Introduction
There is a lack of available research on the breeding biology of South Pacific passerine species (Stirnemann et al. Reference Stirnemann, Potter, Butler and Minot2016). A review of worldwide variation in avian clutch size, found that data were available for only 17% of species in Oceania, compared to 57% elsewhere (Jetz et al. Reference Jetz, Sekercioglu and Böhning-Gaese2008). Basic information about the biology of these species is essential for our understanding of life-history variation, for estimating population growth rates and predicting the response of species to future environmental change (Hau Reference Hau2001, Jetz et al. Reference Jetz, Sekercioglu and Böhning-Gaese2008, Hau et al. Reference Hau, Ricklefs, Wikelski, Lee and Brawn2010). Island species are especially vulnerable to extinction, due to a combination of limited geographic range, small population sizes, and low resistance to introduced predators and pathogens (Steadman Reference Steadman2006). French Polynesia is an important biodiversity hotspot, hosting a number of endemic land birds, with 20 species being globally threatened with extinction, including six listed as ‘Critically Endangered’ (BirdLife International 2016a). It is essential to have a good understanding of the elements underpinning population dynamics for endangered species, so that conservation priorities, actions and strategies can be appropriately targeted or implemented (Ha et al. Reference Ha, Butler and Ha2010; Stirnemann et al. Reference Stirnemann, Potter, Butler and Minot2016).
The Tahiti Monarch or O’mamao Pomarea nigra Sparrmann 1786 is a ‘Critically Endangered’ species endemic to the island of Tahiti, French Polynesia (BirdLife International 2016a). In 1998, the known range of this year-round territorial land bird was restricted to four valleys containing isolated populations of 5–7 birds each, for a total of only 25 birds (Blanvillain et al. Reference Blanvillain, Salducci, Tutururai and Maeura2003). Another remote population of 33 birds was discovered in 2001 (Blanvillain et al. unpubl. data). This remote and inaccessible population occurred above several waterfalls higher up the Maruapo valley, and was only included in the recovery programme in 2009, by which time only 12 birds remained. The species recovery programme was initiated in 1998. At first, it focused on rodent control, because the main threat to the monarch, as for many other oceanic island birds (Atkinson Reference Atkinson and Moors1985, Wace Reference Wace1986), is predation by ship rats Rattus rattus (Robertson et al. Reference Robertson, Hay, Saul and McCormack1994; Thibault et al. Reference Thibault, Martin, Penloup and Meyer2002; Blanvillain et al. Reference Blanvillain, Salducci, Tutururai and Maeura2003). Two introduced bird species: the Common Myna Acridotheres tristis and Red-vented bulbul Pycnonotus cafer were identified as additional threats (Thibault et al. Reference Thibault, Martin, Penloup and Meyer2002, Blanvillain et al. Reference Blanvillain, Salducci, Tutururai and Maeura2003, Ghestemme Reference Ghestemme2011) as they preyed on nests and/or disturbed breeding pairs (Blanvillain et al. Reference Blanvillain, Salducci, Tutururai and Maeura2003). A review of the global impact of introduced birds was shown to be negative in only 10 out of 94 reports examined; seven of these concerned their impact on island birds (Baker et al. Reference Baker, Harvey and French2014). Consequently invasive bird control was addressed, with effective management following the inclusion of improved technical knowledge from 2012 (Saavedra et al. Reference Saavedra, Ghestemme and Blanvillain2012).
Since the beginning of the recovery programme, ornithologists have monitored nests in order to determine changes in breeding success that may have resulted from the conservation measures that were undertaken (Blanvillain et al. in prep). In the present article, we focused on the description of the Tahiti Monarch’s reproductive biology and present the main results and observations on the breeding biology, behaviour and the parental efforts of the Tahiti Monarch collected in 1998–2002 and 2008–2015. We compared our results with those of the Rarotonga Monarch Pomarea dimidiata (Saul et al. Reference Saul, Robertson and Tiraa1998), as information is available from only one other Pomarea monarch species in eastern Polynesia. We also discuss how slow life history strategies such as those of the Tahiti Monarch may considerably affect conservation management options. Further investigation may be necessary for the conservation of this species, as it belongs to an endemic genus close to extinction (Cibois et al. Reference Cibois, Thibault and Pasquet2004).
Methods
Study areas
Tahiti (17°38’S 149°30’W), French Polynesia, is a tropical volcanic island, the highest and largest of the 14 islands of the Society Islands group. It reaches 2,241 m elevation and covers 1,042 km2, with most of the rugged, basaltic interior being covered in forest. On both the mainland and the peninsula, the shape of the ancient volcanoes has given a relatively regular distribution to valleys and dividing ridges that radiate from the central peaks.
