for each species are plotted (Table 3).
The ibis was mostly found in lowlands, but we obtained 12 records above 600 m (five during point counts, plus seven occasional records), from the Lembá and Ana Chaves river basins. These include three records above 900 m, south of Ana Chaves Peak, one of which refers to two juvenile individuals found near a nest at 935 m (Figure S2). We have also detected it in secondary forest: twice during the systematic point counts (out of 21 points with confirmed presence) and 16 times from the occasional records (out of 114 records). The fiscal occurs only in native forest, preferring areas between 400 and 1,300 m even though its altitudinal range can extend from 185 to 1,504 m. The presence of the grosbeak in montane forest was confirmed several times at distinct locations: Ana Chaves Peak (929, 951 and 1,258 m), Morro Provaz (1,231 m), Morro de Dentro (1,309 m) and Lagoa Amélia (1,397 m; Figure S2). A significant number of records considering that higher altitude forests cover only 85 km2 of São Tomé and that four out of 16 point counts with grosbeak were at altitude (25%), while only 170 out of the 1,680 point counts were at altitude (10%). We also detected the grosbeak in secondary forest: twice during the systematic point counts (out of 16 points with confirmed presence) and thrice from the occasional records (out of 23 records).
Discussion
The comprehensive survey of São Tomé forests has allowed, together with information gathered from other researchers, a significant improvement on our knowledge about the distribution, ecology and conservation status of the São Tomé Dwarf Olive Ibis, Fiscal and Grosbeak.
Distribution
We have greatly increased the number of confirmed locations for all of São Tomé’s Critically Endangered bird species, and gained a better understanding of the distribution of their potential habitats. We found that the potential distribution of both the ibis and the fiscal are much more restricted than previously assumed, having changed from 213 to 127 km2 and from 260 to 117 km2, respectively. The grosbeak, on the other hand, seems to be more widespread, with surveys having extended its potential range from 88 to 187 km2. The ibis also seems to have strong seasonal changes in distribution, being restricted to just 65 km2 during its breeding season in the gravanito.
All target species are strongly restricted to the south of the island, and notably to the south-west. The ONP covers most of the areas with high potential for the three species, but leaves out 38 km2 (82.7%) of the top 25% priority areas (Figure 2d). These include around 20 km2 between the Iô Grande river and Maria Fernandes Peak to the east of ONP, and smaller areas next to Juliana de Sousa (west), Caué river and Monte Carmo (south border), and Calvário and Nova Ceilão (north-east; Figure S2). The ONP is well located for the protection of São Tomé’s Critically Endangered bird species, especially considering that it aims to protect many other biodiversity components.
Habitat associations
We confirmed that the three target species are strongly linked to the occurrence of native forest. In addition, the ibis is associated with areas with a higher number of trees, and the fiscal with areas with a lower number of trees at intermediate altitudes.
The ibis seems to have a preference for dense forests, in flat areas and with large trees (Margarido Reference Margarido2015). However, the species is not restricted to lowland, as we have found it at 950 m. The fiscal occurs only in native forest, preferring areas with less trees at mid-elevation. This confirms that the fiscal is not a lowland species, and further suggests that it is associated with more open areas within the closed-canopy forest (Lewis Reference Lewis2015). These habitat associations make sense in light of the more open habitats used by other shrike species (Yosef Reference Yosef, del Hoyo, Elliott and Christie2008). In locations with these specific characteristics the fiscal can be fairly abundant, but it is hard to estimate population sizes, since large extents of area predicted as suitable for its occurrence are extremely difficult to access (Lewis Reference Lewis2015). The occurrence of the grosbeak at higher altitudes was confirmed in new locations, as was its occasional appearance outside the ONP and in secondary habitats near native forest (Solé et al. Reference Solé, Alberto, Samba, Santana and de Lima2012).
Threats
We confirmed that all target species are strongly linked with the occurrence of native forest, indicating that the protection of this forest is key to ensuring the long-term survival of three bird species. Habitat degradation has been listed as a key threat to their survival (Ndang’ang’a et al. 2014) and it seems to have a negative impact on all species: the fiscal is restricted to native forest, while the ibis and the grosbeak appear to occur at lower densities in secondary forests, but only if these are mature and in the vicinity of native forest.
The ibis’s association with lowland forests in flat areas and with large trees makes it especially prone to human activities that also tend to occur in these areas, such as hunting, logging and deforestation. The apparent concentration of the ibis around the south-west of the ONP during the breeding season is particularly concerning, since this region is being targeted for development, including a recently implemented 30-km2 oil palm monoculture and a proposed large hydroelectric dam (de Lima et al. Reference de Lima, Sampaio and Buchanan2013b, Azevedo Reference Azevedo2015). Of São Tomé’s Critically Endangered bird species, the ibis is also the only one targeted by hunters (Carvalho et al. Reference Carvalho, Palmeirim, Rego, Solé, Santana and Fa2015). Recent interviews revealed that subsistence hunters might be killing hundreds of ibises every year, clearly surpassing any previous estimates and making this a key threat to its survival (Sampaio et al. Reference Sampaio, de Lima, Ward-Francis and Havery2016).
