Introduction
In the tropics, predator abundance and diversity are high and nest predation is also naturally high, affecting up to 80% of nests (Robinson et al. Reference Robinson, Robinson, Robinson and Brawn2000, Stutchbury and Morton Reference Stutchbury and Morton2001). Like other passerines (Murphy Reference Murphy1983, Martin Reference Martin1995), high rates of nest predation have been reported for several cerrado (dry savanna woodland) birds, such as Lesser Elaenia Elaenia chiriquensis (67% of nests lost to predation; Medeiros and Marini Reference Medeiros and Marini2007), White-banded Tanager Neothraupis fasciata (61%; Duca and Marini Reference Duca and Marini2011) and Chapada Flycatcher Suiriri islerorum (88%; França and Marini Reference França and Marini2009). In fragmented areas, these already high predation rates seem to be inflated for some species, such as the Chapada Flycatcher, causing its population to decline (França and Marini Reference França and Marini2009).
Several management strategies have been applied to reduce nest predation rates and increase reproductive success of wild birds, such as the use of electric fences to protect nests of colonial marine birds (Minsky Reference Minsky1980, Spear et al. Reference Spear, Schweitzer, Goodloe and Harris2007) and protective cages around the nests of terrestrial birds (Moseby and Read Reference Moseby and Read2006, Isaksson et al. Reference Isaksson, Wallander and Larsson2007). Other strategies aimed at increasing reproductive success range from the use of artificial nests, translocation of nestlings to new nest sites (Priddel et al. Reference Priddel, Carlile and Wheeler2006) to artificial insemination (Blanco et al. Reference Blanco, Wildt, Hofle, Voelker and Donoghue2009). Since conservation and management programmes for threatened species are costly and resources are limited, it is important to ensure the cost-effectiveness of such interventions.
Artificial incubation of eggs collected from natural nests of wild birds reduces the period that eggs are exposed in the wild and thus reduces predation risk (Kuehler et al. Reference Kuehler, Lieberman, McIlraith, Everett, Scott, Morrison and Winchell1993, Reference Kuehler, Kuhn, Kuhn, Lieberman, Harvey and Rideout1996, Reference Kuehler, Lieberman, Oesterie, Powers, Kuhn, Kuhn, Neslon, Snetsinger, Herrmann, Harrity, Tweed, Fancy, Woodworth and Telfer2000, Reference Kuehler, Lieberman, Harrity, Kuhn, Kuhn, McIlraith and Turner2001). So in habitats or regions where nest predation is high, artificial incubation is a potential management tool for increasing reproductive success and population size.
Birds’ eggs have all the resources necessary for embryo development (Visschedijk Reference Visschedijk1991) but also require proper heating, humidity and periodic turning (Klimstra et al. Reference Klimstra, Stebbins, Heinza, Hoffmana and Kondradc2009). Artificial incubation creates different conditions from those of natural nests, so a test of artificial egg incubation with cerrado birds is desirable and timely since climate in the region has strong seasonal variation in precipitation (and thus air humidity) (Nimer Reference Nimer1979), birds breed from the end of the dry season through the rainy season (Marini and Durães 2001, Marini et al. 2009b), and average temperature is predicted to increase by 3.5oC by 2100 due to climate change (Marini et al. 2009a). Since artificial incubation is still considered an imperfect science and hatching success may be low (Klimstra et al. Reference Klimstra, Stebbins, Heinza, Hoffmana and Kondradc2009), incubation variables need to be adjusted for each species. Also, since it may not be possible to first test methodologies with endangered species, the use of a common species is recommended.
