Introduction
Given the current rate of worldwide habitat and biodiversity loss (Baillie et al. Reference Baillie, Hilton-Taylor and Stuart2004, Hails Reference Hails2008) and the associated rise in the need for population status information, rapid population assessment techniques are becoming increasingly important. Declining populations in vulnerable ecosystems are especially in need of strategic monitoring to make informed decisions about conservation actions (Sinclair et al. Reference Sinclair, Fryxell, Caughley, Sinclair and Fryxell2009). Non-invasive survey techniques using reliable signals are a preferred option for population assessment in threatened species (e.g. Karanth and Nichols Reference Karanth, Nichols, Tilson and Nyhus2010), whereby species need to be distinguishable. This can be near impossible among some closely related species or in systems with cryptic species and/or hybrid individuals (Dawson and Efford Reference Dawson and Efford2009, Lambert and Mcdonald Reference Lambert and Mcdonald2014). In such cases, individuals can often only be identified to species using genetic analysis, which requires sampling and sequencing and hence are costly and time-consuming procedures (Hebert et al. Reference Hebert, Penton, Burns, Janzen and Hallwachs2004). Therefore, it is highly desirable to identify traits by which individuals of a species can be clearly and efficiently classified in the field.
Animal vocalisations are widely used to estimate their abundance given the benefit of sound travelling across water and vegetation with little attenuation (Marten and Marler Reference Marten and Marler1977). For this reason, both the presence and distance of an animal can be scored by an observer from afar (Scott et al. Reference Scott, Ramsey and Kepler1981). Animal vocalisations have been used in ecological surveys assessing species abundance across taxa including amphibians (e.g. Driscoll Reference Driscoll1998), cetaceans (e.g. Marques et al. Reference Marques, Thomas, Ward, DiMarzio and Tyack2009) and birds (e.g. Dawson and Efford Reference Dawson and Efford2009, O’Connor et al. Reference O’Connor, Sulloway and Kleindorfer2010c, Dvorak et al. Reference Dvorak, Vargas, Fessl and Tebbich2012). Sometimes two closely related species may be morphologically similar but produce different vocalisations (Toews and Irwin Reference Toews and Irwin2008), but at other times vocal differences may be insufficient to justify their use as a species indicator. Given that 10–16% of bird species regularly hybridise (Grant and Grant Reference Grant and Grant1992, Ottenburghs et al. Reference Ottenburghs, Ydenberg, Van Hooft, Van Wieren and Prins2015), hybridisation is an additional factor that increases challenges associated with acoustical monitoring.
Darwin’s Finches are a model system for evolutionary biology with evidence for evolution and speciation by natural selection in the wild (e.g. Grant and Grant Reference Grant and Grant2014a), but also for species and population decline due to anthropogenic impacts (O’Connor et al. Reference O’Connor, Sulloway and Kleindorfer2010c, Dvorak et al. Reference Dvorak, Fessl, Nemeth, Kleindorfer and Tebbich2012). Human activity on the Galápagos has resulted in introduced species and pathogens (Parker et al. Reference Parker, Buckles, Farrington, Petren, Whiteman, Ricklefs, Bollmer and Jimenez-Uzcategui2011) as well as habitat loss from increasing human population and agricultural activity (Watson et al. Reference Watson, Trueman, Tufet, Henderson and Atkinson2010). There is consensus about the importance of surveys to monitor endemic populations (Dvorak et al. Reference Dvorak, Vargas, Fessl and Tebbich2004, Reference Dvorak, Fessl, Nemeth, Kleindorfer and Tebbich2012, O’Connor et al. Reference O’Connor, Sulloway and Kleindorfer2010c), but Darwin’s Finches are difficult to identify at the best of times given shifts in morphology from interspecific competition (Schluter et al. Reference Schluter, Price and Grant1985) and rapid evolution (Grant and Grant Reference Grant and Grant2014). Darwin’s Finches also regularly hybridise (McKay and Zink Reference McKay and Zink2014), as has been shown in ground finches Geospiza spp. (Grant and Grant Reference Grant and Grant1997, Grant et al. Reference Grant, Grant, Keller, Markert and Petren2003, Reference Grant, Grant and Petren2005a) and tree finches Camarhynchus spp. (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a). Identifying changes in gene flow between Darwin’s finch species and populations is both challenging and necessary to inform our understanding of evolutionary dynamics in this rapidly evolving system.
This study assessed the performance of acoustic survey techniques in a species group with hybridisation. We analyse song in Small Tree Finch C. parvulus, Medium Tree Finch C. pauper, and the recently discovered Camarhynchus hybrid group (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a) on Floreana Island, Galápagos Archipelago. While the Small Tree Finch is listed as ‘common’ and occurs on other islands, the Medium Tree Finch is endemic to Floreana and listed as ‘Critically Endangered’ (IUCN 2015). Morphological differences between these two parental species are slight (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a) which renders visual identification in the field inaccurate. Avian surveys that rely on song activity in the field are now being used in land surveys across the Galápagos Islands (Cunninghame et al. Reference Cunninghame, Ortiz-Catedral and Fessl2012). To inform Galápagos land bird surveys, we need to establish that song can be used to distinguish the three tree finch groups on Floreana Island. This study compares song characteristics between Camarhynchus tree finches on Floreana Island to test if species and the hybrid birds differ in song. Using these insights, we apply established techniques to assess changes in avian abundance from 2004 compared with 2008 and 2013. In addition to comparing population trends in Darwin’s Finches, we analyse population trends in other bird species in our survey of the Scalesia forest, which is important habitat for tree finches. The findings across species creates a larger context for considering patterns in the tree finches.