At the beginning of the recovery programme in 1998, Tahiti Monarchs were restricted to four valleys (Papehue, Orofero, Tiapa and Maruapo) located in Paea and Punaauia districts, in the south-west of the mainland (Figure 1). By 2010 the population in the Orofero valley had disappeared. These birds are territorial with territories of 1–2 ha depending on the habitat and population density (Pratt et al. Reference Pratt, Bruner and Berrett1987). All Tahiti Monarch territories were located between 80 and 400 m with adjacent hillsides 200–400 m high. The study area included four valleys (total length: 16 km) covering a total area of 1,451 ha (BirdLife International 2016b). However, the total area used as monarch territories across the three valleys where remnant populations still existed in 2015 came to a mere 50 ha (Figure 1).

Figure 1. The area of the monarch’s breeding valleys in Tahiti Island (above); and localities of Maruapo, Papehue and Tiapa valleys in Punaauia and Paea districts (below). Monarch territories in 2015 are represented by black spots (●). They covered 50 ha in 2015.
Bird census and nest monitoring
From 1998 to 2002, bird census and nest monitoring began in August or September and lasted for approximately six months (Blanvillain et al. Reference Blanvillain, Salducci, Tutururai and Maeura2003) when no more active nest was found. Valleys were visited at least once a week throughout this period. From 2008, we endeavoured to visit the territory at least once weekly during the peak of the breeding period, then monthly during the rest of the year. We aimed to find all nests and monitor their progress. Most of the observations were done by a single person with the aid of binoculars. Between 1998 and 2002 visits to each territory lasted at least one hour. Between 2008 and 2016, because the number of territories and pairs monitored increased, the time at each territory varied from 10 minutes to 2.3 hours, until a nest was found or the behaviour of the pair elucidated.
The weather was an important limiting factor for regular visits, especially in Maruapo and Tiapa valleys, which are dangerous to access during heavy rainfall. The rainy season in Tahiti (according to data from Punaauia District) usually takes place from December to March while the dry season is from June to October (Figure 2).
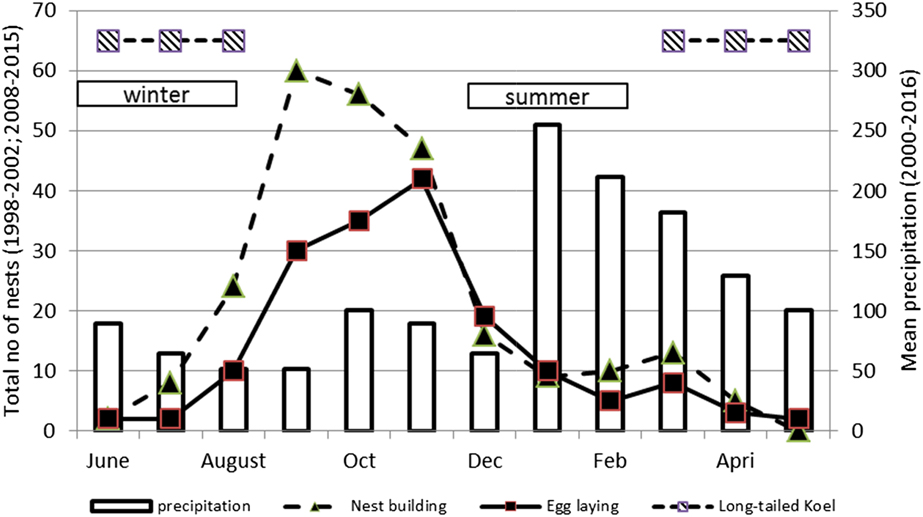
Figure 2. Rainfall and timing of ‘nest-building’ and ‘egg-laying’ for the Tahiti Monarch. The number of nests built and eggs laid per month during the first study period (1998–2001) and the second period (2008–2015) are combined; Timing of “winter” and “summer” and presence of the Long-tailed Koel Urodynamys taitensis on the island of Tahiti according to Holyoak and Thibault (Reference Holyoak and Thibault1984) are also shown.
The Tahiti Monarch has three distinct plumages: orange during the first and second year of their life, mixed during the third year as the black feathers progressively replace the orange ones (starting from the head), and fully black at four years old (Blanvillain et al. Reference Blanvillain, Salducci, Tutururai and Maeura2003). A monarch was considered a young adult if it had orange or mixed plumages and as an adult (> 4 years) if it had fully black plumage.
In 1998, five Tahiti Monarchs were captured and banded with individual colour-ring combinations. Between 2008 and 2015, a further 32 monarchs were captured, colour-marked and genetic sexing was conducted for 22 of them.
Although most Tahiti Monarchs were territorial, a small proportion was observed only transiently, probably because they didn’t succeed in defending a territory. These birds were considered as floater birds.
The Tahiti Monarchs nest in inaccessible tree-tops and our priority was to leave them undisturbed because of the critical situation of the species. To estimate the parental efforts, we focused only on indirect measures (Wright et al. Reference Wright, Both, Cotton and Bryant1998), such as activities during the nest-building and pre-incubation phases, nest occupation, and parental food provisioning rates.