In this paper, we report on management strategies employed with the Lesser Elaenia. This is a migratory and frugivorous passerine and an appropriate species for such experimental evaluation since it nests at very high densities in the cerrado of central Brazil (Paiva Reference Paiva2008), it does not desert after clutch manipulations (Sousa Reference Sousa2008) and it is not endangered. Preliminary field tests were carried out in 2008 and experiments conducted in 2009 and 2010, with the goal of evaluating whether various methods of clutch manipulation were an efficient and cost-effective method of improving breeding success. We considered a management tool efficient if it produced a large number of extra fledglings (compared with natural nests) for the least cost (Wilson et al. Reference Wilson, McBride, Bode and Possingham2006, Duca et al. Reference Duca, Yokomizo, Marini and Possingham2009). We had one treatment and two controls (Table 1). We tested the effects of management strategies on hatching rate, fledgling productivity, daily survival rates (DSR) of eggs, nestlings and nests, and nest success. We also evaluated the financial cost and benefit of three different management strategies of artificial egg incubation with reintroduction of nestlings to the original nest (Table 1).
Table 1. Schematic representation of the tested management strategy in this study and the three different strategies (one tested and two hypothetical) used to estimate managements costs and benefits.

Methods
Study area
The study was located at the Estação Ecológica de Águas Emendadas (15º29’–15º36’S, 47º31’–47º41’W; mean elevation 1,040 m asl), Distrito Federal, Brazil, a 10,500-ha fragment of cerrado vegetation (Silva Jr. and Felfili Reference Silva and Felfili1996). We searched for nests in a 100 ha (1 km x 1 km) permanent plot located > 1 km from the edge of the reserve, mostly in woodland cerrado (‘cerrado sensu stricto’) and open cerrado (‘cerrado ralo’ or ‘campo cerrado’) (Ribeiro and Walter Reference Ribeiro, Walter, Sano and Almeida1998). The climate of the cerrado region is strongly seasonal, with a cool, dry winter and a warm, rainy summer. Further details are given in Nimer (Reference Nimer1979) and Borges and Marini (Reference Borges and Marini2010).
Study species
The study species was the Lesser Elaenia, a passerine with a common clutch size of two eggs (Medeiros and Marini Reference Medeiros and Marini2007). It is migratory (Marini and Cavalcanti Reference Marini and Cavalcanti1990), mostly frugivorous (Marini and Cavalcanti Reference Marini and Cavalcanti1998) and very abundant at our study site during its breeding season, from September through late December (Paiva Reference Paiva2008). Nests are open cup structures constructed in bushes or trees located in cerrado sensu stricto (Medeiros and Marini Reference Medeiros and Marini2007). Nests were located by searching in the vegetation or by following birds with nest material. The incubation period lasts on average 14 days and the nestling period averages 15 days. Further nesting details are provided in Medeiros and Marini (Reference Medeiros and Marini2007).
Experimental design
We searched for nests during the breeding season, from September to December, 2009–2010. Empty nests and one-egg nests found were monitored every two days or every day, respectively, to record the exact day of clutch completion. We manipulated clutches with two eggs that were completed within 24 h of each other (hereafter “manipulated”). We transferred natural eggs to an incubator and replaced them with artificial plaster eggs of similar size and colour. Egg replacement had the objective of avoiding nest abandonment by parents and allowing the reintroduction of new-born nestlings to their original nest. We monitored nests daily, in order to replace predated eggs by new ones as soon as they were predated. We used the same daily monitoring scheme with another group of nests (hereafter “control I”), but without any clutch manipulation. Since the monitoring frequency of nests may increase nest predation and abandonment (Major Reference Major1989, Bolduc and Guillemette Reference Bolduc and Guillemette2003), we tested the effect of nest monitoring frequency comparing control I with another group of non-manipulated clutches (hereafter “control II”) monitored every 3-4 days.
We replaced natural eggs by artificial eggs to try to avoid nest abandonment by parents while natural eggs were in the incubator. We replaced both eggs of each nest with two plaster eggs immediately after the second egg was laid. Since parents maintain nest sanitation by removing egg shells, faecal sacs and alien material (Blair Reference Blair1941, Nethersole-Thompson and Nethersole-Thompson Reference Nethersole-Thompson and Nethersole-Thompson1941), we placed a small green leaf inside the nest to evaluate egg acceptance. If the leaf was removed from the nest up to 24 hours after its placement and parents were seen near the nest, we considered eggs accepted and the nest active. After being replaced by plaster eggs, we protected natural eggs with cotton wool and stored them inside individual acrylic vials with holes in the lid to allow gas exchange. We transported eggs to a portable incubator located in a dark incubation room up to 50 min away from the field site.
