Introduction
Species restoration through reintroduction and reinforcement is widely considered as an effective method for the conservation of threatened animals (IUCN/SSC 2013). High breeding success and successful recruitment into the breeding population are essential for the establishment and persistence of reintroduced populations (Sarrazin and Barbault Reference Sarrazin and Barbault1996, Kemp et al. Reference Kemp, Norbury, Groenewege, Comer, Armstrong, Hayward, Moro and Seddon2015). These approaches have been increasingly implemented over the last half-century, but many attempts have failed (Berger-Tal et al. Reference Berger-Tal, Blumstein and Swaisgood2020). Owing to intrinsic (e.g. social attraction within the breeding population) and extrinsic factors (e.g, human activities) or environmental stochasticity, these expectations have not been fulfilled in some cases (Margalida et al. Reference Margalida, Heredia, Razin and Hernández2008, Oro et al. Reference Oro, Martínez-Abraín, Villuendas, Sarzo, Mínguez and Carda2011).
Accurate knowledge of dispersal behaviour can be a more crucial factor in determining reintroduction success across multiple stages of the process than other issues such as learning, foraging, competition, and social behaviour (Berger-Tal et al. Reference Berger-Tal, Blumstein and Swaisgood2020). Reintroductions can fail due to the immediate and continued dispersal of released individuals away from the reintroduction site where the chances of survival and pair formation are reduced (Rickett et al. Reference Rickett, Dey, Stothart, O’Conner, Quinn and Weihong2013, Richardson et al. Reference Richardson, Doerr, Ebragimi, Lovegrove, Parker, Armstrong, Hayward, Moro and Seddon2015). However, reintroductions also might also fail due to a lack of dispersal of the offspring of founders due to intraspecific interactions, as well as a lack of long-term genetic and demographic exchange with other populations (Matthysen Reference Matthysen2005, Richardson et al. Reference Richardson, Doerr, Ebragimi, Lovegrove, Parker, Armstrong, Hayward, Moro and Seddon2015).
The ‘Endangered’ Oriental Stork Ciconia boyciana is an endemic bird of the Far East. This species is migratory, breeding in Eastern Russia and Northern China, and wintering in South China, South Korea, and Japan; however, some birds are residents in the latter areas (BirdLife International 2018). The population in Japan was declared extinct in the wild in 1971, following a drastic decline due to overhunting, habitat loss, pollution, and inbreeding (Hyogo Park of the Oriental White Stork 2011). Reintroductions were initiated in Japan in 2005 through successful captive breeding programmes. The official recovery plan, “Grand Design for Reintroduction of the Oriental Stork”, included an adaptive management approach (Hyogo Park of the Oriental White Stork 2011). The survival rate, age structure, and abundance of the reintroduced population were subsequently reported (Ezaki et al. Reference Ezaki, Mitsuhashi and Ohsako2016, Ezaki and Ohsako Reference Ezaki and Ohsako2019).
Sutherland et al. (Reference Sutherland, Armstrong, Butchart, Earnhardt, Ewen, Jamieson, Jones, Lee, Newbery, Nichols, Parker, Sarrazin, Seddon, Shah and Tatayah2010) emphasised the importance of documenting reintroduction project plans and routinely publishing follow-up results to improve the success of this conservation strategy. Here, we report new findings on the demographic composition of multiple generations, post-release and natal dispersal, and the determinants of breeding success of the Oriental Stork population reintroduced in Japan. We hypothesized that one of the key determinants of breeding success is the density-dependent effect through post-release and natal dispersal.
Methods
The Oriental Stork is a monogamous bird that breeds annually. Reintroduced birds start to breed from three years of age and have a longevity of proximately 20 years in the wild (Ezaki and Ohsako Reference Ezaki and Ohsako2019). Breeding pairs often nest on artificial structures, such as utility poles and nest towers, and display exclusive territories of approximately 100 ha surrounding the nest (Ezaki and Ohsako Reference Ezaki and Ohsako2019). Clutches contain an average of three to four eggs. Storks often feed in shallow water, such as paddy fields.
