Introduction
The introduction of exotic animals is one of the most important factors contributing to the reduction in biological diversity and extinctions on oceanic islands (Coblentz Reference Coblentz1990, Dobson and Foufopoulus Reference Dobson and Foufopoulus2001, Steadman Reference Steadman2006). Exotic animals can damage native species both directly and indirectly through mechanisms such as predation, the introduction of disease and interspecific competition (Eguchi and Amano Reference Eguchi and Amano2004, Gurnell et al. Reference Gurnell, Wauters, Lurz and Tosi2004, Smith and Carpenter Reference Smith and Carpenter2006, Kurle et al. Reference Kurle, Croll and Tershy2008). In particular, exotic rat species, including Brown Rats Rattus norvegicus, Ship Rats R. rattus and Pacific Rats R. exulans, have now reached more than 80% of the world's oceanic islands and island groups (Atkinson Reference Atkinson and Moors1985). Major declines or extinctions of endemic forest birds followed the invasion and spread of Ship Rats on Lord Howe Island and the Hawaiian Islands (Hindwood Reference Hindwood1940, Recher and Clark Reference Recher and Clark1974, Atkinson Reference Atkinson1977). Campbell (Reference Campbell1991) suggested that the abundance of introduced Ship Rats might adversely affect the diversity of avian communities on seven islands of the Antillean Cays. Predation by Rattus spp. is considered a major cause of avian extinction in insular habitats (King Reference King and Moors1985). However, little is known about factors related to rats other than predation, which are associated with the reduction in the bird diversity of islands. Introduced Ship Rats flexibly used burrows, nest boxes or tree holes to rest or breed in a wide range of habitats, such as coastal areas, cultivated land and forests (Barba and Gil-Delgado Reference Barba and Gil-Delgado1990, Tobin et al. Reference Tobin, Sugihara, Koehler and Ueunten1996, Pye et al. Reference Pye, Swain and Seppelt1999, Lambrechts et al. Reference Lambrechts, Bourgault, Mennerat, Galan, Cartan-Son, Perret, Doutrelant and Charmantier2007, Whisson et al. Reference Whisson, Quinn and Collins2007). Accordingly, introduced Ship Rats have the potential to affect insular avian communities not only through predation but also through interspecific competition for nesting sites in various environments.
Ship Rats were considered to have arrived with building materials for a sugar plant on oceanic Minami-daito Island in the northwest Pacific in 1917 (Editorial Committee of the History of Minami-daito Village 1990). In this paper, we document the construction of arboreal dome-shaped nests in trees in hedgerows along sugarcane fields and the utilization of open-cup Bull-headed Shrike Lanius bucephalus nests for roosting or rearing the young by Ship Rats on Minami-daito Island. Nest-site characteristics of Ship Rats in the hedgerows raise the possibility of overlap with arboreal nesting bird species, i.e. the Brown-eared Bulbul Ixos amaurotis (synonym Hypsipetes amaurotis borodinonis; Ornithological Society of Japan 2000) and the Bull-headed Shrike. We therefore investigated the overlap of nest-site characteristics among these three species in an agricultural habitat. We also quantified the frequency with which rats remodelled the open-cup nests of bulbuls and shrikes.
Methods
The study was conducted on Minami-daito Island (25°50′N, 131°14′E, 30.57 km2 in area) between February and August in each of the years 2002–2006. Minami-daito is an oceanic island located in the northwest Pacific c. 360 km east of Okinawa Island. Most forest cover was removed after humans settled there in 1900, and agricultural fields, mainly sugarcane Saccharum officinarum, now cover c. 60% of the island. Hedgerows composed of Alexandrian Laurel Calophyllum inophyllum and Garcinia Garcinia subelliptica trees function as windbreaks in the fields. The agricultural landscape is homogeneous due to the limited range of vegetation and its simple structure.