Eggs were set in an incubator (Premium Ecológica - IP35 PDA) and artificially incubated and hatched (∼14 days) at 37.5oC and 55% air humidity. Eggs were set horizontally, and mechanically turned through 180º on their longitudinal axis every two hours. We candled eggs every three days to monitor embryo development. On the expected day before hatching, we removed eggs from incubator rollers and placed them in a cloth “nest” inside the incubator to avoid nestling death after hatching by getting trapped in the roller.
After hatching, we fed new-borns with a commercial nestling food on average every 40 min until reintroduced to the nest. We usually removed nestlings from the incubator every day at 08h00. For nestlings born after 08h00, removal from the incubator occurred the next day at 08h00. Thus, most nestlings stayed in captivity for less than 24 hours after hatching. We transported nestlings and fragments of their egg shells in an insulated box at 37oC. To simulate hatching, we replaced plaster eggs with the new-born nestlings and egg shells. We considered nestlings accepted by parents if they were still in the nest and egg shells were removed after 30 min.
We introduced nestlings into nests of adoptive parents when their original nests had been lost (predated or abandoned) during the incubation period. This allowed us to return nestlings to nature instead of sacrificing them. We selected adoptive parents from a pool of dozens of other natural nests found and monitored in the study area. To avoid rejection by adoptive parents or siblings (O’Connor 1978) we used only nests with one or two nestlings at the same developmental stage (± 1 day) of the orphan nestlings. Each nest with adopted parents received one or two nestlings, to a maximum of three nestlings per nest. We marked introduced, or reintroduced, nestlings on the tarsus with non-toxic paint.
We monitored nests of both artificially incubated eggs (manipulated) and naturally incubated eggs (control I) daily, and naturally incubated eggs (control II) every 3–4 days, until eggs hatched. Since clutch manipulations were restricted to the egg period, once nestlings had been reintroduced to their nests, we monitored all nests (manipulated and controls I and II) every 3–4 days. We classified nest fates as: 1) successful, when incubation lasted at least 14 days or when nestlings reached at least 13 days of age (minimum incubation period or minimum age for a egg to hatch or a nestling to fledge, respectively; Medeiros and Marini Reference Medeiros and Marini2007); 2) depredated, when nest contents were found damaged or disappeared before the minimum expected age of eggs/nestlings to fledge successfully; 3) abandoned, when adults were not observed attending the nest and eggs were cold.
Management considerations
To evaluate the viability of the management strategy, we calculated acceptance rate of plaster eggs by parents, as well as acceptance of reintroduced nestlings. We calculated the percentage of nests with plaster eggs that were still active after predation events and replacement of predated eggs with new plaster eggs. We also calculated the percentage of naturally and artificially incubated eggs that did not hatch. Lastly, we quantified the number of eggs and nestlings lost due to handling.
To evaluate overall management cost we summed the cost of field expenses with labour (∼ US$ 58.48/day), fuel for cars (∼ 100 km/day, US$ 0.58/km), as well as products used to make artificial eggs, to transport eggs and nestlings, food for nestlings, and the incubator. We estimated the cost for the artificial incubation methodology used in this study, as well as for egg replacement, with or without adoptive parents. We also estimated the cost for the hypothetical methodology of artificial incubation and egg replacement, but without replacement of depredated artificial eggs, i.e. after the predation of a nest with artificial eggs, the nest would be presumed to become inactive.