Material and methods
Study site
The study site is situated at the base of the Cerro Pajas volcano on Floreana Island, Galápagos (173 km2, 1°28’S, 90°48’W) and consists of humid highland forest dominated by the endemic tree Scalesia pedunculata. By 2010, 38% of the Scalesia habitat had been degraded through clearing for human settlement and agriculture (Watson et al. Reference Watson, Trueman, Tufet, Henderson and Atkinson2010). Our long-term study of Darwin’s finches reveals growing threats from introduced plants, including creeping vines, which could destabilise the system and require careful monitoring. Despite these challenges, this remnant Scalesia forest on Floreana Island is the largest on the Galápagos Islands; it has virtually disappeared from San Cristobal and Isabela Islands and only 1–2% remains on Santa Cruz Island (Watson et al. Reference Watson, Trueman, Tufet, Henderson and Atkinson2010, Dvorak et al. Reference Dvorak, Fessl, Nemeth, Kleindorfer and Tebbich2012). The Scalesia forest appears to be the preferred nesting habitat for tree finches (Peters and Kleindorfer Reference Peters and Kleindorfer2015, Kleindorfer et al. Reference Kleindorfer, Peters, Hohl, Sulloway, Weis and Sol2016), though tree finches have been observed to sing from tall (> 10 m) non-Scalesia trees both within and adjacent to Scalesia habitat, and have been observed in the agricultural zones adjacent to the Scalesia forest (O’Connor et al. Reference O’Connor, Robertson and Kleindorfer2010b, O’Connor unpubl. data).
Study species
We analysed song characteristics of male Small Tree Finch C. parvulus, Medium Tree Finch C. pauper, and birds of the recently identified hybrid group, which are the result of pairings between C. parvulus and C. pauper (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a). We aimed to record a comparable number of songs for each of the three genetic groups (for details on genetic analyses see the extended methods in the online supplementary material); this balanced sampling was not achieved because we could only determine genetic assignment after data analysis and after song recordings had been made in the field. In total, we analysed morphology and song recordings from nine C. parvulus, 19 C. pauper, and 49 hybrid birds. While C. parvulus exists on several other Galápagos Islands, the Critically Endangered C. pauper only occurs on Floreana Island (Lack Reference Lack1983, Grant Reference Grant1986).
Comparing song between tree finches
We recorded the song of birds that had previously been colour-banded, measured and that were later assigned to a genetic group using analysis of nine microsatellite loci following Kleindorfer et al. (Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a) and Peters (Reference Peters2016). Darwin’s finches do not appear to change their behaviour in the presence of human observers, and we were able to record song at close range (< 10 m) using either a Sony DCD-100 DAT recorder or a Sony WMD6 Cassette Recorder with Sennheiser ME 80 directional microphone in 2006, and a Marantz solid state recorder (model PMD661MKII) with either a Telinga Twin Science parabolic microphone or a Røde Precision broadcast-grade long shotgun microphone (model NTG8) from 2010 onwards. Recordings were made in the years 2006, 2010, 2013 and 2014 during the start of the breeding season between 07h00 and 10h00, which is the time of peak singing activity (Christensen et al. Reference Christensen, Kleindorfer and Robertson2006). Like the song of other Darwin’s finch species, tree finch song is simply structured and consists of one repeated syllable constituting a song (Bowman et al. Reference Bowman, Berson and Leviton1983). We recorded up to 15 repetitions of the song of each individual bird. Since the song of tree finches is highly repeatable within individuals (Christensen et al. Reference Christensen, Kleindorfer and Robertson2006), we selected the five best quality recordings per bird and used the mean of each song parameter for subsequent analysis with Raven Pro Version 1.4 for Mac OS X (http://www.birds.cornell.edu/raven). We measured and analysed the following song parameters: song duration (s), minimum frequency (Hz), maximum frequency (Hz), frequency bandwidth (Hz; calculated by subtracting the minimum frequency from the maximum frequency), dominant frequency, number of syllables, maximum number of syllables per song and trill rate (number of syllables/s). Spectrograms were created using a -24dB cut-off criterion relative to the peak power of the vocalisation with visual adjustment, following Podos (Reference Podos2001) and Goodale and Podos (Reference Goodale and Podos2010).
Morphological analysis
Birds of the hybrid group have been reported to have intermediate body size between the smaller-bodied C. parvulus and the larger-bodied C. pauper, but there is much overlap in morphology (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a). We examined the following morphological variables across genetic groups: beak-head (from tip of beak to back of head), culmen length (from tip of beak to base of skull), beak-naris (from tip of beak to naris), beak depth, beak width, tarsus length and wing length (all measurements in mm). We compared morphology across genetic species/groups using analysis of variance (ANOVA), and in combination with acoustic variables using discriminate function analysis (see below). To graph the relationship between beak and body size, we used morphology factor scores using principal components analysis (PCA). Two components were extracted (Eigenvalues > 1) that explained 82% and 87% of the variance respectively: PC Body Size had high factor loadings for tarsus length (0.91) and wing length (0.91); PC Beak Size had high factor loadings for beak-head (0.95), beak-naris (0.92), culmen length (0.92), beak depth (0.92), and beak width (0.91). Therefore, birds with higher factor scores were larger in beak and body size.