The relative parental effort for both sexes was also considered. Sex was determined by both genetic sexing and mating behaviour. All DNA sexing analyses was performed by the laboratory LABOFARM (Loudéac, FRANCE). DNA was extracted from two or three feathers using the Adiapure Feather commercial kit (ADIAGENE, France), and sex chromosomes were then amplified using methods described by Griffiths et al. (Reference Griffiths, Double, Orr and Dawson1998). When the individual’s sex could not be identified during data collection, only the overall parental behaviours were considered. Therefore, we performed the analysis either by combining all data, or by considering each sex separately.
Five distinct phases were observed: (a) nest-building; (b) pre-incubation; (c) incubation; (d) nestling; and (e) fledging. The frequency of visits to the nest was used as a measure of feeding frequency during the nestling stages, and nest attendance time was recorded (Laiolo et al. (Reference Laiolo, Bignal and Patterson1998). The activities were compared between sexes and analysed in respect to days from hatching. Only the subset of nests that were monitored at regular intervals, were used to calculate the duration of each breeding phases (Table 1). The location of the nests used to establish duration and intervals are presented in Table 2.
Table 1. Duration of reproductive phases observed for the Tahiti Monarch. No. days ± SE of each phase are presented. For percentage, SE = square root (pq/n – 1); p = %; q= (1-p). For mean, SE = square root (variance/n). The Pre-incubation phase includes only nests that have a pre-incubation period.

Table 2. Location and number of nests or young used to establish the duration of the different reproductive phases in Tahiti Monarch.
All statistical analyses were carried out using R statistical software (R Development Core Team 2012). Pearson’s Chi-squared tests were used for comparison between qualitative variables and the Wilcoxon-Mann Whitney Test to establish comparison between quantitative variables because this non-parametric test was more accurate for test sample size < 30. We considered that male and female behaviours at the nest were independent of each other. For brooding time, all samples were < 25; the ‘exact’ confidence limit was calculated using Table of Clopper-Pearson.
Results
Between six and 22 pairs of Tahiti Monarchs were observed per year for the 12 seasons surveyed (a cumulated total of 146 pairs), although only 2–13 pairs (56%) produced hatchlings (a cumulated total of 82 pairs). Tahiti Monarchs are monogamous, with pair bonds of variable duration. Pairs are sharing nest-building, incubation and chick-rearing activities. Replacement nests (up to seven nest-building attempts in one breeding season) were common in case of nest failure. After a first successful fledging, only four adult pairs produced a second brood, which only occurred several months later. As these pairs repeated those efforts year after year, they represent 10 of the 82 pairs able to produce hatchlings (12%) during the study period.
Mating
Mating occurs mainly during the pre-incubation phase but was observed over the entire reproductive phase and also observed to occur without any nest-building activity. After a mutual pursuit the Tahiti Monarchs mated either on a branch near the completed nest, up to 15 m away from the nest, or sometimes on the ground. Courtship feeding was observed, but the males often ate the prey themselves. Several beak to beak contacts were also observed between the sexes. Courtship displays involved the male flapping his wings near the female, in the same position as the mounting posture, but with the head slightly more upright and calling. Several short vocalisations were associated with courting, which were different from territorial calls.
Nest-building
Tahiti Monarch nests tend to be constructed in a fork of branches, usually made of three twigs, not far from an umbrella of leaves, which protects the nests from rain and sunlight. Their nests are open cups (about 9 cm across and 4 cm deep; 6 cm across and 3 cm deep for internal cup measurements) built mostly with moss. The nest cup is lined with small fronds of fine ferns (Adiantum trapeziforme or A. raddianum). The nest contains a ‘paste’ composed of very small wood and leaf fragments, which seem to have been regurgitated by the birds. White silk from spiders’ webs is often scattered over the outer surface, sometimes small leaves, helping to camouflage the nests and improving its cohesion are added. Lichens are also used.
Between 1998 and 2002, the location and position of the nests in the trees were recorded systematically. A total of 66 nests were built by 19 different pairs in 41 trees during this period, with 40 nests (62%) built in 22 native mara Neonauclea forsteri) and 23 (35%) in 21 specimens of the invasive African tulip-tree Spathodea campanulata. These two trees were the dominant canopy species during the study period. Occasionally, pairs re-nested in the same tree (up to four times for mara, three times for tulip-tree), but only specific mara trees attracted the same or different pair(s) for several consecutive years. Nests were generally located close to the middle of the territory; 77% of nests were in a tree overhanging a stream, the trunk being located less than 3 m from the stream. The height of the nests above the ground, estimated visually by the same observer in order to reduce bias, averaged 13.0 ± 0.7 m (range 5–21 m) and 2.5 m under the top of the tree canopy. The trees used were medium to tall trees (11–30 metres high; 35–224 cm circumference at 1 m high for mara; 6–25 m high; 48–117 cm circumference at 1m high for tulip tree).
The other species of tree used during both periods (between 1998 and 2002, and then between 2008 and 2015) were, by decreasing importance: Aleurites molucaana, Rhus taitensis, Hibiscus tiliaceus and Claoxylon taitense. Individual trees of both Aleurites and Rhus were also used by the same pair on several occasions between 2008 and 2015.