Between 2005 and 2019, 73 captive storks were released in Japan. Since 2005, 53 storks (29 males, 24 females) have been released in the Hyogo Prefecture at nine sites in Toyooka, two sites in Yabu, and two sites in Asago (Table 1, Figure 1). Since 2015, a further 11 (seven males, four females) storks were released at Noda in the Chiba Prefecture, and nine (five males, four females) storks were released at Echizen in the Fukui Prefecture (Table 1, Figure 1). Half of the birds that were released before 2009 (n = 25, mean ± SD of age: 3 ± 2 years old) were adults (older than three years old); however, the remaining birds across all years of release were primarily juveniles (younger than one year old). Hard (n = 27) and soft (n = 46) release methods, which represent the immediate release of birds from captivity into the wild or a short acclimatisation period at a release site, respectively, were used (Naito et al. Reference Naito, Kikuchi and Ikeda2011). All released storks were marked with a unique colour ring combination for individual identification. Nearly all (99%) descendants were captured on nests and marked with colour rings approximately one month before fledging. The sex of these birds was determined using DNA extracted from blood samples or rachis feathers on the methods of Murata et al. (Reference Murata, Itoh, Ogawa and Mizuno1998). Hyogo Park of the Oriental White Stork is the ringing centre where all observation records by the general public of ringed storks are collected. Survival in the wild was defined as whether living records were collected within a year or the bird was taken into captivity due to injury or inbreeding avoidance. The filial generation was defined based on the older generation when the breeding pair consisted of storks of different generations, for example, the generation of bird born from a pair of F1 male and F2 female was defined as F3. A total of the mixed generations accounted for 42% in the filial generation in 2019.
Table 1. Release history of Oriental Stork in Japan during 2005–2019.


Figure 1. Demographic composition of the reintroduced Oriental Stork population in Japan from 2005 to 2019. F1 corresponds to filial first, F2 to filial second, F3 to filial third, and F4 to filial fourth generations.
During the study period (2005–2019), a total of 27 pairs fledged at least one chick and only one released bird paired with an unringed wild bird that had most likely migrated from Russia or China. Only two pairs regularly visited a captive cage in Hyogo Park of the Oriental Stork to steal food meant for hand-reared birds. Due to these abnormal effects on the dispersal pattern, we excluded these birds from the following analyses. In the individuals that survived to over three years old (i.e. sexually matured birds), the proportion of breeders was compared between released birds (male: n = 21; female: n = 17) and wild-born birds (male: n = 18; female: n = 42) using a χ 2 test. The straight-line distance for post-release (from release sites to first breeding sites, male: n = 12; female: n = 10) and natal dispersal (from birthplaces to first breeding sites, male: n = 13; female: n = 15) of breeding birds was compared using a Mann–Whitney U test. Different filial generations of wild-born birds were combined for this analysis because of small sample sizes. The number of fledglings represented the breeding success of the storks. A generalized linear mixed model with Poisson errors and log-link function was used to determine the factors affecting breeding success. Breeding year, parent age of males and females, pair duration (number of years elapsed from first breeding success), and pair combination pattern (both released birds, both wild-born birds, released and wild-born birds) were treated as explanatory variable candidates. Inter-nest distance (mean straight-line distance between nests each year) was included as the representative value of the density-dependent effect in the candidates because this variable affected on breeding success of a relative species, the White Storks Ciconia ciconia (Nowakowski Reference Nowakowski2003). Significant correlations were found between male age, female age, and pair duration (r > 0.8, P < 0.01 in each case); thus, male and female age were removed from the subsequent analysis. Pair identity was included as a random effect. Model selection was performed using the Akaike information criterion (AIC). The model with the smallest AIC was selected as the simplest best-fitting model, and models with ∆AIC < 2.0 were considered to be competing models with little difference from the best model. A dispersion parameter, which was calculated as the available residual deviance divided by the residual degrees of freedom, was used for detecting the presence of overdispersion (Crawley Reference Crawley2007). Statistical analyses were performed using R 4.0.0 software (R Core Team 2020) and the glmmML package (Broström Reference Broström2020).
Results
During the growth process of the reintroduced population, a total of 216 birds fledged in the wild, seven birds were taken into captivity, 55 birds died (0-year-old: n = 27, 1-year-old: n = 9, 2–14 years old: n = 19), and 35 birds (0-year-old: n = 23, 1-year-old: n = 4, 2–6 years old: n = 8) went missing during the study period of 15 years. The F1 generation occurrence corresponded to two, F2 to seven, F3 to 11, and F4 to 14 years after initial reintroduction (Fig. 1). The proportion of released birds in the reintroduced population continued to decrease after the occurrence of F1. Wild-born birds accounted for 74% of the population in 2019 (n = 191; released: 26%; F1: 31%; F2: 31%; F3: 11%; F4: 1%), when 17 pairs displayed breeding success.