The fiscal was the only species restricted to native forest. This might suggest it is the most sensitive to habitat degradation but, on the other hand, its preference for rugged inaccessible areas makes it less vulnerable to the direct impact of human activities.
Despite the many new records, the grosbeak remains the least seen and most mysterious of São Tomé endemic bird species. The significant change in the number of records between seasons (two birds detected during the gravana, against 18 during the gravanito) suggests that the scarcity of records might be due to its shyness and not necessarily due to a very low population size. Therefore, the grosbeak might be more widespread and numerous than previously thought (Jones and Tye Reference Jones and Tye2006).
During fieldwork we detected several introduced animal species (e.g. mona monkey Cercopithecus mona, African civet Civettictis civetta, black rat Rattus rattus, black cobra Naja melanoleuca and feral pig Sus scrofa) in the vicinity of areas where the Critically Endangered birds occur. There is no solid evidence that these exotic species are having a negative impact on the birds, and the impact of non-native species on São Tomé’s native biodiversity remains little studied as a whole (Dutton Reference Dutton1994, Ndang’ang’a et al. Reference Ndang’ang’a, Ward-Francis, Costa, de Lima, Palmeirim, Tavares, Buchanan, Carvalho, Melo, Dallimer and Valle2014). Nevertheless, introduced species are well known for having strong negative impacts on island species (Trevino et al. Reference Trevino, Skibial, Karels and Dobson2007), and the precautionary principle advises care until such fears are disproved. Many of these exotic animals are likely to prey on birds, others, like many of the invasive plants or the feral pig, might degrade the overall quality of habitat. The quinine plant Cinchona ledgeriana, for instance, might pose a serious threat to the fiscal, since it occupies the understorey (Diniz et al. Reference Diniz, Fernandes, Martins, Moreira and Paiva2002), which this bird needs to be open for hunting (Jones and Tye Reference Jones and Tye2006, Lewis Reference Lewis2015).
IUCN Red List conservation status
The revised potential distributions presented here might necessitate a review of the species’ IUCN Red List status. The ibis is currently classified as ‘Critically Endangered’ due to having a declining population, fewer than 250 mature individuals and being confined to a single location (criterion C2a(ii); IUCN 2001, 2013). The species is restricted to less than 100 km2 during the breeding season and to a single location, with an inferred continuing decline in the area, extent and quality of its habitat, and in the number of mature individuals, so it should retain its status, under different criteria (B1a,b(iii,v)), even if its population is larger than previously assumed.
The fiscal and the grosbeak are both currently classified as Critically Endangered due to having extremely small population sizes, with fewer than 50 mature individuals (criterion D; IUCN 2001, 2013). Our observations, together with those of other authors (Solé et al. Reference Solé, Alberto, Samba, Santana and de Lima2012, Ndang’ang’a et al. Reference Ndang’ang’a, Ward-Francis, Costa, de Lima, Palmeirim, Tavares, Buchanan, Carvalho, Melo, Dallimer and Valle2014, Lewis Reference Lewis2015), suggest that their population sizes may be higher. If this proves to be the case, the category of ‘Endangered’ will perhaps be more appropriate, due to their extents of occurrence being smaller than 5,000 km2 and restricted to a single location, with an inferred continuing decline in the number of mature individuals and in the area, extent and quality of their habitats (criterion B1a,b(iii,v)).
Priorities for future research
Most of the areas where the native forest persists are difficult to access due to the very rugged terrain and very high annual rainfall. These natural conditions have guaranteed protection from human interference, but have also made it difficult to study their ecosystems and species. This study is part of the most intensive systematic biological survey of these remote areas, during which data on other taxa (e.g. terrestrial vertebrates, land snails and plants) was also collected, improving knowledge of their distribution, ecology and conservation status (e.g. de Lima et al. Reference de Lima, Maloney, Simison and Drewes2016). These surveys have collected extensive evidence of the importance of the native forest for maintaining many of the island’s endemic species, but have also shown that each species relies on specific areas of the forest for its survival. For instance, while the ibis is associated with high tree density in the lowlands, the fiscal seems to prefer lower tree densities at intermediate altitudes. These specific habitat associations pose a challenge to conservation prioritisation and intervention, as they demand a differential treatment of ecosystems to ensure the persistence of multiple biodiversity components. It is therefore crucial to keep improving our understanding of the relationship between species and ecosystems.