Survey methods
We conducted point count surveys in February 2004, 2008 and 2013 using the variable circular plot method (for details see Martin et al. Reference Martin, Paine, Conway, Hochachka, Allen and Jenkins1997, O’Connor et al. Reference O’Connor, Sulloway and Kleindorfer2010c), which has been widely used to census Galápagos birds (e.g. Dvorak et al. Reference Dvorak, Vargas, Fessl and Tebbich2004, Reference Dvorak, Fessl, Nemeth, Kleindorfer and Tebbich2012, O’Connor et al. Reference O’Connor, Sulloway and Kleindorfer2010c). We used a total of 15 point counts separated by 200 m along the walking trail to the inner crater of Cerro Pajas volcano, which covered the longest possible transect through the largest remnant patch of Scalesia forest in the archipelago. At each point we conducted a 5-min survey during which we recorded the following: GPS coordinates, species, and estimated distance of bird from observer (to the nearest 5 m). During the survey the observers changed orientation from 0° to 90°, 180° and 270° to ensure 360° coverage. All surveys were conducted early in the breeding season between 06h00 and 11h00. Due to the dense vegetation of the Scalesia forest habitat, visual census data are unreliable. Therefore, records of birds were included in the analysis only if they were heard, which also avoided the counting of non-singing females. In 2004 and 2008 small numbers of Large Tree Finches C. psittacula had been recorded on Floreana (13 and one, respectively) (O’Connor et al. Reference O’Connor, Sulloway and Kleindorfer2010c), but recent genetic and morphological analyses suggest that C. psittacula did not occur on Floreana Island in 2004 and is likely locally extinct (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a). We therefore reanalysed the survey data from 2004 and 2008, and reclassified the records of C. psittacula as C. pauper as these two species both produce song with slower trill rate (Bowman Reference Bowman, Bowman, Berson and Leviton1983), and previously recorded C. pauper were likely identified incorrectly as C. psittacula. Following song and morphology analysis (see results) we treated C. parvulus and birds of the hybrid group as one entity (referred to as C. parvulus/hybrid group) for demographic analysis, given that it is not possible to distinguish these groups by song or morphology. Observers were Kleindorfer in 2004, O’Connor in 2008 and Kleindorfer and Peters in 2013, who are all familiar with the resident bird species and their songs; the three observers had spent at least two weeks calibrating distance estimates and bird song identification prior to conducting the surveys. In 2013, both observers conducted the survey at the same time (after training for calibrated field identification in 2012 and 2013).
Male population density calculation
Male population densities were estimated for all bird species. Density estimates (number of birds/km2) and detection probability estimates were explored using DISTANCE 6.0 (Thomas et al. Reference Thomas, Buckland, Burnham, Anderson, Laake, Borchers, Strindberg, El-Shaarawi and Piegorsch2006) but our dataset did not meet the assumptions required for analysis. Detection numbers were low for all species (all < 60) due to sampling restrictions inflicted by patchy and limited habitat, and minimum detections recommended for calculating reliable density estimates using DISTANCE are 60–100 (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). Since we were particularly interested in temporal abundance trends, we wanted to ensure comparability across years and use the same method for all years. We therefore calculated male population density estimates (number of birds/km2) using the inflection-point-per-species method following Reynolds et al. (Reference Reynolds, Scott and Nussbaum1980). Inflection points (the distance after which the detection rate steeply descends) varied across years and species as specified in Table S3 in the supplementary material and only birds observed within these ranges were included in population density and size estimate calculations. We obtained the number of birds/km2 by dividing the total number of birds observed by the total observation area (area of circle with the inflection point as radius), and then dividing the result by 15 (number of survey points). Because two observers were used in 2013, we calculated bird densities for this year using the average of their two values per species. The density of the Galápagos Flycatcher Myiarchus magnirostris needs to be interpreted with caution; due to their curious nature, these birds often follow observers and can be easily double counted (Dvorak et al. Reference Dvorak, Fessl, Nemeth, Kleindorfer and Tebbich2012).The singing activity of the Dark-billed Cuckoo Coccyzus melacoryphus can be very low and is not considered a reliable cue to detect cuckoo presence (Dvorak et al. Reference Dvorak, Fessl, Nemeth, Kleindorfer and Tebbich2012), and therefore our calculated density could be an underestimate. We are aware of the large group-size differences of Smooth-billed Ani Crotophaga ani which cause problems using point count surveys (Dvorak et al. Reference Dvorak, Fessl, Nemeth, Kleindorfer and Tebbich2012). Given that C. ani is a predator of Darwin’s finches (O’Connor et al. Reference O’Connor, Dudaniec and Kleindorfer2010a, Connett et al. Reference Connett, Guézou, Herrera, Carrión, Parker and Deem2013) we included this species in our analyses but interpreted results with caution.
Avian population size estimates
We estimated the maximum male population size for tree finches only, as their preferred nesting is in Scalesia forest, which occurs at Cerro Pajas and Asilo de la Paz, while the other species also breed elsewhere on the island. Estimates were based on the maximum size of the available suitable habitat, 22.5 km2 (O’Connor et al. Reference O’Connor, Sulloway and Kleindorfer2010c). This area comprises Floreana’s entire highland habitat (25 km2) and excludes 2.5 km2 that have been cleared for agriculture. Of the 22.5 km2 non-agricultural highland area, about 3.71 km2 (16.5%) is dominated by Scalesia, including the study site at Cerro Pajas (2.4 km2) (O’Connor et al. Reference O’Connor, Sulloway and Kleindorfer2010c). Thus, we sampled from 65% of the remnant Scalesia forest. We conducted the survey in the Cerro Pajas area and used these data to estimate density for the known Scalesia habitat (3.71 km2) as well as the total highland habitat (22.5 km2). We assume that Darwin’s finch density will differ across Scalesia patches (landscape variation in density was not a focus of this study); it is likely that our total maximum population size estimate will overestimate rather than underestimate tree finch numbers because Scalesia dominates just 16.5% of the total highland area.