There were no significant differences in the proportion of breeders between the released and wild-born male birds (χ 2 = 0.97, P = 0.33) and female birds (χ 2 = 2.63, P = 0.11) throughout the study period. Pairs that were composed of released birds bred at the nest sites closer to the release sites compared to that of pairs of both wild-born birds as well as pairs of released and wild-born birds (Figure 2). Post-release dispersal distances were shorter than natal dispersal (Z = 3.08, P < 0.01), especially for F1 and F2 (Figure 3). Different release methods were combined in this analysis as there was no difference in the distances travelled between hard and soft release (Z = 0.38, P = 0.71).
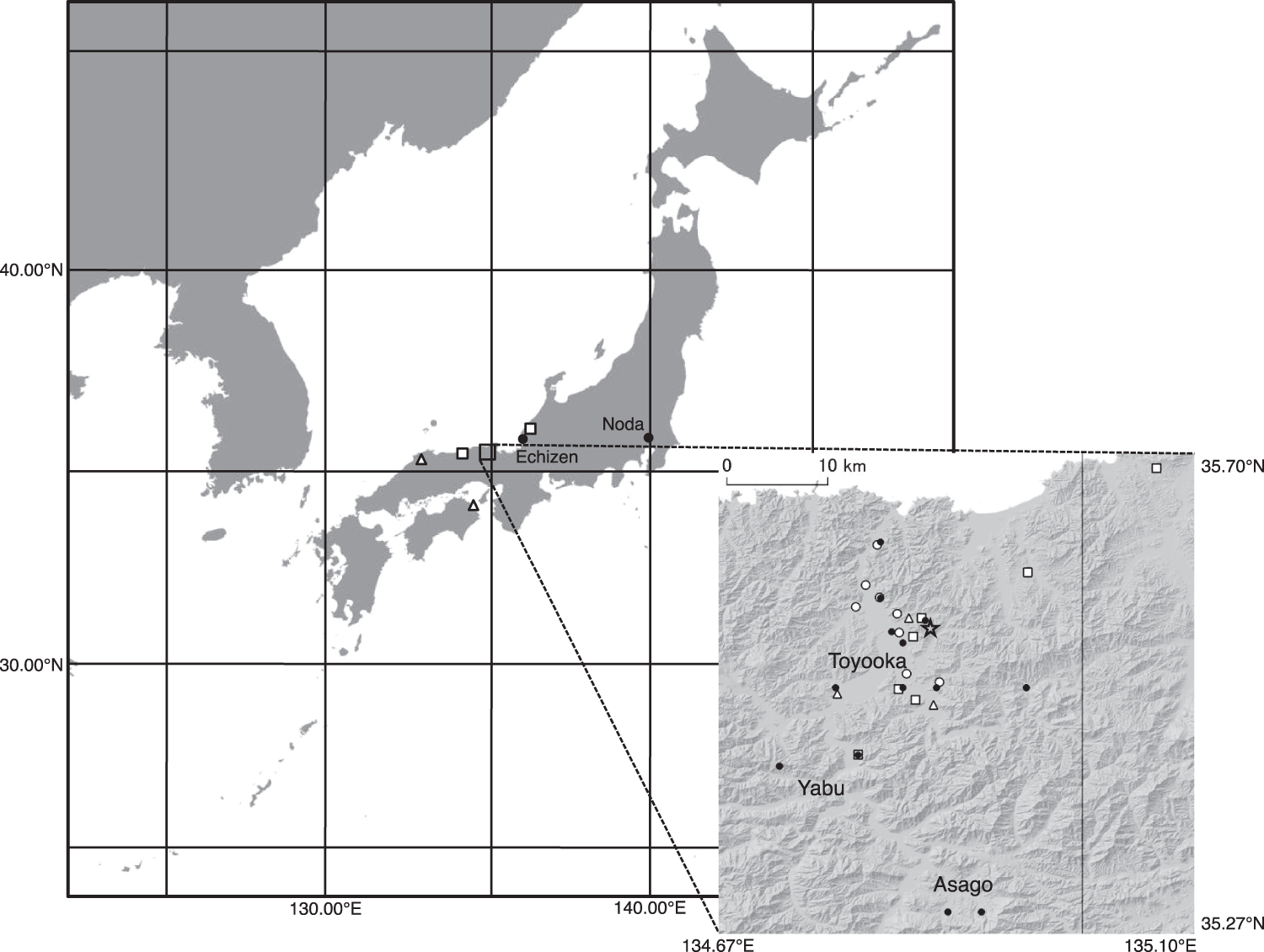
Figure 2. Breeding distribution of the reintroduced Oriental Stork population from 2005 to 2019. Open circles correspond to the breeding sites of pairs of both released birds, triangles correspond to those of pairs of released and wild-born birds, and squares correspond to those of pairs of both wild-born birds. Filled circles and the star represent the release sites and the Hyogo Park of the Oriental White Stork, respectively.
