Introduction
The Lesser White-fronted Goose Anser erythropus, hereafter LWfG, is ‘Critically Endangered’ according to the IUCN Red List (Birdlife International 2013) and is on the Red Data Book of the threatened animals of Greece (Legakis and Maragou Reference Legakis and Maragou2009). The global population size is estimated at 28,000–33,000 individuals (Jones et al. Reference Jones, Martin, Barov and Nagy2008). The Fennoscandian population, however, is on the verge of extinction. It is estimated that about 20–30 pairs (60–80 individuals) comprise the total population in the Nordic countries (Fox et al. Reference Fox, Ebbinge, Mitchell, Heinicke, Aarvak, Colhoun, Clausen, Dereliev, Faragó, Koffijberg, Kruckenberg, Loonen, Madsen, Mooij, Musil, Nilsson, Pihl and van der Jeugd2010), which breed in northernmost Norway, and usually winter in Greece (Jones et al. Reference Jones, Martin, Barov and Nagy2008).
One of the most important winter habitats is Kerkini Lake (an inland freshwater wetland in northern Greece) where LWfG usually stay from early October to the middle or end of December (Lorentsen et al. Reference Lorentsen, Øien and Aarvak1998, Kazantzidis and Naziridis Reference Kazantzidis and Naziridis1999, Vangeluwe Reference Vangeluwe, Aarvak and Timonen2004, Panagiotopoulou et al. Reference Panagiotopoulou, Tsougrakis, Naziridis, Makriyanni, Tolvanen, Oien and Ruokolainen2009). At that time, LWFG move to the Evros Delta (the easternmost wetland in Greece, on the border with Turkey) where they usually remain until the spring migration to the breeding grounds (late February–early March). The reason for this regular within winter shift remains unknown and raises conservation questions since Kerkini Lake is a wildlife refuge while the Evros Delta is the most popular wetland among waterfowl hunters in Greece, where many Greater White-fronted geese Anser albifrons are shot every winter. At the Evros Delta, LWfG usually forage in mixed flocks with other goose species, mainly Greater White-fronted Goose, which is the main game species in the area (Kazantzidis et al. Reference Kazantzidis, Vasiliadis, Ilias and Makriyianni2015). Hence, the probability of accidentally shooting LWFG individuals from these mixed flocks, which constitutes the main threat of the European population of this species (Jones et al. Reference Jones, Martin, Barov and Nagy2008), is very high.
Among many factors capable of shaping the distribution of birds, the availability of winter food supplies is of vital importance (Johnson and Sherry Reference Johnson and Sherry2001, Folmer et al. Reference Folmer, Olff and Piersma2010). This is the case for the Asiatic population of LWfG in China, which relies on highly specific, but spatially restricted heterogeneous recessional grasslands of East Dongting Lake (Wang et al. Reference Wang, Fox, Cong, Barter and Cao2012, Reference Wang, Fox, Cong and Cao2013). As well as the food availability, the nutrient content of foods has been well documented to influence the choice of habitats and patches by many goose species (Bos et al. Reference Bos, Drent, Rubinigg and Stahl2005), with a few exceptions (Chudzińska et al. Reference Chudzińska, van Beest, Madsen and Nabe-Nielsen2015). Geese are highly likely to respond to relatively small differences in nutritional quality of food items available to them in potential feeding habitats or when choosing between patches within habitats due to their relatively small body size, their short digestive tract and high food passage rates (Sedinger Reference Sedinger1997). Availability, nutritional and energetic quality of the food supply plays a major role in their accumulation of fat and nutritional stores to enable them to withstand harsh weather conditions during winter and in preparation for the spring migration back to their breeding areas (Owen and Black Reference Owen and Black1990, Newton Reference Newton1998). Furthermore, availability and nutrient content of winter food supplies may affect subsequent breeding success (Inger et al. Reference Inger, Harrison, Ruxton, Newton, Colhoun, Gudmundsson, McElwaine, Pickford, Hodgson and Bearhop2010, Morrissette et al. Reference Morrissette, Bêty, Gauthier, Reed and Lefebvre2010). Given this background, investigating the factors that shape the spatial distribution of flocking foragers is of vital importance in effectively conservation planning for this species.
There are few data available on the diet composition and selection of LWfG in northern Europe during spring, summer and autumn (Lorentsen and Spjøtvoll Reference Lorentsen and Spjøtvoll1990, Aarvak et al. Reference Aarvak, Øien and Nagy1996, Niemelä and Markkola Reference Niemelä, Markkola, Tolvanen, Ruokolainen, Markkola and Karvonen1997, Markkola et al. Reference Markkola, Niemelä and Rytkonen2003). Grasses were the most important food category for the LWfG, whereas consumption of dicotyledons occurred at a relatively low level. However, knowledge of LWfG’s diet composition and selection during winter is extremely poor. Grasses were also the main food resource for LWfG based on the analysis of nine droppings collected in Evros Delta during the wintering period of 2005–2006 (Karmiris et al. Reference Karmiris, Kazantzidis, Panagiotopoulou, Tolvanen, Oien and Ruokolainen2009). With the exception of this note on the wintering diet of LWfG, there is not a single published study on this topic for the Fennoscandian population of this species. An assessment of foods selected by LWfG is crucial for understanding their feeding ecology and essential for implementing appropriate management of their wintering habitat. Thus, it is of vital importance to broaden our scientific knowledge of the diet composition and selection of the LWfG at Kerkini Lake, where the species usually spends more than half of the wintering period.