Statistical analyses
Male population density estimates were calculated in Microsoft Office Excel following Reynolds et al. (Reference Reynolds, Scott and Nussbaum1980); all other statistical analyses were performed using IBM® SPSS® Statistics version 22. Graphs were produced using SigmaPlot version 12.0. We analysed distinctiveness of song and morphology between genetic groups using (i) ANOVA and Kruskal-Wallis test; and (ii) a discriminant function analysis (DFA). We examined data for normality before using parametric tests. Because data were collected across years, we first assessed if song and morphology differed across years using multivariate analysis of variance for the interaction effect of ‘year’ and ‘genetic group’. Three variables were transformed to meet assumptions of normality: maximum frequency (reflect and square root transformation), maximum number of syllables (reflect and logarithmic transformation) and beak width (inverse transformation). We checked the data for homogeneity of variance using Levene’s test. The variables for minimum frequency, duration, and number of syllables showed homogenous variances and were analysed using ANOVA with Tukey HSD post-hoc test. The variables trill rate, maximum number of syllables, maximum frequency and frequency bandwidth violated the assumption of homogeneity of variance (all P < 0.05) and were therefore analysed using Welch’s ANOVA with Games-Howell post-hoc tests. The variables - number of syllables, dominant frequency, beak length head, beak length naris and beak depth - violated assumptions of normality and were analysed using Kruskal-Wallis tests for independent samples with pairwise comparisons as post-hoc tests.
Since many variables were strongly correlated (Pearson’s correlation > 0.8) violating a key assumption of DFA, we first performed a principal component analysis (PCA) and used PC scores (varimax rotation) instead of raw variables in the DFA. PCA produced one morphology variable accounting for 75.1% of variance, and three acoustic variables accounting for 82.3% of variance in the original dataset. We performed the DFA using all four variables and examined the significance of discriminant models using F-tests (Wilk’s Lambda).
Results
Species determination based on morphology and song
There was no significant interaction effect between ‘year’ and ‘genetic group’ for morphology (MANOVA: Pillai’s Trace = 0.71, F35,310 = 1.46, P = 0.05); therefore we pooled data across years for morphological analysis. Camarhynchus pauper was significantly larger in all analysed variables (post-hoc tests all P < 0.04, Table 2, Figure 1), but C. parvulus and hybrid birds were morphologically indistinguishable (post-hoc tests all P > 0.79, Table 2, Figure 1). These results were consistent with results from discriminant function analysis: cross-validated DFA including vocal and morphological variables was able to correctly assign 93.8% of C. pauper males, but could only assign 57.1% of C. parvulus and 54.5% of hybrid males (Wilk’s Lambda = 0.32, χ28 = 58.07, P < 0.001).

Figure 1. The association between body size and beak size (factor scores form principal component analysis) in 77 Darwin’s tree finches that have been genetically assigned and for which we also have song recordings. The three recognised populations on Floreana are C. pauper, C. parvulus, and the hybrid group that arose from pairings between female C. pauper and male C. parvulus. The large-bodied C. pauper can be identified based on morphology, but the small-bodied C. parvulus overlaps with the morphology of hybrid birds. Some hybrid birds appear large like C. pauper.
We obtained a total of 325 song recordings from 77 genetically identified Darwin’s finches across four years (2006, n = 14; 2010, n = 22; 2013, n = 36; 2014, n = 5; Table 1). There was no significant interaction effect of ‘year’ and ‘genetic group’ (MANOVA: Pillai’s Trace = 0.464, F30,325 = 0.936, P = 0.576) and therefore we pooled the data across years. Tree finch Camarhynchus spp. song did not differ significantly between genetic groups for the variables maximum frequency and song duration. However, there were significant differences across genetic groups in minimum frequency, dominant frequency, frequency bandwidth, number of syllables, maximum number of syllables and trill rate (ANOVA: minimum frequency F2,76 = 16.75, P < 0.01, Welch’s ANOVA: frequency bandwidth F2,76 = 8.08, P = 0.003, trill rate F2,20.647 = 14.49, P > 0.001, maximum number of syllables F2,20.077 = 5.04, P = 0.016, Kruskal-Wallis test: dominant frequency F2 = 21.81, P < 0.001, number of syllables F2 = 9.18, P = 0.010, Table 1). Effect size was calculated using eta squared (minimum frequency = 0.31, frequency bandwidth = 0.11, trill rate = 0.16, maximum number of syllables = 0.06). Post-hoc comparison showed that C. pauper had a lower minimum and dominant frequency, a broader frequency bandwidth, fewer syllables, and a slower trill rate than C. parvulus and hybrid birds (all P < 0.04), but there was no significant difference between the song of C. parvulus and hybrid birds (all P > 0.5) (Figure 2).
Table 1. Male song characteristics in three tree finch Camarhynchus spp. genetic groups. Data are shown as mean ± SE (95% CI), statistical results are shown for Kruskal-Wallis test* and ANOVA. The sample size per genetic group is shown in brackets. Songs were recorded from colour-banded birds in the field and retrospectively assigned to species/group after laboratory analysis of genetic samples.
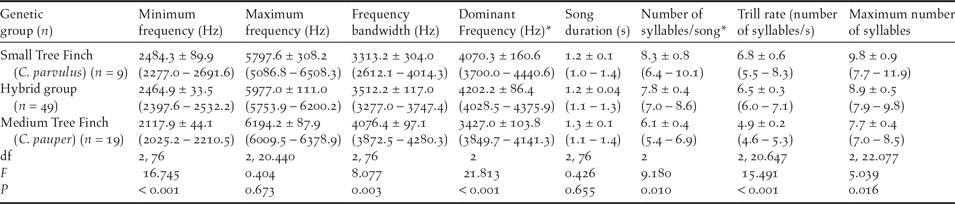
Table 2. Male morphology shown as mean ± SE (95% CI) per genetic group of tree finches Camarhynchus spp. for which we also analysed song recordings. Statistical results are shown for Kruskal-Wallis test* and ANOVA; post-hoc tests showed that C. parvulus and birds of the hybrid group were statistically indistinguishable from each other, but smaller than C. pauper (see results).