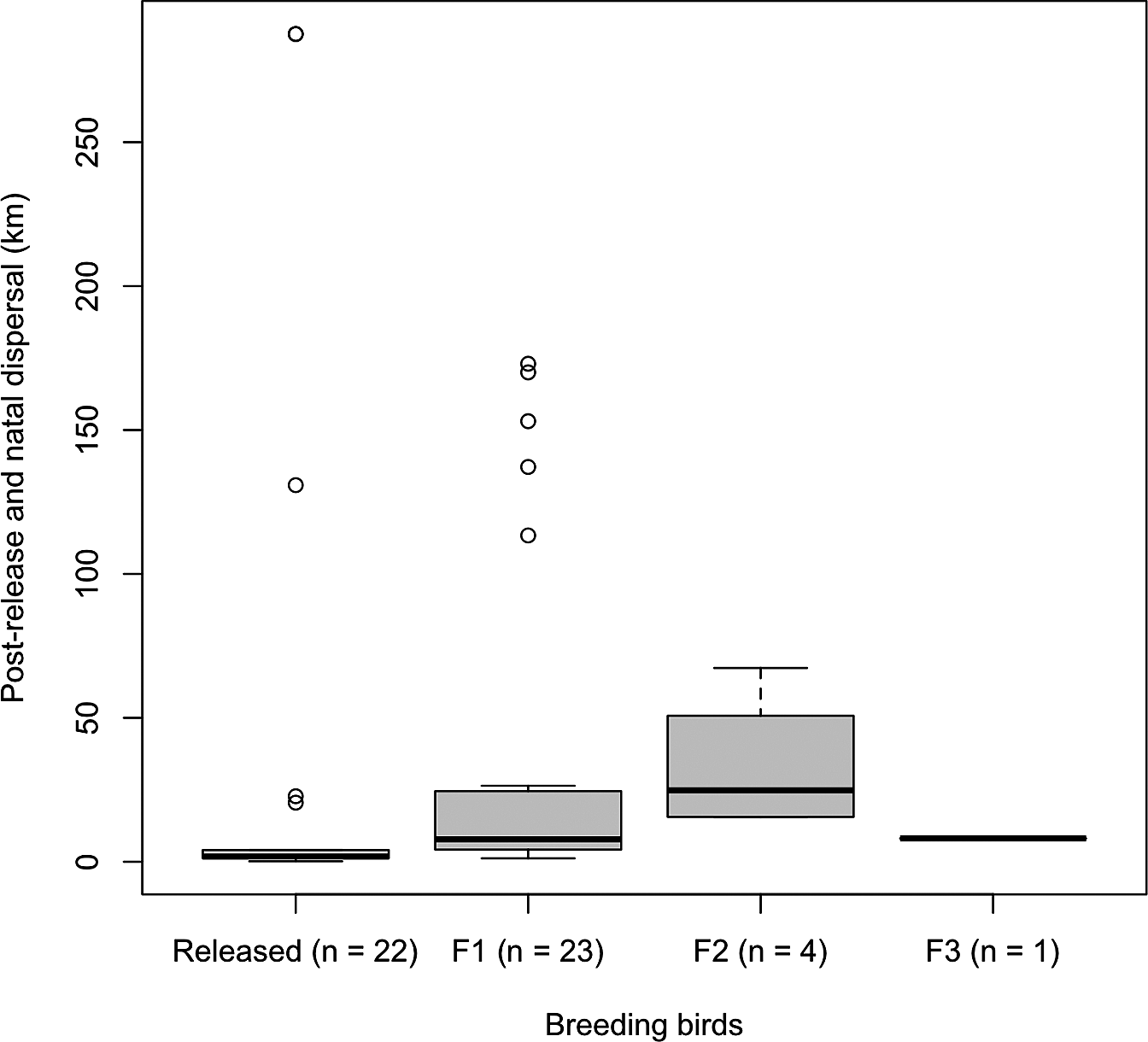
Figure 3. Straight-line distance of post-release (from release sites to first breeding sites) and natal dispersal (from birthplaces to first breeding sites, F1–F3) of reintroduced breeding Oriental Storks.