This study directly addresses the crucial issue of the diet selection of LWfG in its wintering habitats at Kerkini Lake during winters 2012–2013 and 2013–2014. The null hypothesis tested in this study was that the LWfG showed no significant selection in relation to the availability of food plants. The present study also aimed to reveal the possible reasons (food quantity and/or nutrient content) for the movement of the LWFG from Kerkini Lake to Evros Delta in the middle of the winter feeding period and to suggest measures that will encourage LWFG to remain at a safe wintering area not subject to hunting (i.e. Kerkini Lake).
Study area
Kerkini Lake is a freshwater reservoir created in 1932, mainly for irrigation and flood control purposes, after the construction of a dam along the Strymon River c.10 km south of the border with Bulgaria (Figure 1). In 1982, a higher dam and dykes were constructed along the eastern shore of the lake. Kerkini Lake is a National Park included in the list of the wetlands of international importance for waterbirds (according to the Ramsar convention) and is a Special Protection Area (SPA) where goose hunting is prohibited.
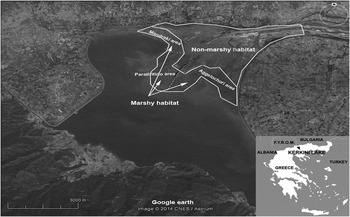
Figure 1. Location map of Kerkini Lake, northern Greece, with available habitats (Google Earth).
The study area was defined as a grassland habitat in the northern and eastern parts of Kerkini Lake. In this area, two major habitats were identified: marshy (no more than 300–400 m away from the shoreline) and non-marshy habitat (more than 400 m away from the shoreline). Due to the lake’s operation as an irrigation reservoir, its water level fluctuates by 5 m and its surface usually decreases from 75 km2 to 50 km2 yearly (higher levels in May–June and lowest in August–September). As a consequence, the marshy habitat area gradually decreases during the period that the LWfG spends at Kerkini Lake each year (usually from October to December), which primarily depends on precipitation rates during this period. The marshy freshwater habitat is dominated by plant species adapted to grow under these conditions, such as Echinochloa crus-galli, Paspalum paspalodes, Ranunculus spp. and species of the Cyperaceae family. Other goose species, such as the Greater White-fronted Goose, Greylag Anser anser and, occasionally, a few (2–3 individuals) of escaped Egyptian Goose Alopochen aegyptiacus also use the same habitat for feeding and often graze together with LWfG.
The remainder of the study area comprises non-marshy grassland dominated mainly by Paspalum paspaloides, Cynodon dactylon and Xanthium strumarium. This habitat is the main feeding area of about 2,000 free-grazing water buffaloes Bubalus bubalis, an expanding agricultural practice which is a very economically important activity both at the local and the national level (Cazacu et al. Reference Cazacu, Rotsios and Moshonas2014). Buffaloes avoid feeding on the marshy area near the shoreline (Karmiris et al. Reference Karmiris, Platis, Kazantzidis and Papachristou2016a). As a result of this activity the area is subject to disturbance from the presence of livestock sheds, herdsmen and livestock guard dogs, supplementary feeding points for stock, etc.
Materials and methods
Habitat use
The relative use of the two available habitats (marshy and non-marshy ones) by the LWfG at Kerkini Lake was based on visual observations of the LWfG flock during the wintering periods 2012–2013 and 2013–2014. The size of the flock (number of individuals) and the type of feeding habitat were recorded on 3–4 days weekly during the daytime, throughout the period that LWfG were present in this area, i.e. from early October to middle December in both wintering periods, as well as during February and early March 2014 (BirdLife Norway and WWF Finland 2016).
Food availability
Relative cover (%) of each plant species in the marshy habitat was estimated on nine field samplings (100 0.25 m2 square quadrats at each sampling site) when dropping collection was also undertaken (Cook and Stubbendieck Reference Cook and Stubbendieck1986). Sampling was conducted at a regular interval from early October to mid-December in 2012 and 2013, the period when LWfG winter at Kerkini Lake. Availability of each plant species was based on the relative cover of vegetation in its feeding area. This was estimated by excluding mosses, bare soil and those plant species which were not consumed by LWfG at all, mainly Bidens tripartita, Cirsium sp., Conyza canadensis, Euphorbia villosa and Xanthium strumarium (Markkola et al. Reference Markkola, Niemelä and Rytkonen2003).
Dropping collection
Fresh droppings from LWfG were collected at Kerkini Lake during the wintering periods mentioned earlier (Appendix S1 in the online supplementary material). We observed the LWfG flock with a telescope without causing disturbance and we located the exact feeding place of the birds. In the cases when the LWfG flock was not mixed with other goose species, we went in situ and collected only fresh droppings. On all collection dates, the number of droppings used in analysis was less than the number of birds in the flock, in order to minimise the possibility of including different droppings belonging to the same individual. In a few cases, a pile of several droppings was found in the field, in which case only one dropping was analysed to estimate diet composition for the same reason. In total, during the first and the second wintering period in the marshy habitat at Kerkini Lake, 65 and 181 droppings of LWfG were analysed, respectively. Each dropping was preserved separately in a plastic bag.