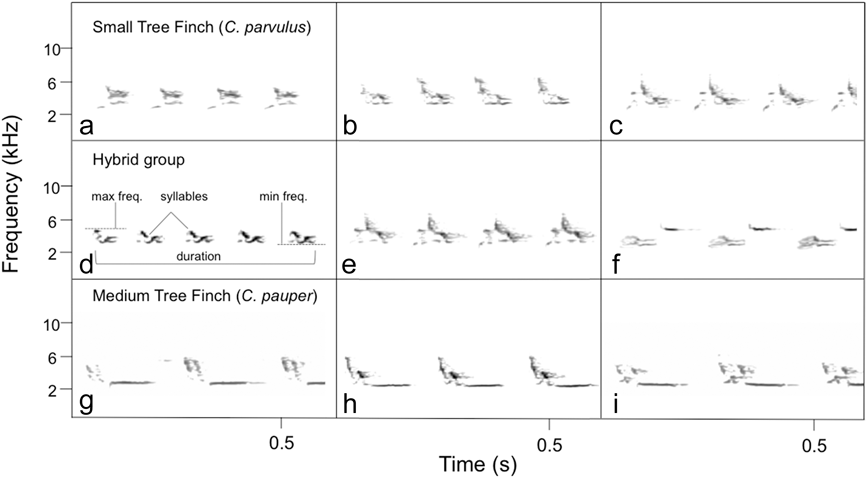
Figure 2. Spectrograms of song in: (a-c) Small Tree Finch Camarhynchus parvulus, (d-f) hybrid group, and (g-i) Medium Tree Finch C. pauper. Each spectrogram represents ∼0.6 seconds of song of one male; we chose this partial representation to include three males per genetic group and visualise the difference in trill rate between C. pauper vs. C. parvulus and hybrid. The song of C. parvulus and hybrid birds could not be statistically distinguished, while the song of C. pauper had slower trill rate, fewer syllables and lower minimum frequency (see Table 1). One spectrogram of a complete song per genetic group is provided in the supplementary material.
Avian population density and population size estimates
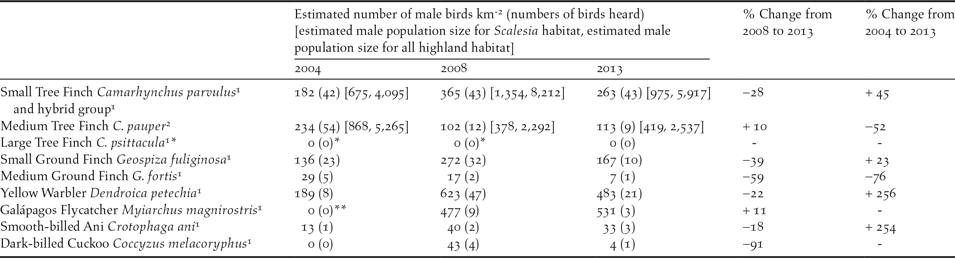
Using the respective inflection points per species and year as a threshold for data inclusion, our avian surveys at Cerro Pajas generated 362 bird records from nine species across the three survey years (2004 = 133, 2008 = 152, 2013 = 77; Table 3). As shown in Table 3, C. pauper abundance declined by 52% from 2004 to 2013, and C. parvulus/hybrid group numbers increased by 45%. Two other species showed patterns of decline: Medium Ground Finch G. fortis (-76%) and Dark-billed Cuckoo Coccyzus melacoryphus (-95%). Four other highland species showed patterns of increase: Galápagos Flycatcher Myiarchus magnirostris (+11%), Small Ground Finch Geospiza fuliginosa (+23%), Yellow Warbler Dendroica petechia (+256%), and Smooth-billed Ani Crotophaga ani (+254%). Neither the Warbler Finch Certhidea fusca nor the Large Tree Finch C. psittacula were detected.
Table 3. Estimated male population density of bird species in the highlands of Floreana Island during 2004, 2008, and 2013. Data are from singing males monitored using the circular plot method. The highland population estimate [shown in brackets] is given for Scalesia forest (3.71 km2) and for total highland habitat (22.5 km2) for males only in both tree finch groups; total population estimates were not calculated for the other species, as they do not predominantly nest in the highlands or in Scalesia forest.
Current IUCN status: 1Least Concern, 2Critically Endangered.
* O’Connor et al. (Reference O’Connor, Dudaniec and Kleindorfer2010) noted 13 (2004) and one (2008) singing C. psittacula, but findings by Kleindorfer et al. (Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a) suggested the C. psittacula was locally extinct; in this study, the C. psittacula males heard in 2004 and 2008 are considered to be C. pauper males.
** Galápagos Flycatchers were seen (but not heard) in 2004; therefore the relative increase from 2004 to 2013 is due to the occurrence of vocalising flycatchers and likely does not represent changes in population size.