The Brown-eared Bulbul of the Daito Islands is an endemic subspecies (Ornithological Society of Japan 2000), and there have been naturally established populations of Bull-headed Shrikes on Minami- and Kita-daito islands since the 1970s (Takagi Reference Takagi2000, Matsui et al. Reference Matsui, Hisaka and Takagi2006). To describe the arboreal nesting and utilization of open-cup bird nests by Ship Rats, we searched for and observed nests of Ship Rats, Brown-eared Bulbuls and Bull-headed Shrikes in the hedgerows along the sugarcane fields. Since we have not observed them re-use previously constructed nests, we considered that Brown-eared Bulbuls and Bull-headed Shrikes build new nests at each breeding attempt, like other open-nesting passerines (see Friesen et al. Reference Friesen, Wyatt and Cadman1999; Marshall et al. Reference Marshall, Glover, Buechi and VanDruff2001). To quantify the frequency with which rats remodelled bird nests, between 4 and 16 August 2005, we revisited all bulbul and shrike nests that we had found in 2005 and checked whether these nests had been remodelled by Ship Rats. Shrike nests were monitored every 3–6 days until the nest fledged or failed. We measured nests to compare their shapes among Ship Rats, Brown-eared Bulbuls and Bull-headed Shrikes. Measurements included nesting height (from ground to nest base), nest cup width and length (wall-to-wall inside the oval nest), cup depth (from rim level to the bottom of the cup), nest width and length (wall-to-wall outside the oval nest), exterior nest height (from nest base to cup lip) and bottom thickness (exterior nest height minus cup depth). For dome-shaped nests with an entrance at the side, we also measured the entrance width and length. We recorded nest tree species and measured the height and diameter at breast height (dbh) of the nest tree. All data are expressed as mean ±SD (n) unless noted otherwise.
Results
Arboreal nesting of Ship Rats
We observed three breeding attempts of Ship Rats on trees in hedgerows along the sugarcane fields in 2002 and 2005. The first breeding attempt was made in a dome-shaped nest on a Garcinia tree in a hedgerow along the sugarcane field. One adult rat and more than two naked rat pups emerged from the nest on 9 May 2002. It was inferred that the rats had constructed the nest because we had never seen such a dome-shaped bird nest on Minami-daito Island.
The second breeding attempt was made in a remodelled Bull-headed Shrike nest. One adult rat and two young rats emerged from the nest (nest tree species Casuarina equisetifolia; nesting height, 406 cm; nest tree height, 1,255 cm; dbh, 39 cm) on 8 August 2005. The first shrike egg was laid in the nest in early March 2005, and all the eggs were depredated during the incubation period. When we found the three rats on 8 August 2005, the nest had been repositioned on its side and the materials of the nest-cup had been brought out of the nest and used to elongate the entrance. In addition, the opening of the nest-cup was narrowed and shaded with dead horsetail leaves. The nest was dome-shaped.
The third probable breeding attempt was also made in a remodelled Bull-headed Shrike nest. Two adult rats emerged from a used Bull-headed Shrike nest (nest tree species, Alexandrian Laurel; nesting height, 328 cm; nest tree height, 824 cm; DBH, 27 cm) on 3 June 2005. The first shrike egg was laid in the nest on 11 February 2005, and the nestlings were depredated by a Japanese Weasel Mustela itatsi in late March. An adult rat was found in the nest when we inspected the nest on 4 July. The nest was dome-shaped with an entrance in the side. Chips (1–2 cm) of green leaves of Alexandrian Laurel were found in the nest cup.
Nest usurpation by Ship Rats
We found that rats usurped shrike nests without signs of remodelling. We observed rats sitting in two shrike nests during the pre-laying period in 2006, and the shrikes subsequently abandoned these nests, but the two nests had not been remodelled when we re-checked the nests on 10 and 11 July 2006, respectively.