Dietary composition
Microhistological analysis of droppings is the most frequently used method to estimate the diet composition of wild and tame herbivores (Paola et al. Reference Paola, Cid, Brizuela and Ferri2005). This technique causes minimal disturbance to animals in feeding studies of secretive and endangered species (Holechek and Gross Reference Holechek and Gross1982a), such as the LWfG. It is based on the identification of fragments of epidermal tissue in the faeces of herbivores that are assigned to dietary species by comparison with parts of identified plant species which are available to herbivores (Litvaitis et al. Reference Litvaitis, Titus, Anderson and Bookhout1996). The relative frequency of each species can then be compared with the availability of each species in the field.
The dropping samples were oven-dried at 60°C for 48 hours, grounded, mixed thoroughly and sieved through a 1-mm mesh screen to ensure particle uniformity. Five microscopic slides were prepared per dropping. Twenty systematic fields per slide were examined for particle frequency, with a field defined as the area visible on a microscope slide at 100 x magnification. The relative frequency of each plant species was calculated as its frequency divided by the sum of frequencies of all species. Relative frequency accurately estimates relative percentage of dry weight composition of diets (Holechek and Gross Reference Holechek and Gross1982b). Only particles containing epidermal tissue were considered. Hairs and trichomes were disregarded, unless they were attached to identifiable epidermal tissue. Each plant species identified in the droppings was assigned to one of the following forage classes: (1) grasses, (2) other graminoid species (Cyperaceae and Juncaceae families), (3) aquatic plants, i.e. submerged, emerged and amphibious species that occur on permanently or seasonally wet environments and (4) forbs (all other broadleaved herbs present in the non-marshy grassland area).
The most common plant species presented in the marshy habitat at Kerkini Lake (about 60 species) were also collected in plastic bags and pots. Special attention was taken to collect several plant parts (stems, flowers, fruits, etc.) when these were available. Microscopic slides containing the epidermal tissue of the plant parts were then prepared for comparative purposes. The same pattern in diet composition of LWfG was observed during 2012–2013 and 2013–2014, as was expected, since LWfG used the same habitat (the marshy habitat near the shoreline) in both wintering periods; therefore data were combined over years.
Differential digestibility of different food items may bias potential estimates of herbivore diets, particularly when shrubs or forbs are a major component of the diet (Gill et al. Reference Gill, Carpenter, Bartmann, Baker and Schoonveld1983, Leslie et al. Reference Leslie, Vavra, Starkey and Slater1983). In grazers however, microhistological analysis of faeces is considered an accurate and precise method to estimate the diet composition (Bartolomé et al. Reference Bartolomé, Franch, Gutman and Seligman1995, Mohammad et al. Reference Mohammad, Pieper, Wallace, Holechek and Murray1995) and the usefulness of calculating correction factors to deal with differential digestibility is questioned (Paola et al. Reference Paola, Cid, Brizuela and Ferri2005). According to Alipayo et al. (Reference Alipayo, Valdez, Holechek and Cardenas1992), the problems of bias can be alleviated to a great extent by the systematic training of the observer(s) using the procedure described by Holechek and Gross (Reference Holechek and Gross1982a,Reference Holechek and Grossb), rather than applying correction factors that compensate for potential differential digestibility of various food items. In this study, it was assumed that results from the microhistological analysis of LWfG droppings are reasonably accurate, because all recommended techniques were incorporated into the analyses.
Diet selection
Selection indices (ŵi) for each one of the forage categories in both study areas, as well as for every plant species identified in the LWfG’s droppings (except those species that constitute less than 1% of the diet) at Kerkini Lake, were calculated as:
![]() ${\mathord{\buildrel{\lower3pt\hbox{$\scriptscriptstyle\frown$}}\over w} _i} = {{{o_i}} \over {{p_i}}}$
, where oi is the proportion of used resource units and pi is the proportion of available resource units. The standardised selection index Bi (Krebs Reference Krebs1999) was also calculated according the formula:
${\mathord{\buildrel{\lower3pt\hbox{$\scriptscriptstyle\frown$}}\over w} _i} = {{{o_i}} \over {{p_i}}}$
, where oi is the proportion of used resource units and pi is the proportion of available resource units. The standardised selection index Bi (Krebs Reference Krebs1999) was also calculated according the formula:
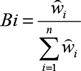 $Bi = {{{{\mathord{\buildrel{\lower3pt\hbox{$\scriptscriptstyle\frown$}}\over w} }_i}} \over {\sum\limits_{i = 1}^n {{{\mathord{\buildrel{\lower3pt\hbox{$\scriptscriptstyle\frown$}}\over w} }_i}} }},$
where Bi is the standardized selection index for species i, and ŵi is the selection index for species i. Standardized selection indices for all forage resources add up to 1 and in essence give the probability of selection of forage resource i in case of equal availability of all resource categories. We tested the null hypothesis of no selection using the G-test (Krebs Reference Krebs1999):
$Bi = {{{{\mathord{\buildrel{\lower3pt\hbox{$\scriptscriptstyle\frown$}}\over w} }_i}} \over {\sum\limits_{i = 1}^n {{{\mathord{\buildrel{\lower3pt\hbox{$\scriptscriptstyle\frown$}}\over w} }_i}} }},$
where Bi is the standardized selection index for species i, and ŵi is the selection index for species i. Standardized selection indices for all forage resources add up to 1 and in essence give the probability of selection of forage resource i in case of equal availability of all resource categories. We tested the null hypothesis of no selection using the G-test (Krebs Reference Krebs1999):
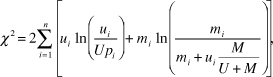 ${\chi ^2} = 2\sum\limits_{i = 1}^n {\left[ {{u_i}\ln \left( {{{{u_i}} \over {U{p_i}}}} \right) + {m_i}\ln \left( {{{{m_i}} \over {{m_i} + {u_i}{M \over {U + M}}}}} \right)} \right]} ,$
where χ2 is the Chi-squared value with (n – 1) degrees of freedom, ui is the number of observations using resource i, mi is the number of observations of available resource i, U is the total number of observations of use (i.e.