Discussion
Main findings for song analyses and population estimates
Hybridisation created a considerable obstacle for species detection using acoustic surveys in tree finches due to the similarity between the song of hybrid birds and C. parvulus. For this reason, song cannot be used to estimate the abundance of C. parvulus and hybrid birds separately but could detect the combined ‘C. parvulus/hybrid’ group. Song of the critically endangered C. pauper differed from that of the C. parvulus/hybrid group in several variables, and therefore song can be used to monitor its abundance. However, there is one caveat: cross-validated discriminant function analysis including vocal and morphological variables only correctly assigned 93.8% of C. pauper males (and incorrectly assigned 6.3% of males to C. pauper when in fact they were hybrid birds). Inspection of Tables 1 and 2 and Figure 1 shows how this error could arise given some cases of overlap between C. pauper and hybrid birds in morphology and song. The statistical results of this study (combined with our unpublished field calibration trials) support the view that overall, morphology and song can be used to distinguish ∼ 94% of genuine cases of C. pauper. Aware of these caveats, using acoustic surveys, C. pauper declined by 52% across the decade from 2004 to 2013 (with 10% increase since 2008), while the C. parvulus/hybrid group increased by 45% (with 28% decrease since 2008). These results underscore the warranted conservation concern for the critically endangered C. pauper. Because we cannot distinguish C. parvulus from hybrid birds using song or morphology, only genetic analysis can reveal the population trends for C. parvulus relative to the hybrid group.
Differences in song and morphology in tree finches
Compared with C. parvulus/hybrid birds, song of C. pauper had slower trill rate (fewer syllable/s), fewer syllables per song, broader frequency bandwidth, lower minimum frequency, and lower dominant frequency. Camarhynchus pauper was significantly larger in all analysed morphological variables, but differences occurred across a gradient. Inspection of Figure 1 shows that some hybrid birds were indistinguishable from C. pauper in morphology, and C. parvulus/hybrid birds could not be distinguished morphologically.
The lack of difference in song between C. parvulus and hybrid birds has several possible explanations including small sample size (9 vs. 49), lack of time or selection for divergence, and the role of vocal tutors for learning of song type. Despite our efforts to sample equally from all three tree finch groups, post-hoc genetic assignment revealed the high relative abundance of birds of the hybrid group on Floreana Island, which explains the higher number of recorded hybrid songs (n = 49). Using morphology (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a) and genetics (Peters Reference Peters2016), the observed tree finch hybridisation is largely the result of C. pauper females pairing with C. parvulus males. Darwin’s Finches learn song from a male tutor which is usually their father (Grant and Grant Reference Grant and Grant1996), therefore it is likely that hybrid sons would have learned their song from their C. parvulus fathers and would sing a C. parvulus song. A similar pattern of song learning, and hence a possible mechanism for backcrossing in favour of the paternal genetic lineage, was previously shown in Geospiza hybrids (Grant and Grant Reference Grant and Grant1997, Reference Grant and Grant2014b). These possibilities require further investigation.
Survey results for tree finches
Our survey results show fluctuations in numbers in both C. parvulus/hybrids (overall 45% increase from 2004 to 2013 but 28% decline from 2008 to 2013) and C. pauper (overall 52% decline from 2004 to 2013 but 10% increase from 2008 to 2013). The Galápagos Islands provide compelling case studies for novel evolutionary trajectories as the result of climate impacts in a single year (e.g. Grant and Grant Reference Grant and Grant1993). A four-year drought prevailed on the Galápagos from 2003 to 2007, and then followed years with high rainfall conditions in 2008, 2011 and 2012 (CDF Meteorological Database, http://www.darwinfoundation.org/datazone/climate/). Therefore, the decline in C. pauper from 2004 to 2008 may be the result of drought-induced mortality, and the post-2008 increase may the result of rainfall-induced nesting recruitment. It is also possible that we are detecting more unpaired singing C. pauper across the years, rather than an increase in C. pauper males and females (Kleindorfer et al. in review) The pattern is more difficult to interpret in the C. parvulus/hybrid group, because they are indistinguishable using acoustic surveys. It is likely that hybridisation increased post-rainfall, but perhaps hybrids are unable to establish – which remains to be tested.
According to criteria established by IUCN, C. parvulus is classified as ‘Least Concern’ (The IUCN Red List of Threatened Species. Version 2014.3; www.iucnredlist.org). However, hybridisation among tree finches makes its actual status on Floreana Island uncertain. To date we have insufficient information on the makeup of the hybrid group, but unpublished data suggest that the hybridisation extends well beyond F1. Genetic introgression from C. pauper to C. parvulus was previously suspected (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a) and ongoing analyses show evidence for extensive asymmetrical gene flow towards C. parvulus (Peters Reference Peters2016). The acoustic and morphological similarity of C. parvulus and the hybrid group presented here supports the scenario that backcrossing has already occurred and the hybrid group does not consist of first generation hybrids but rather comprises later generation hybrids and introgressed individuals (see also Derégnaucourt et al. Reference Derégnaucourt, Guyomarc’h and Richard2001). A reliable classification of the conservation status of the Floreana C. parvulus population will depend on results of detailed genetic analyses.
Floreana Island has the longest history of human settlement and activity (Lack Reference Lack1983, Steadman Reference Steadman1986, Watson et al. Reference Watson, Trueman, Tufet, Henderson and Atkinson2010) and the highest number of species extinctions across the Galápagos Archipelago. Three bird species (Large Ground Finch, Geospiza magnirostris; Sharp-beaked Ground Finch, G. difficilis and Floreana Mockingbird, Nesomimus trifasciatus) have become locally extinct over the past 200 years (1835–2005) (Grant et al. Reference Grant, Curry and Grant2000, Reference Grant, Grant, Petren and Keller2005b, Merlen Reference Merlen2013). The Warbler Finch Certhidea fusca (Grant et al. Reference Grant, Grant, Petren and Keller2005b), the Vermilion Flycatcher Pyrocephalus rubinus (O’Connor et al. Reference O’Connor, Sulloway and Kleindorfer2010c), and C. psittacula (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a) are either currently locally extinct or likely to become locally extinct in the near future (discussed below). The Vegetarian Finch Platyspiza crassirostris was only heard once by our group in the highlands in 2010, and hence could also be considered very rare (Kleindorfer pers. obs.).