Nest building took between four and 22 days with a mean of 11.2 ± 1.3 days (Table 1; n =15 nests). Both sexes participated in nest-building (4.6 ± 0.9 trips/hour for males vs. 5.0 ± 1.1 for females; 19 hours of observations; ns, Wilcoxon rank- ann Whitney Test). Some 7.5% of nests, were built on a pre-existing nest (either recently built or built the previous year). The shortest duration for nest-building (four days) was in an old nest that was re-built, the longest one (22 days) corresponding to a behaviour very similar to a pre-incubation phase (see below), although material continued to be brought to the nest on a regular basis. Another pair spent 28 days building a nest prior to abandoning it.
Pre-incubation phase and nests abandoned
During the eight breeding seasons of the study period, 238 nests were discovered. Of these, 38 nests were already abandoned: 19 nests between 1998 and 2001 that were mostly built before the start of the survey period and 19 nests between 2008 and 2015 at which no birds were observed to visit. Thus only 200 nests were monitored (Figure 3).
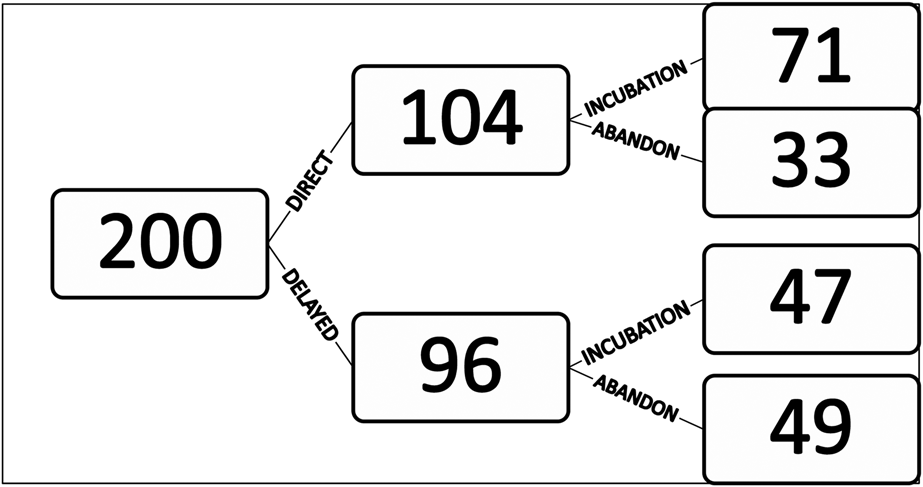
Figure 3. Total number of nests monitored in 1998–2002 and 2008–2014 (n=200) and fate of those nests, including direct or delayed incubation and abandonment.
Observations of behaviour at the nest indicated that 33 nests (16%) were abandoned soon after their construction and for 71 nests (34%), an egg was laid immediately. A further 96 nests (46%) showed a pre-incubation phase of 18.9 ± 1.9 days (range 3–62 days; n = 47 nests) during which birds just visited the nest cup irregularly (Table 1). Of these nests, 49 (51%) were eventually abandoned. An egg was eventually laid and incubation commenced in the remaining 47 nests.
The pre-incubation phase was longer in nests which were finally abandoned than for nests that eventually held a breeding attempt: 23.4 ± 3.0 days (ranges 9–62 days, n = 20 nests) versus 15.5 ± 2.2 days (ranges 3–48 days; n = 29 nests), P = 0.01; Wilcoxon rank-Mann Whitney Test.
Bird behaviour during the pre-incubation phase was variable with some nests being only irregularly and briefly visited in the time before an egg was laid or the nest abandoned. In 46 of the 96 nests which showed a pre-incubation phase, transient incubation-like behaviours were observed before the nest was either abandoned (22 nests) or egg-laying occurred (24 nests). This behaviour involved birds remaining in the nest for longer periods than a simple nest visit.
Incubation-like behaviour was mostly very easy to establish. It occurred when the nest was ‘incubated’, then left unattended regularly or for too long (over 20 consecutive minutes), or when the ‘incubation sessions’ included a succession of short nest attendance periods. It was sometimes difficult to differentiate between incubation-like behaviour and incubation. In cases of doubt, we considered it was incubation.
Influence of the age of the bird or delayed reproduction on the fate of the nest
There is no apparent benefit of the pre-incubation phase on the subsequent incubation phase. An egg was laid in 49% of nests with a pre-incubation phase but 68% of nests where a pre-incubation phase was not observed (Figure 3; Pearson’s Chi-squared test; χ2 = 7.69; df = 1, P < 0.01).
The age of pair had a significant effect on the outcome of nest: 23% of nests built by adult pairs were abandoned without any egg being laid, compared with 62% of nests built by pairs that included one or more young adults (Table 3; χ2 = 30.4, df = 1, P < 0.001). Age of bird did not influence the number of nests that experienced a pre-incubation phase (Pearson’s Chi-squared test; df = 1, χ2 = 1.31; ns).