${\chi ^2} = 2\sum\limits_{i = 1}^n {\left[ {{u_i}\ln \left( {{{{u_i}} \over {U{p_i}}}} \right) + {m_i}\ln \left( {{{{m_i}} \over {{m_i} + {u_i}{M \over {U + M}}}}} \right)} \right]} ,$
where χ2 is the Chi-squared value with (n – 1) degrees of freedom, ui is the number of observations using resource i, mi is the number of observations of available resource i, U is the total number of observations of use (i.e.
![]() $\sum {{u_i}}$
), M is the total number of observations of availability (i.e.
$\sum {{u_i}}$
), M is the total number of observations of availability (i.e.
![]() $\sum {{m_i}}$
) and n is the number of resource categories.
$\sum {{m_i}}$
) and n is the number of resource categories.
Standard errors of selection indices were calculated using the formula:
![]() ${s_{\bar w}}_i = \sqrt {{{\left( {1 - {o_i}} \right)} \over {U{o_i}}} + {{\left( {1 - {p_i}} \right)} \over {{p_i}M}}} ,$
where
${s_{\bar w}}_i = \sqrt {{{\left( {1 - {o_i}} \right)} \over {U{o_i}}} + {{\left( {1 - {p_i}} \right)} \over {{p_i}M}}} ,$
where
![]() ${s_{\bar wi}}$
is the standard error for a selection index and the other terms as defined above. 95% confidence intervals (CI) for selection indices were calculated using the Bonferroni correction as:
${s_{\bar wi}}$
is the standard error for a selection index and the other terms as defined above. 95% confidence intervals (CI) for selection indices were calculated using the Bonferroni correction as:
![]() ${\mathord{\buildrel{\lower3pt\hbox{$\scriptscriptstyle\frown$}}\over w} _i} \pm {z_{0.0125}}{s_{\bar wi}}$
for the four forage categories, and
${\mathord{\buildrel{\lower3pt\hbox{$\scriptscriptstyle\frown$}}\over w} _i} \pm {z_{0.0125}}{s_{\bar wi}}$
for the four forage categories, and
![]() ${\mathord{\buildrel{\lower3pt\hbox{$\scriptscriptstyle\frown$}}\over w} _i} \pm {z_{0.03125}}\;{s_{\bar wi}}$
for the 16 plant species which were identified in the LWfG droppings and their correspondent percentage in the diet composition was equal or above 1%.
${\mathord{\buildrel{\lower3pt\hbox{$\scriptscriptstyle\frown$}}\over w} _i} \pm {z_{0.03125}}\;{s_{\bar wi}}$
for the 16 plant species which were identified in the LWfG droppings and their correspondent percentage in the diet composition was equal or above 1%.
Confidence intervals of selection indices that do not include the value 1 indicate significant selection. If confidence intervals overlap with unity then the selection index does not differ from a value for α = 0.05, i.e. there is no selection for or against the forage category. Indices of selection were then estimated based on the ratio of diet composition to the relative availability of food item to the LWfG. Values of indices above and below 1 indicate significant selection for or against a plant species respectively (Krebs Reference Krebs1999).
Food nutrient content
During 2015, based on the diet selection data, we collected representative samples of the four most highly selected plant species (Echinochloa crus-galli, Cyperus esculentus, Scirpus lacustris and Ranunculus sceleratus) and the four forage categories (grasses, other graminoids, aquatic plants and forbs) in the middle of November (i.e. about the mid-point of the traditional time that LWfG stay at Kerkini Lake) and at the end of December (i.e. after their departure from Kerkini Lake, which usually happens in the middle of December), in order to detect any effects of the nutrient content of available foods for the LWfG on the departure time from Kerkini Lake. These samples were collected separately for each plant species and forage category in three representative sites in the marshy area, oven-dried (55°C for 48 hrs), ground in a Wiley mill (1-mm screen) and used for the determination of nitrogen (AOAC 1990; crude protein (CP) was determined by N x 6.25), neutral detergent fibre (NDF), acid detergent fibre (ADF) and acid detergent lignin (ADL) (Goering and van Soest Reference Goering and van Soest1970, van Soest et al. Reference van Soest, Robertson and Lewis1991).
Differences among vegetation categories and plant species and collection periods in the levels of their chemical composition were determined using analysis of variance. The data on vegetation categories and plant species were analysed separately. Each analysis consisted of four forage types (either four vegetation categories or four plant species) and two periods. The three sites where forage samples were collected were a random factor while forage types and periods were the main fixed effects. When F-tests indicated significant differences, means were compared using LSD (P ≤ 0.05).