Remodelling of open-cup bird nests by Ship Rats
We found 8 rat nests, 11 bulbul nests and 193 shrike nests in 2005 (Figure 1a–c). We examined whether 10 bulbul nests and 128 shrike nests were remodelled by Ship Rats. No bulbul nests (n = 10) and 22.7% (29/128) of the shrike nests were remodelled. The shapes of the 29 remodelled shrike nests were classified into the following three types: (1) materials brought out of the nest-cup (Figure 1d, 51.7%, n = 15); (2) rotated to a more horizontal orientation (Figure 1e, 10.3%, n = 3); (3) remodelled into a dome shape (Figure 1f, 37.9%, n = 11).
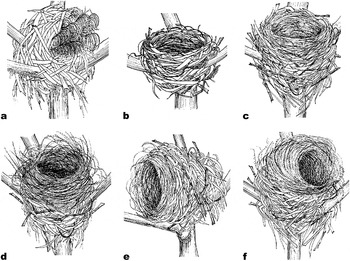
Figure 1. (a) Ship Rat nest. (b) Brown-eared Bulbul nest. (c) Bull-headed Shrike nest. (d) Shrike nest reshaped by removal of materials from nest-cup. (e) Repositioned shrike nest. (f) Shrike nest remodelled into a dome shape.
Out of the 29 remodelled shrike nests, four (13.8%) were remodelled after breeding failure unrelated to Ship Rats, and three (10.3%) were remodelled after the shrike nestlings had fledged. We found pieces of eggshells in two remodelled shrike nests (6.9%), and inferred that Ship Rats had remodelled the nests after egg depredation. We could not determine the breeding stages in which 20 nests (69.0%) were remodelled.
Nest shape and materials
Ship Rats built dome-shape nests (Figure 1a) and Brown-eared Bulbuls and Bull-headed Shrikes constructed open-cup nests on branches (Figure 1b,1c). We investigated the nest materials in two rat nests. Rat nests were composed of only Poaceae plant leaves that were more than 1 cm wide (e.g. sugarcane or Chinese Silvergrass Miscanthus sinensis). Entrances to the dome-shaped nests were concealed by c. 4–8 ears of Chinese Silvergrass (Figure 1a). Shrike nests that had been remodelled into dome shapes were significantly larger than non-remodelled shrike nests (Table 1). The shapes of bulbul and shrike nests were very similar to each other (Figure 1b, c), but the bottom of shrike nests was significantly thicker than that of bulbul nests (Table 1). The mean weights of bulbul and shrike nests were 19.9 ± 2.9 g (n = 3) and 22.8 ± 4.3 g (n = 3), respectively. We could separate nest materials of bulbuls and shrikes into nest-cup and other materials. The weight of the nest-cup material of shrikes (7.4 ± 0.5 g) was approximately two times that of the bulbul nest-cup material (3.6 ± 1.2 g); the weights of the other materials were similar for bulbuls (16.3 ± 3.3 g) and shrikes (15.3 ± 4.1 g).
Table 1. Dimensions of (A) Ship Rat nests, (B) Bull-headed Shrike nests remodelled by ship rats into a dome shape, (C) non-remodelled Bull-headed Shrike nests and (D) Brown-eared Bulbul nests in 2005.
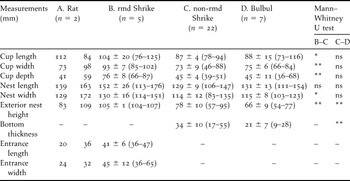
ns P > 0.05, *P < 0.05, **P < 0.01, A measured values; B-D mean ± SD (max–min).