Table 3. Number of nests where incubation or nest abandonment was observed in pairs of adult birds or in pairs that included one or more young adults (YA).

This effect of age on breeding parameters was similar in nests with and without pre-incubation phases: where there was no pre-incubation phase 16% of nests built by adult pairs were abandoned compared to 56% of nests built by pairs with one or more young adults; (χ2 = 16.7, df = 1, P < 0.001). In nests with a pre-incubation phase: 34% of nests built by adult pairs were abandoned compared to 67 % built by pairs with one or more young adult (χ2 = 10.6, df = 1, P < 0.002).
Incubation and egg-laying phase
Nest-building occurred throughout the year but increased between August and December with a peak in September (Figure 2). Egg-laying also occurred year-round but usually increased between August and January and peaked around November, two months after the peak of nest-building (Figure 2). Replacement clutches of failed pairs and second clutches from successful pairs explains the small peak observed in March. A few clutches were laid between April and July.
The incubation phase was 13.6 ± 0.3 days with a range of 13–15 days (Table 1, n = 7 nests). A total of 65 hours of observations were collected on 28 different nests during the incubation phase in order to determine parent’s behaviour: 40 hours on pairs of undetermined birds and 25 hours on sexed pairs. During daytime, birds visited the nest at a rate of 3.8 ± 0.1 times/hour and percentage time at nest was 97 ± 2%. Both sexes incubated eggs. During this phase females spent 56 ± 10% of observation time incubating and male 39 ± 10% (P = 0.005, Wilcoxon-rank-Mann Whitney test). On the three instances when birds were successfully monitored until dusk, the last bird on the nest at dusk was always female. Tahiti Monarchs most likely lay one egg per clutch (see above). Egg shells found on the ground were white with small purple spots.
Nestling phase
The 118 nests incubated resulted in the hatching of 109 chicks. At the nestling phase, only one chick was seen per nest, and only one fledged young was produced per nest (n = 94). The nestling phase lasted 15.5 ± 0.7 days with a range of 13–20 days (Table 1, n = 11 nests). A total of 76 hours of observations were collected on 27 different nests during the nestling phase: 44 hours on pairs of undetermined birds; 32 hours on sexed pairs. During this phase, birds visited the nest at a rate of 10.3 ± 0.2 trips per hour. The nest attendance (brooding) time remained over 50% until five days after hatching, but dropped to 10% by day 10 after hatching (Figure 4). Both parents fed the nestling, but after the first week after the egg hatched, the female spent about twice as much time brooding compared with the male (65% of time vs 35% of time; Qobs = 240.5; P = 0.01, Wilcoxon-rank-Mann Whitney test).
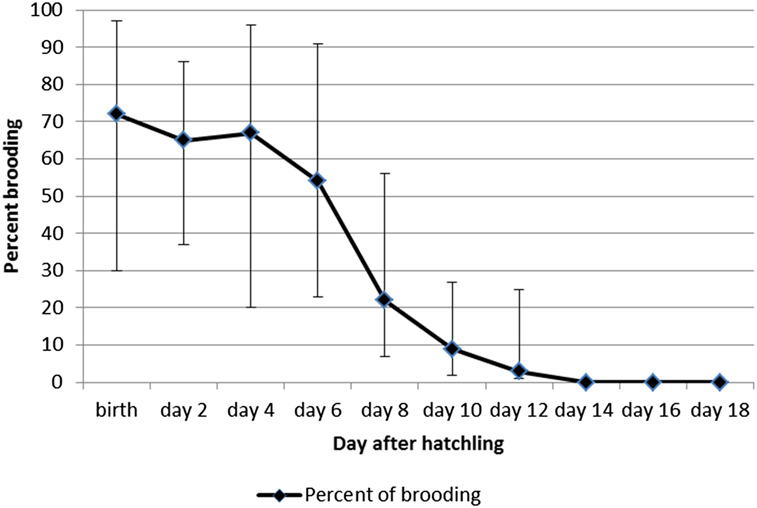
Figure 4. Percentage (%) of time spent in nest attendance (brooding) after hatching for the Tahiti Monarch. Exact confidence limits are shown as vertical bars.
Fledging phase
Parents feed the young for 74 ± 5 days after leaving the nest (range 42–174 days, Table 1; n = 48 young). Independence was sometimes difficult to establish as some young remained in their parents’ territory for several months. They were able to hunt by themselves, but were still fed by adults quite regularly. Conversely some fledglings were fully independent at 42 days post-fledging. Dispersal of juveniles was often local at first, although other joined the floater population. They remained alone or paired with older birds usually displaying orange or mixed plumages.