Results
Feeding habitat
The feeding habitat of LWfG at Kerkini Lake was exclusively (100%, n = 69) the marshy grassland from below the water line (less than 5 cm deep) to 300–400 m away from the shore. During the study period, LWfG were distributed at the north-eastern part of the lake, mainly at the sites of ‘Aggelochori’ and ‘Paratiritirio’ and only rarely at the Mandraki area (Figure 1). The mean flock size (± SE) was 43 ± 3.0 (n = 31) individuals in 2012–2013 and 45 ± 2.7 (n = 38) in 2013–2014. Greater White-fronted, Greylag and Egyptian Geese were also recorded foraging at the same marshy habitat in mixed flocks with the LWfG (in c.60% of the cases), especially during the second half of the wintering period (December–March). Additionally, many duck species (Anas spp.) were also recorded foraging in the deeper parts of the marshy habitat.
Food availability and nutrient content
Grasses were the most available forage to LWfG (47.6%) and total monocotyledons constituted more than the half of the total available food resource (Figure 2). The remainder was comprised of aquatic plants (17.4%) and forbs (23.3%). Aquatic species, such as Polygonum persicaria, Limosella aquatica, as well as species thriving in damp, wet soils, such as Echinochloa crus-galli, Paspalum paspalodes, Filaginella uliginosa, Amaranthus blitus, Cyperus michelianus were the most available species to foraging LWfG.
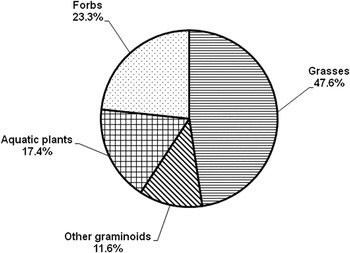
Figure 2. Food availability of the major vegetation categories in the marshy habitat at Kerkini Lake from October to mid December 2012 and 2013. Data are based on 900 plots (0.5 x 0.5 m) on nine different dates.
The relative proportions of the major plant categories (grasses, other graminoids, aquatic plants and forbs) present in vegetation cover were more or less stable during October and November, but all showed substantial reductions during December (Figure 3). The same trend was also observed amongst the four most highly selected plant species by LWfG and all were effectively absent by the end of December. Subsequently, the relative proportions of bare soil and mosses showed the opposite trend reaching their highest values in December. It is noteworthy, that the departure of LWfG from Kerkini Lake (mid-December) coincided with the dramatic reduction in the coverage of all plant species but especially the selected plant species within its feeding habitat.
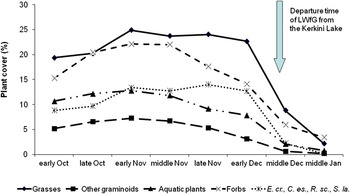
Figure 3. Temporal changes in cover (%) of major vegetation categories and the four most highly selected species (Echinochloa crus-galli – E.cr., Cyperus esculentus – C. es., Scirpus lacustris – S. la. and Ranunculus sceleratus – R. sc.) in the marshy habitat at Kerkini Lake from early October 2013 to the middle of January 2014. Data are based on eight field surveys.
Chemical composition of the vegetation categories and plant species at the two sampling dates is shown in Table 1. There were significant differences among categories in their CP (F 3, 6 = 12.71, P = 0.005), NDF (F 3, 6 = 119.54, P < 0.001), ADF (F 3, 6 = 35.54, P < 0.001) and ADL (F 3, 6 = 13.91, P < 0.004) contents; and among species for all variables as well (F 3, 6 = 131.43, P < 0.001; F 3, 6 = 619.87, P < 0.001; F 3, 6 = 316.39, P < 0.001; F 3, 6 = 9.28, P = 0.011, respectively). The averaged CP content across periods of Ranunculus sceleratus (227.1 g/kg DM) was higher than Echinochloa crus-galli (118.4 g/kg DM), Cyperus esculentus (105.3 g/kg DM) and Scirpus lacustris (68.6 g/kg DM). Also, Ranunculus sceleratus had the lowest values of NDF (340.1 g/kg DM) and ADF (269.8 g/kg DM), whereas Echinochloa crus-galli showed the lowest ADL content (57.3 g/kg DM). The averaged CP (F 1,8 = 6.13, P < 0.05) and NDF (F 1,8 = 31.95, P < 0.001) concentrations across plant species varied with time but this was not the case for ADF (F 1,8 = 3.49, P > 0.05) and ADL content (F 1,8 = 0.03, P > 0.05); a decrease in both CP and NDF contents from November (136.3 and 530.3 g/kg DM, respectively) to December (123.5 and 483.0 g/kg DM, respectively) was found. There were significant vegetation categories x period interactions for CP (F 1,8 = 11.25, P = 0.003), NDF (F 1, 8 = 8.24, P = 0.008), ADF (F 1, 8 = 4.63, P < 0.05) and ADL (F 1,8 = 5.51, P < 0.05). However, significant differences (P < 0.05) between periods were found only for CP of forbs, NDF of other graminoids and forbs, ADF of grasses and forbs and ADL of forbs (Table 1). There were no effect of plant species x period interactions on concentrations of all four chemical compounds (P > 0.05; Table 1) indicating that the nutrient content of the four most preferred plant species remained fairly constant through the whole experimental period.