To estimate costs with fuel and labour, we first determined field effort (number of days) for each methodology using the following assumptions: (1) two people search for nests and one undertakes all the other activities; (2) each person is able to find five nests per day, totalling 10 nests found per day; (3) nests are found when nest building was half completed, and the interval to the laying of the second egg is eight days; (4) the nest searching period consists of consecutive days, including weekends and holidays; (5) the incubation period lasted 14 days and nestling period 15 days; (6) trips to the study site were daily after the first nestling was returned to the nest, since after the first egg hatched, hatching became daily and at least one nestling was returned to the nest; (7) after a nestling was returned to the nest, monitoring occurred every 3–4 days. We estimated costs for each methodology considering a manipulation of 30 nests each. To estimate the number of days required to search for nests, we first estimated the number of nests necessary to have 30 manipulated nests + nests to be used as adoptive parents (when necessary). We considered a nest predation rate of 9.6% until laying of the second egg (for manipulated nests) and 57% for the incubation period (for nests with adoptive parents), based on field data obtained during nest monitoring in this study. Then, we divided the number of required nests by 10, which is the maximum number of nests found in one nest-searching day. Finally, we obtained the number of days required to search for enough nests to manipulate and use as adoptive parents.
Statistical analyses
We compared hatching rate (number of eggs hatched by number of eggs incubated per nest) (Mayfield Reference Mayfield1975) among clutch-manipulated and control I nests, and control I and control II nests, using a Mann-Whitney U-test. We compared fledgling productivity (number of fledglings per nest; Ricklefs and Bloom Reference Ricklefs and Bloom1977) among clutch-manipulated and control I nests, and control I and control II nests, using a Chi-square test. We calculated the apparent success (percentage of successful nests) and did not use Mayfield (Mayfield Reference Mayfield1975) or logistic-exposure (White and Burnham Reference White and Burnham1999) methods for clutch manipulation because nests with eggs artificially incubated were either successful or not. We compared the apparent success among clutch-manipulated and control I nests using a Chi-square test. We set α-level at 5% for all tests, and for all analyses we present means followed by standard error (SE).
We used program MARK (Version 4.3) to estimate daily survival rate (DSR) of eggs, nestlings and the full nest period. To test whether there was any negative clutch manipulation interference on nestling survival after their reintroduction to the nest, we estimated daily survival rates during nestling periods of clutch-manipulated and control I nests. To test the effect of monitoring frequency on breeding parameters, we estimated daily survival rates of eggs and nestling periods separately and also for the full nesting period (egg and nestling periods) of control I and control II clutches. Although the breeding success of birds can vary throughout the breeding season (Nilsson Reference Nilsson1989, Hochachka Reference Hochachka1990), we did not consider temporal variations in DSR because our study period was short. Thus, we used the null model similar to Mayfield’s (Reference Mayfield1961, Reference Mayfield1975) estimate, that considers DSR as constant over the breeding season (Dinsmore et al. Reference Dinsmore, White and Knopf2002). We calculated Mayfield success (1961, 1975) for control I and II, raising DSRs of the null model to the number of days needed to complete the nest cycle (one day of laying + 14 days of incubation + 13 days of minimum duration of nestlings in the nest).
Clutch-manipulated nests were analysed in two ways: a) with use of adoptive parents when natural nests were destroyed during incubation period; and b) the hypothetical case without use of adoptive parents, when nestlings whose original nests have been destroyed and would not be returned to nature (sacrificing).
Results
We monitored 50 manipulated nests (20 in 2009 and 30 in 2010), 40 control I (18 in 2009 and 31 in 2010) and 118 control II (55 in 2009 and 63 in 2010) with two eggs each in the two years.
Management considerations
Considering all nests from both years, there was a 100% acceptance of artificial eggs by parents. Considering all 77 nestlings reintroduced to nests, 96% (n = 74) (91% in 2009 and 100% in 2010) were accepted by parents. Two manipulated nests were abandoned by parents a few days after they had accepted and incubated artificial eggs. Among the 50 manipulated nests with artificial eggs, 64% (65% in 2009 and 63% in 2010) were predated during the egg period. However, because of the replacement of artificial eggs lost by predation, 38% (46% in 2009 and 32% in 2010) of these predated nests were not abandoned by parents, allowing the reintroduction of their own artificially incubated nestlings.