Discussion
This study is the first on the breeding biology of the Tahiti Monarch. It has provided results which will have implications for the future conservation of the species. Although Tahiti Monarchs show some seasonality, with most eggs being laid between August and January, they are able to breed all year round and their breeding season is more protracted than the Rarotonga Monarch, which lives slightly further from the equator at (21014’S) on Rarotonga, Cook Islands (Saul et al. Reference Saul, Robertson and Tiraa1998). In contrast to temperate birds, little is known regarding the environmental cues that stimulate reproductive activity for tropical birds (Hau Reference Hau2001). It might be expected that the reduced variation in photoperiod near the equator would result in a longer breeding season, or that the breeding season is governed by other cues (Curry and Grant Reference Curry and Grant1989). Spotted Antbirds Hylophylax naevioides can measure slight photoperiodic increases and use it to initiate reproductive activity, but they also respond to seasonal changes in food availability (Hau Reference Hau2001). In contrast, Small Ground-finches Geospiza fuliginosa are opportunistic breeders: they do not anticipate the breeding season but depend strongly on rainfall (Wingfield et al. Reference Wingfield, Hahn, Levin and Honey1992, Hau Reference Hau2001). Most Tahiti Monarch reproductive efforts coincided with increasing day-length, but some occurred during decreasing day-lengths. The Tahiti Monarch’s egg-laying peaked just after the start of the rainy season in October and November but before the heavier precipitation that begins in January. Several other tropical monarch flycatchers have been observed to breed mostly during the late dry season to the early part of the wet season (Coates et al. Reference Coates, Gregory, Moeliker, del Hoyo, Elliott and Christie2006). This may be because rain increases insect food abundance (Grant and Grant Reference Grant and Grant1989, Leigh et al. Reference Leigh, Rand and Windsor1996).
There are several similarities in the reproductive features of the Tahiti and Rarotonga Monarchs. Both sexes share the burden of nest building, incubation and brooding although females invest more time in incubation and brooding than males (Saul et al. Reference Saul, Robertson and Tiraa1998). This suggests that in both species, females may be more susceptible than males to predation at the nest. It is possible that a sex ratio bias was present at the start of the Tahiti Monarch recovery programme, which may have slowed down the initial rate of recovery of the species. A sex ratio bias was also reported for the Rarotonga Monarch (Robertson et al. Reference Robertson, Hay, Saul and McCormack1994). As with the Rarotonga Monarch, nest attendance time decreased with increasing nestling age. This probably reflects the increase of the chick thermoregulatory abilities (Royama Reference Royama1966) as well as the need for increased volumes of food as the chick grows, but brooding could also represent a defence against nest predators.
Some differences also exist between those two species. The Tahiti Monarch has a lower clutch size (one nestling versus the two observed for some of the Rarotonga Monarch), slightly longer incubation (14 versus 13 days for the Rarotonga Monarch) and brooding length (13–20 days versus 11–15 days for the Rarotonga Monarch). Adult Rarotonga Monarchs are slightly smaller than Tahiti Monarchs: 23.5 ± 1.2 g and 22.7 ± 1.1 g for adult male and female Rarotonga Monarch respectively (Robertson et al. Reference Robertson, Hay and Saul1993) versus 24.8 ± 1.2 g and 24.5 ± 1.9 g for adults male and female Tahiti Monarch captured during the present study (n = 4 and 7 birds respectively). However, both are tropical island birds and close relatives and this difference in size cannot explain differences in incubation and nestling lengths. We can conclude that the Tahiti Monarch has a lower reproductive potential than the Rarotonga Monarch. As resources in an island ecosystem are likely to be in limited supply (Abbot Reference Abbott1980) and land bird density tend to be higher on islands due to lower nest predation (George Reference George1987), lack of space and the inability to emigrate, a lower productivity strategy in island taxa may decrease the chance of intraspecific competition (Hasegawa Reference Hasegawa1997, Buckley and Jetz Reference Buckley and Jetz2007). For conservationists, this lower productivity suggests that the recovery of the Tahiti Monarch will take longer than for the Rarotonga Monarch.
For other monarch flycatchers (Monarchidae), the incubation phase ranges from 12 to 18 days, but is mostly 14–16 days, and the nestling phase ranges from seven to 20 days but is mostly 12–16 days. Parents continue to feed young for 28–42 days, or more, after the young have left the nest (Coates et al. Reference Coates, Gregory, Moeliker, del Hoyo, Elliott and Christie2006). The Tahiti Monarch, with one of the longest nestling phases (13–20 days) and a mean of 74 days (range 42–174) feeding the young after fledging, invests a lot in the survival of their single offspring. Another island bird, the Mao Gymnomyza samoensis displays extended parental care with both long incubation and nestling phases, and a fledging phase 30–47% longer than that for any other honeyeater species (Stirnemann et al. Reference Stirnemann, Potter, Butler and Minot2016). The authors hypothesised that this slow development rate may be a common feature of tropical island species that have evolved without mammalian predators. Extended parental care in the fledging phase may increase the survival of juveniles, and thus counteract the effects of smaller clutches (Russell Reference Russell2000).