Habitat fragmentation, habitat loss, introduced species and pathogens (Wiedenfeld and Jiménez-Uzcátegui Reference Wiedenfeld and Jiménez-Uzcátegui2008) can be particularly problematic for small and range restricted populations (Simberloff Reference Simberloff1995). Less than 62% of the original Scalesia forest persists on Floreana Island given land clearing for human activities. The remaining Scalesia habitat is under increasing pressure from introduced flora (Mauchamp Reference Mauchamp1997, Rentería et al. Reference Rentería, Gardener, Panetta, Atkinson and Crawley2012) and fauna (Whiteman et al. Reference Whiteman, Goodman, Sinclair, Walsh, Cunningham, Kramer and Parker2005, Jiménez-Uzcátegui et al. Reference Jiménez-Uzcátegui, Carrión, Zabala, Buitrón and Milstead2008), such as black rat Rattus rattus and Norwegian rat R. norvegicus (Grant et al. Reference Grant, Grant, Petren and Keller2005b), domestic cats Felis catus (Jiménez-Uzcátegui et al. Reference Jiménez-Uzcátegui, Carrión, Zabala, Buitrón and Milstead2008), Smooth-billed Ani C. ani (Connett et al. Reference Connett, Guézou, Herrera, Carrión, Parker and Deem2013) and the introduced dipteran Philornis downsi.
Philornis downsi is considered the biggest threat to Darwin’s finch survival and to breeding success in Galápagos land birds in general (Kleindorfer and Dudaniec Reference Kleindorfer and Dudaniec2016). Both parasite intensity and Darwin’s finch mortality have increased across the past decade (Kleindorfer and Dudaniec Reference Kleindorfer and Dudaniec2016). The available data on impacts of P. downsi suggest that low annual recruitment in C. pauper is the main explanation for its critical decline (O’Connor et al. Reference O’Connor, Sulloway, Robertson and Kleindorfer2010d, Peters Reference Peters2016). Given the 45% increase in numbers of the C. parvulus/hybrid group from 2004 to 2013, another factor contributing to the C. pauper decline could be selection favouring hybridisation with C. parvulus. If hybrid birds have higher fitness (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a, Peters Reference Peters2016) and if hybrid offspring backcross with other hybrids or C. parvulus, this will increase recruitment of the C. parvulus/hybrid group rather than the C. pauper group.
Camarhynchus psittacula has always been rare on Floreana Island (discussed in Grant et al. Reference Grant, Grant, Petren and Keller2005b, Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a). Our repeated survey and nest monitoring efforts support the view that C. psittacula is locally extinct on Floreana Island (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a). Because we only surveyed at one location, it is possible that this species exists elsewhere on the island. However, we have traversed the island widely for various reasons, and have not heard C. psittacula song. We tested this in the field by comparing our recordings with historical 1960s recordings from Robert Bowman on Floreana and Santa Cruz Islands (Kleindorfer unpubl. data), and no Floreana tree finch had a strong response to historical C. psittacula song.
Implications for conservation and survey techniques
Given that the majority of songbird species learn song from an adult tutor which is usually their father (Catchpole and Slater Reference Catchpole and Slater2003), hybrids are generally likely to sing the song of their paternal species, and therefore other systems with contemporary hybridisation may show the same pattern we present here. In other systems, frequent hybridisation resulted in genetic and demographic swamping of one or both of the parental species by the hybrids (Rhymer and Simberloff Reference Rhymer and Simberloff1996, Roberts et al. Reference Roberts, Gray, West and Ayre2010). But rare species can benefit from hybridisation, as it increases their often depleted genetic diversity and possibly their fitness and adaptive potential (Baskett and Gomulkiewicz Reference Baskett and Gomulkiewicz2011, Hamilton and Miller Reference Hamilton and Miller2016). Camarhynchus parvulus could constitute an important source of genetic variation for the critically endangered C. pauper. The hybrid group could serve as a genetic reservoir preserving the genes of an endemic and declining species, in which case all three genetic groups and their habitat should be conserved (López-Pujol et al. Reference López-Pujol, Garcia-Jacas, Susanna and Vilatersana2012).
Because hybridisation usually occurs between already closely related species, detection of hybrids is complicated as it often relies on molecular analyses. Backcrosses and later generation hybrids in particular cannot be determined using morphological characters alone (Allendorf et al. Reference Allendorf, Leary, Spruell and Wenburg2001). Characterising hybridisation patterns may therefore require genetic analyses, such as in grey wolf Canis lupus and domestic dog C. familiaris (Andersone et al. Reference Andersone, Lucchini and Ozoliņš2002, Vilà et al. Reference Vilà, Walker, Sundqvist, Flagstad, Andersone, Casulli, Kojola, Valdmann, Halverson and Ellegren2003), and Hawaiian Ducks Anas wyvilliana and introduced Mallard A. platyrhynchos (Fowler et al., Reference Fowler, Eadie and Engilis2009). Hybridisation therefore makes rapid population assessment practically impossible in many species, which is especially problematic when threatened species are involved that require regular monitoring. In the case of the Floreana tree finch group, the distinct song of C. pauper means that acoustical identification can be retained for surveys, which is a significant finding given the critically endangered status of this endemic and declining species.