The percentage of eggs that did not hatch was higher for artificially incubated (18% in 2009 and 25% in 2010) than for natural eggs (0.5% in 2009 and 3.8% in 2010) after three days since the first egg hatched. For natural nests, we considered only the nests in which none of the eggs was predated during the incubation phase, and that after the first egg hatched, the second egg remained unhatched in the nest for at least three days. It was not possible to monitor egg fertility at the start of artificial incubation since eggs were removed from nests immediately after laying.
During all experiments, 22 eggs and one nestling were lost. Of these eggs, 19 (five in 2009 and 14 in 2010) did not hatch and three (two in 2009 and one in 2010) were accidentally broken. The nestling died in 2010 for an unknown reason after it was removed from the incubator.
The cost of manipulating 30 nests, for the methodology with egg replacement after predation events of manipulated clutches, was US$ 6,105.18 (in December 2010) with the use of adoptive parents, and US$ 3,415.12 without the use of adoptive parents (Table 2). The cost of the same methodology for 30 nests without egg replacement is only US$ 2,625.64.
Table 2. Total management costs of the three methodologies: (1) artificial incubation of eggs without egg replacement by artificial eggs; (2) artificial incubation of eggs with replacement of field predated plaster eggs in natural nests during the incubation period, c) artificial incubation, egg replacement and use of adoptive parents when natural nests were destroyed during incubation period.
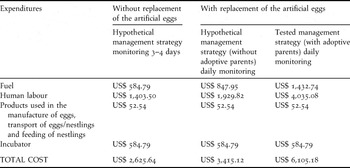
The management cost per fledgling produced for each methodology was: US$ 218.04 for the methodology with egg replacement and adoptive parents (28 fledglings produced); US$ 170.76 for the methodology with egg replacement but without adoptive parents (20 fledglings produced); and US$ 201.97 for the methodology without egg replacement (an estimate of 13 fledglings produced).
Effect of artificial incubation
The average hatching rate increased with management. There was a significant (U = 1,827.0; df = 49, 50; P < 0.001) increase in average hatching rate of eggs incubated artificially (78%) when compared with eggs from control I (36%) (Table 2).
The DSR of nestlings was apparently not affected by management strategy over the two years. DSRs were slightly lower in 2009 and slightly higher in 2010 for eggs incubated artificially with and without adoptive parents than for eggs from control I (Table 3). DSR of nestlings with their natural or with adoptive parents was similar in both years (Table 3).
Table 3. Breeding parameters of Lesser Elaenia Elaenia chiriquensis of control I (n = 49), control II and manipulated (n = 50) nests for 2009 + 2010 (hatching rate, fledgling production, and nest apparent success) and each year separately (daily survival rate and Mayfield nest success).
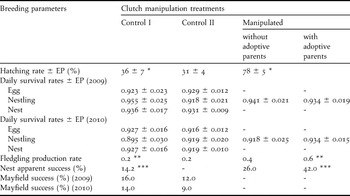
* U = 1827.0; df = 49, 50; P < 0.001
** χ22 = 8.579; P = 0.014
*** χ21 = 8.055; P = 0.002
Fledgling production was low for nests of control I (0.20 fledglings/nest; 0.28 in 2009 and 0.19 in 2010), intermediate for incubated eggs without adoptive parents (0.40; 0.60 in 2009 and 0.27 in 2010) and high for nestlings with adoptive parents (0.60; 0.79 in 2009 and 0.47 in 2010). However, fledgling production from incubated eggs was not significantly higher (χ22 = 1.043; P = 0.594) than from control I nests, using pooled data from both years (Table 3). However, when nestlings from adoptive parents are considered, nests with artificially incubated eggs had significantly higher (χ22 = 8.57; P = 0.014) fledgling production than control I (Table 3).