Mortality of offspring was also significant for the evolution of clutch size (Jetz et al. Reference Jetz, Sekercioglu and Böhning-Gaese2008). In the case of the Tahiti Monarch population, this could reflect a higher adaptation process to predator-free tropical islands than that of the Rarotonga Monarch. The impact of Long-tailed Koel, Urodynamys taitensis may have been lower in Tahiti Monarch, as Tahiti is further away from the koel’s breeding range in New Zealand than is Rarotonga. The koel is the only indigenous nest predator present in both islands (Pratt et al. Reference Pratt, Bruner and Berrett1987). Figure 2 shows how the presence of this potential predator coincides with lower reproductive efforts of the Tahiti Monarch.
Alternatively, what we have observed could be a reflection of a short-term adaptation process, due to a disturbed environment rather than a natural feature of the island bird’s strategy. Less food availability in Tahiti could be the reason why it takes longer for Tahiti Monarch to raise its chick. It could be because of the high rate of introduced plants species present in its habitats (Butaud and Jacq unpubl. report). The Red-vented Bulbul and Common Myna, are both omnivorous and opportunistic introduced species that may also have an impact on the food availability in Tahiti Monarch territories. This suggests that habitat restoration and control of introduced birds may have an impact on its reproductive parameters.
One surprising aspect of Tahiti Monarch reproductive pattern, which has never been reported with the Rarotonga Monarch, was the pre-incubation phase and the incubation-like behaviour observed in some nests. The occupation of empty nests, which in the case of the Tahiti Monarch we presume to be an extended pre-incubation phase, is a widespread but a poorly understood phenomenon. It has been reported in Great Tit Parus major (Dhondt and Eyckerman Reference Dhondt and Eyckerman1978, Berndt and Winkel Reference Berndt and Winkel1979, Ojanen and Orell Reference Ojanen and Orell1981, Carlsson et al. Reference Carlsson, Carlsson, Wallin and Wallin1991, Winkel Reference Winkel1993), European Pied Flycatcher Ficedula hypoleuca, Eurasian Blue Tit Cyanistes caeruleus, Coal Tit Periparus ater, Eurasian Nuthatch Sitta europaea (Järvinen and Väisanen Reference Järvinen and Väisanen1984, Winkel and Hudde Reference Winkel and Hudde1990), and Eurasian Blackbird Turdus merula (Schilde Reference Schilde1986). It has also been recorded in a number of non-passerines (Owen Reference Owen1940, Poulsen Reference Poulsen1953, Gruber Reference Gruber1978, Gudmundsson Reference Gudmundsson1983, Ludwig Reference Ludwig2006). For all these species, this phenomenon is seldom observed, and appears to occur at a much lower rate than we have observed in Tahiti Monarch (0.1% of nests instead of 22.5%). This behaviour also seems frequent in the Fatu Hiva Monarch Pomarea withneyi, which also lays only one egg (authors’ pers. obs.). It is usually assumed that birds sitting on empty nests are unable to lay eggs. A prolonged egg-laying phase and mimicking incubation could be performed by a pair waiting for the cue that determines the start of gonad development in one of the partners. It could be a way to motivate or stimulate the weak partner or a strategy to improve pair-bonding while waiting for the egg-laying cue to occur. In the case of replacement nests, it could also be a necessary period for partners to recover enough fitness to breed. In Tahiti Monarch, the age of the partners seems very important in determining if the nest- building stage is followed by egg-laying, or if the nest is abandoned. Then again, age did not influence the number of nests that experienced a pre-incubation phase. Perhaps this species has learned (or has been naturally selected) to pretend to be nesting to test if the nest site is safe or dry. The Tahiti Monarch’s egg-laying period peaked just after the start of the rainy season.
One possible explanation of this pre-incubation behaviour could be early nest failure due to predation at the incubation stage. However, this would imply that, on 24 occasions, the pairs used the same nest for laying a second clutch despite predation. This explanation would be more difficult to accept than incubation-like behaviour.
Another explanation could be the incubation of infertile eggs. An infertile egg may be removed from the nest and a new clutch initiated, safe in the knowledge that the nest site itself was secure, dry and predator-safe. It is important to collect more data on this behaviour by direct nest observations, to understand its significance, and also to determine if it is possible to reduce the frequency of occurrence, the length of the period, or the relatively high likelihood of abandonment compared with egg laying at the end of the period.
More information is needed on the genetic diversity of the isolated populations of the species, the possible inbreeding effects from this population bottleneck and how this might affect the breeding biology of the species.
As expected from life-history theory (Ricklefs Reference Ricklefs1980, Kulesza Reference Kulesza1990, Stearns Reference Stearns1992), the Tahiti Monarch shows the pattern typical of tropical passerines in having a low reproductive rate with a small clutch and usually only one brood each year. The Tahiti Monarch has low productivity because 1) only 56% or pairs were breeding pairs in any one year, able to lay an egg and produce a chick, 2) only one brood per nest, and for 88% of breeding pairs, only one chick per year was produced and 3) the considerable length of the nesting and fledging phases.