Survey results for other bird species
While this study focused on the Camarhynchus tree finches, we present the findings for other bird species in Table 3. We provide comment here on the introduced, and the very rare or possibly locally extinct species known for Floreana Island. Crotophaga ani was introduced to the Galápagos Archipelago in the 1960s to consume the ticks on cattle; but analysis of gizzard contents found Darwin’s Finch remains instead (Olivares and Munves Reference Olivares and Munves1973, O’Connor et al. Reference O’Connor, Dudaniec and Kleindorfer2010a, Connett et al. Reference Connett, Guézou, Herrera, Carrión, Parker and Deem2013). Therefore, the increase in C. ani could be a threat to populations of songbirds. The extreme drought across the Galápagos from 2003 to 2007 (CDF Meteorological Database, http://www.darwinfoundation.org/datazone/climate/) is suspected to have negatively influenced insectivorous and frugivorous species in particular. The Vegetarian Finch and Vermilion Flycatcher used to be relatively common in the Floreana highland forest, although there is no information about former population size, and abundance has mainly been inferred from statements made by locals and the previously high numbers of collected specimens (P. crassirostris: 48 in 1905/06, three in 1962, one in 1974, P. rubinus: seven in 1888–1891, 133 in 1898–1906 and 10 in 1962) (Wiedenfeld Reference Wiedenfeld2006, O’Connor et al. Reference O’Connor, Sulloway and Kleindorfer2010c, Merlen Reference Merlen2013). We have only one sighting of P. crassirostris since 2004, and individuals of P. rubinus have not been seen since 2008 (Walter Cruz, K. J. Peters pers. comm.). In the case of the Warbler Finch, several targeted surveys by Grant et al. (Reference Grant, Grant, Petren and Keller2005b) during the breeding season in 1979, 1983, 1997, 1999 and 2004 using species-specific playback to stimulate a response, failed to locate any C. fusca on Floreana Island; but O’Connor et al. (Reference O’Connor, Sulloway and Kleindorfer2010c) reported hearing a male C. fusca singing at Asilo de la Paz in 2008. This species is suspected to be locally extinct or at least extremely rare on Floreana, and the fact that this study did not observe any C. fusca supports this view. Several species increased across the decade including Dendroica petechia (+256%), C. ani (+254%) G. fuliginosa (+23%), and Myiarchus magnirostris (+11 %); however, the Ground Finch G. fortis (-76%) decreased in the highland Scalesia. In summary, the strong decline observed in C. pauper surveyed in Scalesia forest is historically paralleled by three local extinctions in Darwin’s finch species on Floreana Island, and warrants concern. Understanding why five land bird species have apparently increased in the same habitat, including two Darwin’s finch species (C. parvulus/hybrid, G. fuliginosa), requires focused inquiry.
Conclusion
Acoustic survey techniques could not reliably detect tree finch hybrids. Song can be used to distinguish the critically endangered C. pauper, but song was the same in common C. parvulus and birds of the hybrid group. Using a comparable survey approach at three sampling times across the decade, the data suggest that C. pauper did not recover from its dramatic decline in abundance following the drought years of 2003 to 2007; rather, it maintained its 52% decline across the decade in spite of modest population stabilisation since 2008. Recent evidence suggests substantial introgression from C. pauper into the C. parvulus population in the Cerro Pajas region (Kleindorfer et al. Reference Kleindorfer, O’Connor, Dudaniec, Myers, Robertson and Sulloway2014a, Peters Reference Peters2016), many P. downsi parasites per nest (O’Connor et al. Reference O’Connor, Robertson and Kleindorfer2010b), naris malformation from P. downsi parasites (Kleindorfer and Sulloway Reference Kleindorfer and Sulloway2016), and no observed nesting success since 2010 (Kleindorfer et al. Reference Kleindorfer, Peters, Custance, Dudaniec and O’Connor2014b, Peters Reference Peters2016). Our second major finding that C. parvulus and hybrid birds generally increased across the decade (but declined by 28% since 2008) requires further investigation as we cannot ascertain actual size estimates for each respective population without genetic analysis, and it could mask an undercurrent of decline in C. parvulus. Repeated bird surveys across the decade show a range of patterns in populations: several species showed a marked increase (including an introduced avian predator), other species showed a noticeable decline (including the locally endemic C. pauper). Floreana Island has a history of local extinctions, which warrants concern for existing species and underlines the need for regular monitoring. Hybridisation may be a driver of biodiversity and adaptive capacity if alleles from rare species are introgressed into common species, but hybridisation can hamper reliable population estimates of common species when the two groups become acoustically and visually indistinguishable. This study highlights the need for urgent conservation measures for the tree finch group on Floreana Island.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270916000630
Acknowledgements
This publication is contribution number 2129 of the Charles Darwin Foundation for the Galápagos Islands. We thank the Charles Darwin Research Station and Galápagos National Park for logistical and research support and TAME airlines for reduced airfares to Galápagos. We are grateful to the community on Floreana Island for their continued support. We thank D. Arango Roldan, R. Bassi, C. Evans, S. Gantefoer, D. Gaspard, K. Gavrilchuck, S. Humberto, M. Louter, J. O’Connor, J. Robertson, M. Schmidt, R. Schubert and T. Seebacher for field assistance. We thank the following organizations for funding: American Bird Conservancy, Australian Federation of University Women (SA), Australian Research Council, Birdfair/RSPB Research Fund for Endangered Birds, Club300 Bird Protection, Conservation International, Earthwatch Institute, Ecological Society of South Australia, Flinders University of South Australia, Max Planck Institute for Ornithology, Mohamed bin Zayed Species Conservation Fund, Royal Zoological Society of South Australia, Rufford Small Grant Foundation, and Winifred Violet Scott Trust. All appropriate ethical approval (Flinders University Animal Welfare Committee E270, E393) and scientific permits (Parque Nacional Galápagos, 012-2004, 013-2005, 013-2006, 008-2008, 011-2010, PC-58-11 (2012), PC-39-12 (2013), PC-15-14 (2014)) necessary to carry out this project were obtained.