In a similar way to the fledgling production rate, the apparent success of nests was low for control I (14%; 22% in 2009 and 10% in 2010), intermediate for incubated eggs without adoptive parents (26%; 40% in 2009 and 17% in 2010) and high for nestlings with adoptive parents (42%; 50% in 2009 and 37% in 2010) (Table 3). Nests from control I, however, differed significantly (χ21 = 8.055; P = 0.002) only from manipulated nests with adoptive parents, and were similar to manipulated nests without adoptive parents (χ21 = 1.443; P = 0.230).
Effect of nest monitoring frequency
Nest monitoring frequency did not affect hatching rate. Even though nests from control I were monitored more frequently than nests from control II, average hatching rate was similar (U = 2,735.0; df = 49, 118; P = 0.522) between control I (36%) and control II (31%) (Table 3).
Nest monitoring frequency apparently also had no effect on average DSR during egg or nestling periods, since differences were small between control I and II and similar, but in opposite directions, between 2009 and 2010 (Table 3). DSRs over all the nest period, however, were also similar, but slightly higher in both years in control I than in control II (Table 3).
Fledgling production was similar between nests with different nest monitoring frequencies. Nests from control I (average of 0.22 fledglings/nest; 0.28 in 2009 and 0.19 in 2010) had similar (χ22 = 2.322; P = 0.313) fledgling production to nests of control II (average of 0.21 fledglings/nest; 0.24 in 2009 and 0.19 in 2010) (Table 3). Fledgling production was lower in 2010 than in 2009 for both treatments.
Contrary to what was predicted, nesting success increased slightly with an increase in nest monitoring frequency. Nests from control I had higher nesting success (16% in 2009 and 14% in 2010) than nests from control II (12% in 2009 and 9% in 2010) (Table 3). Nesting success was lower in 2010 than in 2009 for both treatments.
Discussion
Clutch manipulation efficiency
Our results clearly showed that clutch manipulation is an efficient method of increasing the hatching rate of Lesser Elaenia. Hatching rates are usually low in wild nests of tropical birds (Skutch Reference Skutch1966). A pattern of high hatching rates of artificially incubated eggs has also been obtained in other species such as Oma’o Myadestes obscurus (93%) and Palila Loxioides bailleui (96%) (Kuehler et al. Reference Kuehler, Lieberman, McIlraith, Everett, Scott, Morrison and Winchell1993, Reference Kuehler, Kuhn, Kuhn, Lieberman, Harvey and Rideout1996, Reference Kuehler, Lieberman, Oesterie, Powers, Kuhn, Kuhn, Neslon, Snetsinger, Herrmann, Harrity, Tweed, Fancy, Woodworth and Telfer2000, Reference Kuehler, Lieberman, Harrity, Kuhn, Kuhn, McIlraith and Turner2001).
Although expected and desired, a hatching rate of 100% will seldom be obtained by artificial incubation due to several factors. Eggs might be naturally infertile, and embryos might not develop due to pre-incubation problems (parental genetics, nutrition, maternal age, and environmental conditions; French and Tullett Reference French, Tullett and Tullett1991, Christensen Reference Christensen2001). Failure might also be related to inappropriate incubation conditions (temperature, humidity, oxygen and turning of the eggs; Tullet Reference Tullet1990, Meijerhof Reference Meijerhof1992), and accidental loss due to egg/nestling manipulation (Graber Reference Graber1955, Snelling Reference Snelling1972, Klimstra et al. Reference Klimstra, Stebbins, Heinza, Hoffmana and Kondradc2009).