The Tahiti Monarch also has many life history characteristics resembling other tropical island birds that make it vulnerable to introduced predators. These traits include low maximum reproductive capacity and extended development times (Stirnemann et al. Reference Stirnemann, Potter, Butler and Minot2016).
Proposed conservation strategies
With only 53 adults and not more than 13 breeding pairs remaining in 2015, the Tahiti Monarch is still one of the most endangered bird species in the world. This study suggests the importance of taking into account the life history attributes of a species when determining management strategies. Recovery potential for species with high productivity (multiple eggs and clutches) is much higher than that for species with low maximum productivity (single eggs and clutches per season) (Sæther and Bakke Reference Sæther and Bakke2000). The lower reproductive potential indicates why, given the same conservation actions, the recovery rate of the Tahiti Monarch is likely to be slower than that of even the Rarotonga Monarch (Robertson and Saul Reference Robertson and Saul2007). Clearly, a long-term concerted recovery effort will be required to enable the species to survive.
Species with life history traits that include low maximum annual reproduction often have a longer lifespan than other birds (Ashmole Reference Ashmole1963, Gadgil and Bossert Reference Gadgil and Bossert1970). This was confirmed in Tahiti Monarch with one banded male reaching approximately 23 years of age before disappearing (authors’ pers. obs.). It can be difficult to detect population declines in these species, as high adult survival can mask failures at the breeding stage for many years. Conservationists must be aware of this bias and would, ideally, monitor both nest success and recruitment into the breeding population in order to establish the actual success of their efforts.
The Tahiti Monarch breeds all year round so predator control needs to be maintained throughout the year. As both rats and introduced birds have an impact on reproductive success (Thibault et al. Reference Thibault, Martin, Penloup and Meyer2002, Blanvillain et al. Reference Blanvillain, Salducci, Tutururai and Maeura2003) and the long nesting period is likely to increase nest predation risk, the continued control of both introduced species is the most effective way to save the Tahiti Monarch from extinction. The size of Tahiti Island means that predator control will be more realistic to implement than eradication. While the use of rodent control is well documented (Robertson et al. Reference Robertson, Hay, Saul and McCormack1994), the control of introduced birds to save a species is a new aspect of conservation strategies in the Pacific. There have been several successful eradication programmes of introduced birds (Keitt et al. 2011). In Tahiti, the control methods used are similar to eradication methods: a combination of poisoning, shooting and trapping (Keitt et al. Reference Keitt, Campbell, Saunders, Clout, Wang, Heinz, Newton, Tershy, Veitch, Clout and Towns2011). Furthermore, the local population is involved as a way of obtaining sustainable results in a long-term conservation strategy (Saavedra et al. 2002, Blanvillain et al. in prep.) following the model of the Canberra Indian Myna Action Group Inc., CIMAGI in Australia (http://indianmynaaction.org.au/, verified March 2017).
Conclusion
Due to its low productivity, the critical situation of the Tahiti Monarch and its longevity that may mask negative population trends, maximising its reproductive success is likely to be essential in securing its recovery. This may also be the case for several other endangered birds in the Pacific. The monarch’s low productivity increases the length of time needed for recovery to occur but species with low productivity typically offset this by having high annual adult survival and therefore many years in which to attempt to breed.
Further research into the impact of habitat restoration (through the removal of invasive plant species and reforestation with native species) or the continued control of introduced birds on food availability, and the length of nestling and fledging phases would be of great interest. Further studies of southern tropical island birds could help establish whether the Tahiti Monarch’s reproductive strategy is extreme and explain why this species, and genus, is so susceptible to the introduction of introduced mammals and birds. Studies on this and a range of related tropical Pacific species may help to determine the evolutionary mechanisms behind the development of slow reproductive traits in avifauna as well as clarify potential conservation strategies.
Acknowledgements
The recovery of the Tahiti Monarch was funded by the Délégation à l’Environnement (DIREN) of French Polynesia, the European Union (programmes BEST & BEST 2.0.), BirdLife International, the French minister of environment, the Critical Ecosystem Partnership Fund (CEPF), Pacific Conservation and Development Trust, Secretariat of the Pacific Regional Environment Program, ‘Club 300’ foundation for bird protection, CEPA (Conservation des Espèces et des Populations Animales), ZGAP (Zoologische Gesellschaft für Arten und Populationschutz), the Ligue pour la Protection des Oiseaux (France), the foundation ‘Nature et Découverte’, Paea and Punaauia districts and three local enterprises: EDT, OPT and Vini. We also thank the SOP members, owners of the valleys and inhabitants of Paea and Punaauia district who participated in the fieldwork, the ‘Service de l’Aménagement et de l’Urbanisme’ of French Polynesia for the physical map. Thanks to David Beaune, Mike Britton, Jacques Boubee, Nirmala Withers and the three anonymous referees for critical reading and improvement of this manuscript.