All target species were found more often during the gravanito. This suggests that they become more abundant or easier to detect during this season, and further supports the hypothesis that it coincides with the breeding season for most bird species in São Tomé, as has been previously described (Jones and Tye Reference Jones and Tye2006, Maia et al. Reference Maia, Gascoigne, de Deus and de Lima2014, Azevedo Reference Azevedo2015, Margarido Reference Margarido2015). This finding also indicates that this is most likely the best time of year to census these species. Since resources for monitoring are likely to be scarce, we would prioritise having more robust data for a single season, than dispersing resources in multiple-season assessments, thus maximising detection by surveying when the species are easier to find. Estimating and monitoring population sizes, following specific censuses for each species should be a top research priority, to ensure a better sustained revision of the conservation statuses and provide bases for evidence-based management.
A key priority for further research is to gain a better understanding of the role of potential threats, such as the collection of forest products (timber, charcoal, quarry species, palm wine, medicinal plants) and introduced animal and plant species (de Lima et al. Reference de Lima, Dallimer, Atkinson and Barlow2013a). Tackling the hunting of the Dwarf Olive Ibis is also a top priority, as it seems to be posing an immediate threat to the survival of this species and depends on complex socio-economic drivers (Carvalho et al. Reference Carvalho, Palmeirim, Rego, Solé, Santana and Fa2015, Sampaio et al. Reference Sampaio, de Lima, Ward-Francis and Havery2016). This is essential when considering the species’ restricted breeding range, alongside the fact that hunting pressure may already be limiting the population density in the areas of suitable habitat we have identified.
Implications for conservation
We confirmed that all of São Tomé’s Critically Endangered bird species have a very limited distribution, strongly associated with the occurrence of the best preserved patches of native forest. These are mostly located in the centre and south-west of the island and within the ONP (Salgueiro and Carvalho Reference Salgueiro and Carvalho2007), which despite its legal recognition is weakly enforced (de Lima et al. Reference de Lima, Sampaio and Buchanan2013b). Increasing the effectiveness of the park is key to ensuring the long-term survival of São Tomé’s most threatened avifauna and native forest ecosystems. Nevertheless, it requires significant investment, given the current staff and logistical limitations of the protected area authority. If São Tomé’s tropical forest ecosystems and globally threatened biodiversity are to be protected, particular attention needs to be focused on developing and implementing a rigorous enforcement and surveillance programme, which in turn depends on the identification and development of a sustainable financing approach.
Our work has also shown that even the most threatened species might occur outside the ONP boundaries and use secondary forests. These results are a sign of hope for the future of these species, but should be taken with caution since they represent an improvement on the knowledge about their situation rather than a change in their conservation status. It would be important to better understand the conditions in which they use these areas, to expand suitable habitat through habitat management, control of human activities and expanding the existing network of protected areas in the island. Much of these secondary habitats fall within a proposed buffer zone, which is under threat from large-scale commodity development, while awaiting legal recognition (de Lima et al. Reference de Lima, Sampaio and Buchanan2013b). Improving the protection of key ecosystems outside the only existing protected area and developing an effective management framework for a more sustainable use of resources in edge forest ecosystems is also critical to protect São Tomé’s unique biodiversity.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270916000241
Acknowledgements
We would like to express our gratitude to everyone who contributed to the International Action Plan for the Conservation of Critically Endangered Birds on São Tomé, which was used to guide this work. A special acknowledgment to Eng. Arlindo Carvalho, the General Director for the Environment for supporting our activities in São Tomé. Fieldwork would not have been possible without the help of Silvino Dias, José Malé, Filipe Santiago, Lidiney and countless other Santomeans. A special dedication to “Dakubala”. We thank António Alberto, Nuno Barros, Mariana Carvalho, Martin Dallimer, Hugulay Maia, Stuart Marsden, Martim Melo, Fábio Olmos and Longtong Turshak for sharing their observations. We also thank the three anonymous reviewers for comments that improved an earlier draft of the manuscript. This work is part of BirdLife’s São Tomé and Príncipe Initiative, which was funded by BirdLife’s Preventing Extinctions Programme through the Prentice family (as part of BirdLife’s Species Champion Programme), the Royal Society for the Protection of Birds, the Disney Worldwide Conservation Fund, the U.S. Fish and Wildlife Service Critically Endangered Animals Conservation Fund (AFR-1411 - F14AP00529), the Mohammed bin Zayed Species Conservation Fund (Project number 13256311) and the Waterbird Society Kushlan Research Grant. R.F.L. was supported by a post-doc grant from the Portuguese Government Foundation for Science and Technology “Fundação para a Ciência e para a Tecnologia” (FCT/MCTES - SFRH/BPD/91494/2012).