Estimates of nest survival are an efficient way to evaluate strategies for conservation and management of bird populations (Jehle et al. Reference Jehle, Adams, Savidge and Skagen2004). DSRs of nestlings of control or manipulated nests were relatively similar and suffered small variations between years and treatments, probably due to uncontrolled factors such as predation risk and nest attendance by parents (Skutch Reference Skutch1949), or characteristics of the nesting site (Fontaine et al. Reference Fontaine, Martel, Markland, Niklison, Decker and Martin2007). Thus, we did not find any negative effect due to the presence of observers during nest manipulation. Although DSRs did not vary much among treatments, our results clearly showed that clutch manipulation increased fledgling production. However, fledgling production varied considerably between 2009 and 2010, but was always lower for control I and higher for manipulated nests with adoptive parents (Table 3). These variations between years due to natural environmental factors may cause unpredictability in results or expectations of conservation programmes. The fact that breeding parameters were better with adoptive parents highlights the importance of having a large number of non-manipulated nests available to attain more positive results with this management strategy.
Similar to hatching rate and fledgling production, apparent nest success was lowest for control I nests, and highest for manipulated nests with adoptive parents. Consistent with other breeding parameters, variations between 2009 and 2010 were as high as variations among treatments and limit comparisons with other species. Despite this high variation between years, the success of manipulated nests with adoptive parents was usually much higher than the success of other passerines nesting in the same study area: Suiriri Flycatcher Suiriri suiriri, 19% and Chapada Flycatcher, 14% (Lopes and Marini Reference Lopes and Marini2005); Plain-crested Elaenia Elaenia cristata, 27% (Marini et al. 2009b); and White-banded Tanager, 29% (Duca and Marini Reference Duca and Marini2011). This comparison with other species shows the potential use of clutch manipulation of these species with low breeding success or in species with declining populations, such as Chapada Flycatcher (França and Marini Reference França and Marini2010).
Management considerations
The major advance of our study in relation to others is the demonstration that it is not always necessary to raise nestlings or fledglings in captivity, and thus incur additional costs with fledgling/young reintroduction programmes. By maintaining the female incubating artificial eggs, we were able to return nestlings to a nest soon after hatching, decreasing costs of the management programme and maintaining all the behavioural benefits of parental care. Overall, our results clearly showed that any amount of clutch manipulation had a positive effect on several breeding parameters of Lesser Elaenia. Better results were usually obtained when adoptive parents were used instead of not returning (sacrificing) nestlings without nests. The use of adoptive parents, however, depends on finding a large number of extra non-manipulated nests. This was possible for the Lesser Elaenia since this species is very abundant at our study site, and a large (∼ 8–12 people) field team found 100–300 active nests of this species each year from 2005 to 2010 (Marini unpubl. data). The use of this methodology is limited to species with a population size large enough to have nests that can be used by adoptive parents. Thus, the use of adoptive parents, even though desirable, might not be feasible in species with low population densities or whose nests are difficult to find.
Three parents rejected nestlings reintroduced into their nests. The rejection of these nestlings was probably related to low body condition, a quality apparently assessed by parents. One of these nestlings was not able to completely leave the eggshell, and the others took too long to hatch and appeared to be weak. Also, selective rejection seems the case, since the same parents that rejected one of these nestlings accepted another nestling later.
The use of clutch manipulation may not be a panacea for all species, since some are intolerant of human presence at their nesting site (Gotmark Reference Gotmark and Power1992, Carney and Sydeman Reference Carney and Sydeman1999). Lesser Elaenia accepted artificial eggs for incubation, accepted most reintroduced and introduced nestlings and did not abandon nests because of daily nest monitoring. Some authors have reported higher predation rates on nests frequently monitored (Major Reference Major1989, Martin Reference Martin1993). Our tests indicate that nest monitoring frequency had no negative effect on Lesser Elaenia breeding parameters, as also reported by Cotter and Gratto (Reference Cotter and Gratto1995), Verboven et al. Dechesne (Reference Verboven, Ens and Dechesne2001), and Bolduc and Guillemete (Reference Bolduc and Guillemette2003). Contrary to expected, nests monitored daily (control I) tended to have a higher hatching rate, higher DSR, and higher Mayfield nesting success than nests monitored every 3–4 days. The higher DSR and nestling success might be explained by avoidance of humans by predators.