The breeding success of each pair was positively related to inter-nest distance in every competing model (Table 2). Specifically, an increase in inter-nest distance from 4.1 km to 179.2 km doubled the number of fledglings per nest (Figure 4). The different release methods were combined in this analysis as there was no difference in breeding success between hard and soft released birds (Z = 0.96, P = 0.34). Overdispersion was not detected in any competing model because the dispersion parameter was less than 1.
Table 2. Summary of model selection results for factors affecting the breeding success of each pair (n = 25) of reintroduced Oriental Storks in Japan during 2005–2019. Explanatory variables in the model include breeding year, number of years elapsed from first breeding success (pair duration), pair combination patterns (both released birds, both wild-born birds, released and wild-born birds) and inter-nest distance (mean straight-line distance between nests). Only models with differences in Akaike’s information criterion (∆AIC <2.0) are listed. Akaike weights (wi) are also presented.
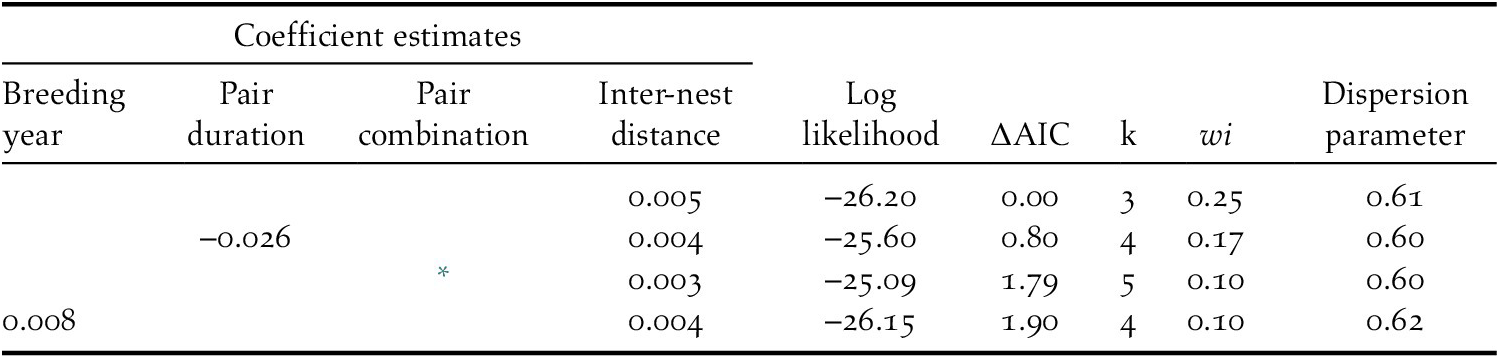
* indicates the coefficients of both released birds (‒0.194) and released and wild-born birds (0.103) compared with that of pairs with both wild-born birds.