Table 1. Chemical composition of the major vegetation categories and the most highly selected plant species by LWfG during the middle of their stay at Kerkini Lake (November) and after their departure (December).

DM= dry matter; CP= crude protein; NDF= neutral detergent fibre; ADF= acid detergent fibre; ADL= acid detergent lignin. LSD0.05 = 36.6, 41.7, 56.9 and 39.9 g/kg to compare vegetation categories x period means for CP, NDF, ADF and ADL, respectively; F-tests did not indicate significant differences for major plants x period means of all items. Asterisks denote vegetation categories when significant (P ≤ 0.05) differences existed between the two tested periods.
Diet composition and selection
At least 33 plant species were recognized and quantified in the droppings of LWfG (Table 2). The main food of LWfG was grass species (especially Echinochloa crus-galli) and other graminoids (mainly species of the Cyperaceae family), which made up more than the 2/3 of the LWfG total diet. Aquatic plants and forbs were also found in the droppings of the LWfG but to a lesser extent (about a quarter of the total diet). Species such as the Echinochloa crus-galli, Paspalum paspalodes, Cyperus spp., Scirpus lacustris, Limosella aquatica and Ranunculus sceleratus constituted important food resources for the LWfG in both wintering periods. These species grow mainly in the marshy habitat, but some of them, such as the Paspalum paspalodes, may also contribute to the vegetation composition of the terrestrial habitat.
Table 2. Diet composition (% dry weight) of the Lesser White-fronted Goose based on 246 droppings at Kerkini Lake, Greece during the wintering periods 2012–2013 and 2013–2014.

* less than 1%.
Twelve species with the highest participation in diet composition accounted for 80% of the total diet (Table 2). These species include Echinochloa crus-galli, Paspalum paspalodes, Cyperus esculentus, C. fuscus, Scirpus lacustris, Limosella aquatica, Ranunculus repens, R. sceleratus, Amaranthus lividus, Lindernia dubia, Portulaca oleracea, Veronica beccabunga. Frequency of occurrence of each one of these plant species in the LWfG droppings was especially high, as more than 90% of the droppings examined contained identified particles of these plant species. These high percentages of frequency of occurrence of the most important food items resulted to a limited variation of the diet composition among LWfG’s droppings.
The most highly selected species by LWfG at Kerkini Lake in both years of the study were Echinochloa crus-galli, Cyperus esculentus, Scirpus lacustris and Ranunculus sceleratus (Table 3). All four species grew in wet soils and their biomass was used as food repeatedly on different days by the LWfG.
Table 3. The most highly selected plant species consumed by Lesser White-fronted Goose at Kerkini Lake, Greece during the 2012–2013 and 2013–2014 wintering periods.

a all values of the selection index ŵ i above the value 1 indicate preferential selection.
b all values of the selection index B i above the critical value 0.062 indicate preferential selection.
Grasses (mainly Echinochloa crus-galli and Paspalum paspalodes) were the only forage category which was significantly selected by the LWfG both in each one of the two wintering periods and in total (Table 4). Forbs (e.g. Lindernia dubia, Portulaca oleracea, Amaranthus lividus and others) were marginally avoided by the LWfG during the first wintering period and in total, but not during the second wintering period. Although other graminoid species were overall positively selected, the selected indices of most of them fell below the critical value that indicates preferential selection. Conversely, aquatic plants were overall avoided relative to their abundance, despite the fact that Ranunculus sceleratus was preferentially selected.
Table 4. Selection indices (ŵ i ± 95% confidence intervals) and standardized selection indices (B i ) of major forage categories for Lesser White-fronted Goose at Kerkini Lake during the 2012–2013 and 2013–2014 wintering periods.
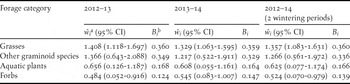
a confidence intervals of the selection indexes ŵ i above or below the value 1 indicate significant selection for or against the forage category respectively.
b all values of the selection index B i above the critical value 0.250 indicate preferential selection.
Discussion
LWfG consumed forage produced by several plant species capable of growing in the marshy habitat at Kerkini Lake. These species are commonly found in temporarily flooded areas in Europe and especially in the Balkans and Eastern Europe, i.e. along the LWfG flyway (Pál et al. Reference Pál, Pinke, Oláh, Csiky and Koltai2006, Stroh Reference Stroh2006, Lukács et al. Reference Lukács, Sramkó and Molnár2013). Although LWfG consumed a variety of plant species at Kerkini Lake, the high frequency of occurrence in LWfG droppings of the 12 most highly consumed plant species showed a considerable dietary selectivity and a limited variation in the diet composition among LWfG’s droppings. This indicates that the individuals in the flock of LWfG consumed more or less the same food items, in similar proportions, on the same feeding grounds.