Several other methodologies were not used in this study, but could have improved management success. For example, playback of vocalisations of parents and siblings during the last days of artificial incubation can stimulate nestlings to break and leave the egg (Kuehler et al. Reference Kuehler, Lieberman, Oesterie, Powers, Kuhn, Kuhn, Neslon, Snetsinger, Herrmann, Harrity, Tweed, Fancy, Woodworth and Telfer2000). Eggs accidentally broken or cracked during manipulation can be repaired with white glue when egg contents are not lost (Mace Reference Mace1987). Lastly, one day before hatching, eggs could have been transferred to a hatcher, a place with better temperature and humidity for a newly-hatched chick than an incubator (Burnham Reference Burnham1983, Klimstra et al. Reference Klimstra, Stebbins, Heinza, Hoffmana and Kondradc2009).
The long dry season of 2010 may also explain some clutch manipulation differences between years. For example, a higher percentage of eggs did not hatch in 2010 than in 2009, most of them transferred to the incubator before the start of the rains. Also, a higher percentage of unhatched eggs were observed in 2010 than in 2009. Embryo death in mid- or late development may be related to low female investment in egg production (Martin Reference Martin1987, French and Tullett Reference French, Tullett and Tullett1991).
Management costs
The methodology of replacing natural eggs with artificial eggs and the use of adoptive parents was financially the most costly. Thus, this methodology is not the most appropriate for management programmes with limited resources. For these programmes, egg replacement without adoptive parents produces the least costly fledglings.
The costs and benefits of producing an extra chick by this methodology might decrease with larger field teams and artificial incubation facilities closer to or at the field sites. Thus, hiring more people to search for nests might increase the efficiency of the methodology and decrease costs, since several other costs are fixed and not related to the number of people. Fuel costs might be decreased if the incubation facilities are at or closer to the field site.
Our methodology integrating in situ and ex situ management might have lower costs than other management methodologies since the immediate return of newly-hatched nestlings to the nest does not require the high additional costs of captive rearing and reintroduction programmes. Two decades ago, it was estimated that the cost of captive breeding programmes of endangered species may already involve around half a million dollars per year per species (Derrickson and Snyder Reference Derrickson, Snyder, Beissinger and Snyder1992). Similar studies using artificial incubation of wild bird eggs have achieved positive results, but at a higher cost compared with this study, as they require the reintroduction of young full-grown birds to their original habitats (Kuehler et al. Reference Kuehler, Kuhn, Kuhn, Lieberman, Harvey and Rideout1996, Reference Kuehler, Lieberman, Oesterie, Powers, Kuhn, Kuhn, Neslon, Snetsinger, Herrmann, Harrity, Tweed, Fancy, Woodworth and Telfer2000, Reference Kuehler, Lieberman, Harrity, Kuhn, Kuhn, McIlraith and Turner2001). Besides the expense, the re-introduction of birds might not be effective and even cause impacts if there is no appropriate planning and nest monitoring after release (review in Marini and Marinho-Filho Reference Marini, Marinho-Filho, Rocha, Bergallo, Sluys and Alves2005). Even well-planned reintroduction programmes might not achieve success, as happened with Spix’s Macaw Cyanopsitta spixii (Bampi and Da-Ré Reference Bampi and Da-Ré1994).
Acknowledgements
This study was partly funded by a research grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq - Brazil and grants from Fundação de Apoio à Pesquisa do Distrito Federal (FAP-DF). We thank CNPq for a researcher fellowship to MÂM and for scholarship to YL. We are thankful to ESECAE’s administration for permission to work in the area, and to IBAMA for authorisations (licences no. 15968-2 and no. 25699-1 - SISBIO). We thank all students from Laboratório de Ecologia e Conservação de Aves at Universidade de Brasília for field support.