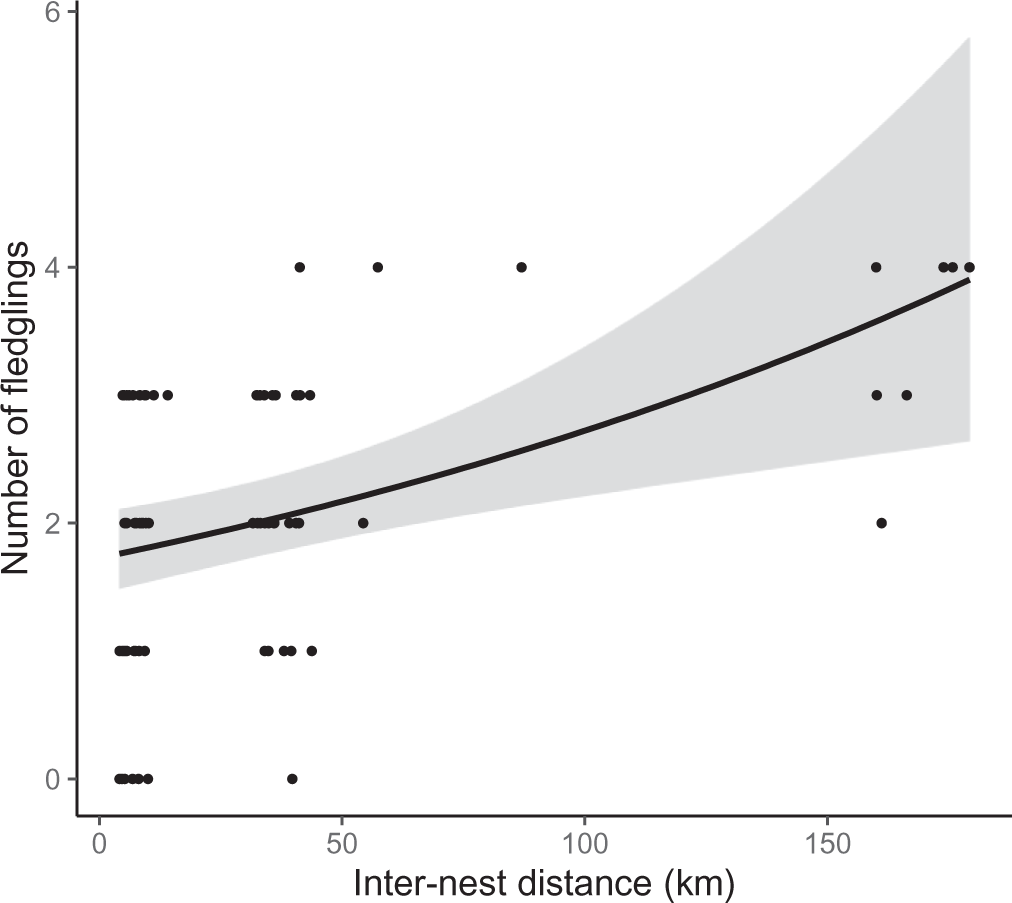
Figure 4. Relationship between inter-nest distance (mean straight-line distance between the nests) and number of fledglings in reintroduced Oriental Storks (n = 25 pairs) from 2005 to 2019. The regression line and 95% confidential interval (grey area) were obtained from the best model (Table 2).
Discussion
Published data on annual changes in demographic composition of reintroduced bird populations are rare, despite providing valuable information. Multiple successive generations are produced by acclimatization and genetic adaptation (Groombridge et al. Reference Groombridge, Raisin, Bristol, Richardson, Ewen, Armstrong, Parker and Seddon2012). This study was able to acquire such information as the reintroduced storks primarily consisted of identifiable individuals which have received major public attention in East Asia due to being regarded as a symbol of wetland biodiversity. In addition, reintroduced Oriental Storks live in areas close to human habitation, including the wild population in the Jewish Autonomous Oblast, Russia (Nikitina et al. Reference Nikitina, Tyagunin and Svetlakov2011) and Yellow River Delta, China (Cheng et al. Reference Cheng, Zhou, Wu and Feng2020).
A key measure of the success of a reintroduction project is the establishment of a self-growing population (Schaub et al. Reference Schaub, Zink, Beissmann, Sarrazin and Arlettaz2009). The establishment of a stable population and meta-population structure connecting other countries were the short- and long-term goals of the Oriental Stork reintroduction projects in Japan, respectively. The increase in population size and decrease in the proportion of released birds over 15 years indicated that the short-term goal of the projects was achieved. Some studies have evaluated next-generation recruitment in reintroduced bird populations based on F1 and F2 generations; however, few studies have reported recruitment based on additional generations. Li et al. (Reference Li, Ye, Wang, Li, Dong, Huo and Yu2018) indicated that the F1 and F2 generations of Crested Ibis Nipponia Nippon, which displays similar ages of first breeding and survival rates to our study species, occurred one and eight years after reintroduction initiation, respectively, through the release of a total of 56 captive birds in China. The approximate correspondence between the two studies is attributed to the similar release numbers and ecological characteristics. Further study of the relationship between the recruitment rate and genetic variation is needed to ensure population persistence, as inbreeding depression and the random loss of genetic diversity ultimately reduce long-term fitness (Jamieson Reference Jamieson2015). To preserve genetic diversity, it is essential to increase the number of migrants from the wild populations of Russia and China. The individual number of Oriental Storks breeding in the wintering and stopover sites of East China has increased (Cheng et al. Reference Cheng, Zhou, Wu and Feng2020). This trend might facilitate the increase in migrants to Japan.