The trend observed in this study (i.e. grasses constitute the main food for the wintering LWfG) has also been reported for this species during the breeding season (Aarvak et al. Reference Aarvak, Øien and Nagy1996, Niemelä and Markkola Reference Niemelä, Markkola, Tolvanen, Ruokolainen, Markkola and Karvonen1997, Markkola et al. Reference Markkola, Niemelä and Rytkonen2003). In general, grasses on rangelands, pasturelands and agricultural crops are considered an important food category by many goose species (Vickery and Gill Reference Vickery and Gill1999, van der Wal et al. Reference van der Wal, van Wijnen, van Wieren, Beucher and Bos2000, Best and Arcese Reference Best and Arcese2009, Soininen et al. Reference Soininen, Hübner and Jónsdóttir2010). Although goose species tend to exhibit more or less constant food preferences in their feeding areas (Summers et al. Reference Summers, Underhill, Howells, Vickery and Jones1996, Gill et al. Reference Gill, Watkinson and Sutherland1997), neighbouring habitats with suitable nutritional resources may attract them away from regularly used habitats (Bos et al. Reference Bos, Drent, Rubinigg and Stahl2005, Fox et al. Reference Fox, Madsen, Boyd, Kuijken, Norriss, Tombre and Stroud2005). Concerning LWfG at Kerkini Lake, the almost exclusive use of the marshy water’s edge as the primary feeding area has been observed, not only during the last two wintering periods, but also consistently for many years prior to this study, with a few exceptions. For instance, LWfG have occasionally used the non-marshy habitat and cereal crops outside the protected area of Kerkini Lake National Park (Kazantzidis and Naziridis Reference Kazantzidis and Naziridis1999). These movements can be considered as an exception to the general rule and may be the consequence of temporal food shortage on the primary habitats of LWfG, but also of the potential temporal changes in the quality of the available food as winter progresses (Cong et al. Reference Cong, Wang, Cao and Fox2012, Wang et al. Reference Wang, Fox, Cong and Cao2013). In the case of Kerkini Lake, however, LWfG departed in mid-December to the Evros Delta (Thrace, eastern Greece), which coincided with the rapid decline in forage availability at the lake. In addition, the nutrient content of the preferred plant species did not change from November to December. For instance, there was no change for both grasses and other graminoids, which made up the 70% of the diet. This supports the opinion that availability of food supplies and not the nutrient content seems to play the major role in feeding habitat choice and the movement pattern of LWfG.
According to Aloupi et al. (Reference Aloupi, Kazantzidis, Akriotis, Bantikou and Hatzidaki2015), a seasonal increase of lead (Pb) and aluminium (Al) was recorded in LWFG’s droppings at Kerkini Lake during the 2013–2014 wintering period, which indicates a gradual increase in soil ingestion by the grazing birds. The same authors claimed that the decrease of the availability and height of grasses (the main food of LWfG) and generally of all available vegetation as winter progresses may explain the gradual increase in soil intake, especially in early December. In the present study, the departure time of birds from the lake, (mid-December in both years) coincided with very low levels of food availability. Food-dependent distributional shifts have been recently recorded in the eastern Asiatic population of LWfG in China, where food constraints seem to regulate its movements and the selection of feeding habitats (Wang et al. Reference Wang, Fox, Cong and Cao2013). However, the threat of accidental shooting posed by moving to Evros Delta is especially great because goose hunting is permitted very close to the main feeding grounds of the LWfG there. Because LWfG usually graze in association with the very similar Greater White-fronted Goose, the main game species in this area, the rarer species is at great risk of being shot. Thus, the provision of adequate food stocks in areas not subject to hunting (i.e. Kerkini Lake) is potentially a valuable ‘tool’ for the conservation of this bird species and its wintering habitats.
Management implications
Marshy habitat at Kerkini Lake can be considered the main wintering habitat for LWfG during the three years of this study, as the birds wintered there for most of the wintering period each year. In addition, LWfG departed from Kerkini Lake to other wintering habitats, such as Evros Delta, when either food availability falls in very low levels or flooding occurred in their main feeding habitat. Consequently, as long as food and habitat resources are available for LWfG, it is very likely that the birds will winter mainly at Kerkini Lake and not at Evros Delta, which will contribute further to minimisation of the theoretical risk of accidental shooting of LWfG at the latter wintering habitat. Thus, future conservation actions should primarily focus on grassland improvement at Kerkini Lake enhancing the availability of food resources for LWfG (mainly grasses) and protection of the feeding habitat from flooding, rather than making the Evros Delta a safer place for LWfG (e.g. by imposing further limitations on goose hunting or other human activities).