In general, reintroduced individuals exhibit greater post-release dispersal than natal dispersal because they face unfamiliar habitats and risk losing their social groups (Richardson et al. Reference Richardson, Doerr, Ebragimi, Lovegrove, Parker, Armstrong, Hayward, Moro and Seddon2015). However, the trend was not observed in the reintroduced Oriental Storks. There are multiple potential reasons for this phenomenon. One reason may be attributed to the fact that the restoration of suitable foraging habitat is suggested to be important for nest site selection in Oriental Storks, as shallow open wetlands with rich aquatic life (Zhou et al. Reference Zhou, Xue, Zhu, Shan and Chen2013, Cheng et al. Reference Cheng, Zhou, Wu and Feng2020). During the 1970s, water connections between the rivers and floodplains or paddy fields in Japan rapidly disappeared due to flood control and agricultural modernization. However, since species reintroductions were initiated, the connections appear to have been restored by eliminating vertical steps and installing fishways that provision prey animals. During 2014–2015, the Ministry of Land, Infrastructure, Transport and Tourism installed 90 fishways that connected rivers and paddy fields in the 34-ha area of Toyooka, where the 22 pairs of Oriental Stork were bred (Minagawa et al. Reference Minagawa, Wakamiya, Takeshita, Sagawa, Kawaguchi, Murase, Tsuzuki, Fukazawa and Ezaki2020). In addition, stork-friendly farming methods (SFFM), which minimise agricultural dependency on chemical fertilisers and pesticides, have been adopted in many paddy fields that produce both rice and aquatic animals (Nishimura and Ezaki Reference Nishimura and Ezaki2019). SFFM-adopted paddy field areas have expanded more than tenfold in the Hyogo Prefecture over the past 15 years. Therefore, post-released storks are likely selecting breeding sites that are close to the release sites. In contrast, wild-born birds should have to disperse farther from their birthplaces to become breeders as the parent birds have exclusive territories (Ezaki and Ohsako Reference Ezaki and Ohsako2019). Another reason for the long dispersal of wild-born birds might be the replenishment of a suitable nesting site. Five new nest towers were installed during 2006–2012 but later, 1–2 nest towers were installed annually in the enlarged area of Figure 2.
Inter-nest distance was selected as the key factor determining the breeding success of the reintroduced Oriental Storks. Some studies have indicated that inter-nest distance and nest density affect the breeding success of White Storks because of intraspecific interference competition, including major fights against intruders (Barbraud et al. Reference Barbraud, Barbraud and Barbraud1999, Nowakowski Reference Nowakowski2003, Denac Reference Denac2006). The same phenomenon was often observed in the reintroduced Oriental Storks.
In conclusion, our Oriental Stork reintroduction projects were classified as partially successful because reintroduced birds survived and bred successfully but were still far from establishing a metapopulation (Ezaki et al. Reference Ezaki, Ohsako, Yamagishi and Soorae2013). Steady recruitment to the next generation is thought to be due to a moderate natal dispersal and the subsequent low breeding density of wild-born birds. However, issues unrelated to foraging habitat restoration are surfacing. One of these problems is the occurrence of collisions with power lines and agricultural materials (e.g. animal-proof nets) and future work to resolve these problems is required to maintain the long-term persistence of the reintroduced population.
Acknowledgements
We are grateful to many people who worked diligently on this project, with special thanks to K. Edo, M. Funakoshi, M. Horie, IPPM-OWS, M. Ito, R. Kuwabara, R. Matsumoto, K. Naito, M. Takagaki, S. Yamagishi, and T. Yoshizawa. We also thank Cao Lei, Phil Atkinson, and anonymous reviewers for their helpful comments. The procedures used in this study were approved by the Ministry of the Environment and the Agency for Cultural Affairs. Funding was provided by the Hyogo Prefecture, the Agency for Cultural Affairs, the Suntory Fund for Bird Conservation, and JSPS KAKENHI Grant Number JP21K06351.