The most promising and feasible solution for this purpose would be the creation of alternative feeding areas for geese (Owen Reference Owen1990, Percival Reference Percival1993, Vickery and Gill Reference Vickery and Gill1999). These areas should be located at the upper parts of the marshy habitat or even more at the boundaries with the non-marshy habitat (i.e. about 300–600 m away from the shoreline), where flooding occurs at a later time in relation to the low elevation parts of the marshy habitat near the shoreline. As coverage of natural vegetation falls below 50% from December onwards, experimental seeding should aim at filling the gaps in availability of the natural vegetation. Hence, seeding should be applied before the arrival of birds at Kerkini Lake in late September– early October), without any preparation of the soil in order to protect the valuable natural vegetation which provides food for geese for many years. This is expected to minimise both the impact of disturbance on birds and the potential interference with free-grazing buffaloes which graze on the non-marshy area. However, Echinochloa crus-galli, which constituted almost the half of the total diet content and was preferentially selected by LWfG, is a warm-season (C4) grass species, intolerant of the usually prevailing low winter temperatures. During this study, the availability of this grass species was almost eliminated from early December onwards, because of freezing. Instead of Echinochloa crus-galli, winter cereals of other cool-season (C3) grasses (e.g. Festuca arundinacea) could be used for that purpose. Cereal crops may constitute a substantial part of the diets of goose species (Vickery and Gill Reference Vickery and Gill1999) and may modify their usual movement pattern (Bos et al. Reference Bos, Drent, Rubinigg and Stahl2005, Fox et al. Reference Fox, Madsen, Boyd, Kuijken, Norriss, Tombre and Stroud2005, Wang et al. Reference Wang, Fox, Cong and Cao2013). LWfG have been recorded occasionally in previous years to feed on the non-marshy habitat and in cereal crops outside of the protected area of Kerkini Lake National Park (Kazantzidis and Naziridis Reference Kazantzidis and Naziridis1999). Seeding cereals and highly palatable cool-season grasses could be an important benefit for LWfG because the availability of such forage could expand the carrying capacity of the site and prolong the length of stay within Kerkini Lake, as well as to reduce movements to other areas outside the protected area of the Kerkini Lake National Park. This may further reduce the accidental shooting of LWfG (the main threat according to Jones et al. Reference Jones, Martin, Barov and Nagy2008), as recorded in 2007, when an adult bird was found shot outside the protected area of Kerkini Lake (Tsougrakis et al. Reference Tsougrakis, Panagiotopoulou, Makriyanni, Tolvanen, Oien and Ruokolainen2009). For these reasons, cereals (e.g. Triticum aestivum, Hordeum vulgare) could be sown under the conditions mentioned above (no preparation of the soil, sowing at the edge of the marshy and non-marshy habitats before the arrival of the LWfG), in order to increase the availability of food from mid-December onwards (i.e. the time that the preferred food cover of natural vegetation is very limited). Under the current hydrological regime, artificially sown plants would not have the opportunity to flower or even more to produce seeds, as this area is usually flooded until February every year, thus potential negative effects on natural vegetation are not likely to occur but reseeding should be carried out every year. Towards this goal, preliminary data from a small-scale seeding trial (ongoing study) indicate that seeding cereals under the mentioned conditions at Kerkini Lake increased the total plant cover and the above ground production and subsequently the availability of food for goose species (Karmiris et al. Reference Karmiris, Platis, Kazantzidis and Papachristou2016b). Furthermore, seeding sites attracted geese within 40 days after seeding and herbage utilisation of the seeded sites was higher than the sites covered by natural vegetation. Because of the maintenance of natural vegetation, such small-scale management actions are not expected to lead to adverse effects by the consumption of non-natural foods (i.e. seeded species). At the moment, no effects on natural vegetation structure and composition and responses of birds have been recorded by the application of the seeding process, but intensive monitoring will also follow in order both to detect any adverse potential effects on birds and to maintain the long-term integrity of the habitat. Further and in-depth investigation of the effects of the seeding process both on natural vegetation and on the bird behaviour during the upcoming years should be a high research priority. This knowledge is required to assist in prioritising multiple management actions for the conservation of the European LWfG population and its habitats.
The periodic flooding of the marshy and the terrestrial grasslands surrounding the northern and eastern parts of Kerkini Lake make them available for wild and domestic herbivores for approximately 6 months per year (from August to January-February). As the marshy habitat is of prime importance for LWfG conservation, the operation of the dam, besides irrigation purposes and flood control of agricultural land, should also target to prevent flood events on the marshy habitat during the period that birds are present at Kerkini Lake (i.e. usually from October to late December, but this time may be extended to the end of the wintering period). Furthermore, because LWfG usually arrives at Kerkini Lake in early October, the various plant species occurring in the marshy habitat should have had time to grow sufficiently to provide food for the LWfG on their arrival. Sprouting of these plants should therefore occur at least 1–1.5 months prior to the arrival of the LWfG at Kerkini Lake, i.e. not later than the end of August. This is more or less what happens at Kerkini Lake due to the current operation of the dam but for other management purposes (crop irrigation and flood control). This regime is considered vital for the LWfG and it is therefore important to safeguard the existing hydrological regime in the future in a way which will incorporate the needs of this species in a flexible way.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270916000393
Acknowledgements
We would like to thank Dr. Theodoros Naziridis, director of the Management Body of Kerkini Lake National Park, for his support and constructive comments during the whole study. Gratitude is also expressed to Kostas Papadopoulos, Panagiotis Chatzigiannidis, Sotiris Moutzelos and Michalis Davis, personnel of the Management Body of Kerkini Lake National Park, for their help in the field. We would also like to thank Ioakim Vassiliadis, Alkmini Mpataka (Forest Research Institute) for the help in the field and for laboratory assistance and Dr. Georgios Menexes for statistical advice. This research was carried out in the framework of the EU LIFE + project “Safeguarding the Lesser White-fronted Goose Fennoscandian population in key wintering and staging sites within the European flyway” (LIFE 10 NAT/GR/000638), which was funded by the European Commission and the Norwegian Directorate for Nature Management. The Forest Research Institute, Hellenic Agricultural Organization “DEMETER” receives support from the Hellenic Ministry of Rural Development and Food.