Overlap of nest-site characteristics
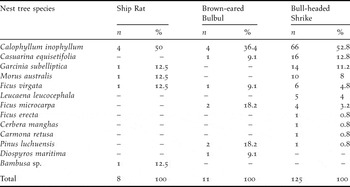
The nesting tree species are shown in Table 2. Out of the five nesting tree species of Ship Rats, 40% and 80% of tree species were used by Brown-eared Bulbuls and Bull-headed Shrikes for nesting, respectively. Nesting height from the ground to the base of the nest differed significantly among rat nests (309 ± 87 cm, range 222–475 cm, n = 8), bulbul nests (493 ± 187 cm, range 221–824 cm, n = 10), remodelled shrike nests (295 ± 154 cm, range 111–641 cm, n = 28) and non-remodelled shrike nests (319 ± 155 cm, range 129–792 cm, n = 97) (Kruskal–Wallis test, H = 9.93, df = 3, P = 0.019). In contrast, nesting height did not differ significantly between bulbuls and rats (Mann–Whitney test, U = 14.0, Z = −2.31, P = 0.021, but P > 0.05 with sequential Bonferroni correction according to Rice Reference Rice1989), bulbul nests were significantly higher than remodelled shrike nests (U = 53.0, Z = −2.88, P = 0.003, P < 0.05 with Bonferroni correction) and non-remodelled shrike nests (U = 216.5, Z = −2.87, P = 0.004, P < 0.05 with Bonferroni correction). The height of the nesting tree did not vary significantly among rat nests (649 ± 195 cm, range 389–923 cm, n = 8), bulbul nests (810 ± 263 cm, range 438–1,311 cm, n = 11), remodelled shrike nests (606 ± 281 cm, range 255–1,255 cm, n = 28) and non-remodelled shrike nests (678 ± 310 cm, range 226–1,838 cm, n = 97) (Kruskal–Wallis test, H = 4.65, df = 3, P = 0.199). DBH also did not vary significantly among rat nests (15 ± 10 cm, range 6–34 cm, n = 8), bulbul nests (20 ± 9 cm, range 5–7 cm, n = 11), remodelled shrike nests (16 ± 12 cm, range 3–47 cm, n = 28) and non-remodelled shrike nests (19 ± 14 cm, range 1–93 cm, n = 97) (Kruskal–Wallis test, H = 2.52, df = 3, P = 0.471).
Table 2. Nest tree species of Ship Rats, Brown-eared Bulbuls, and Bull-headed Shrikes on Minami-daito Island in 2005.
Discussion
On Minami-daito Island, Ship Rats constructed dome-shaped nests in trees in hedgerows along sugarcane fields and utilized open-cup bird nests to breed. Arboreal dome-shaped nests, as well as tree-cavity nests of Ship Rats, were also observed in Mauritius (L. Woolaver pers. comm.). Ship Rats have been recorded using nest boxes filled with leaves, mainly to rest but occasionally to breed, in eastern Spain (Barba and Gil-Delgado Reference Barba and Gil-Delgado1990). Lambrechts et al. (Reference Lambrechts, Bourgault, Mennerat, Galan, Cartan-Son, Perret, Doutrelant and Charmantier2007) observed Ship Rats building a nest on top of unfinished nests of Blue Tits Parus caeruleus and Great Tits P. major in nest boxes. However, we could not find records showing that Ship Rats bred in remodelled open-cup bird nests. In contrast, the construction of arboreal nests by Ship Rats is considered to be common, but nest sites of Ship Rats for breeding have not been sufficiently described in the wild.
Introduced Ship Rats have the ability to use a wide range of spaces, including the ground and trees, in different habitat types, e.g. forest, grasslands and orchards (Dowding and Murphy Reference Dowding and Murphy1994, Hooker and Innes Reference Hooker and Innes1995, Tobin et al. Reference Tobin, Sugihara, Koehler and Ueunten1996, Pye et al. Reference Pye, Swain and Seppelt1999, Whisson et al. Reference Whisson, Quinn and Collins2007). Radio-tracked Ship Rats were mostly arboreal in tall (up to 30 m) forests in New Zealand (Hooker and Innes Reference Hooker and Innes1995). Whisson et al. (Reference Whisson, Quinn and Collins2007) observed Ship Rats using tree hollows at heights of 2 m or more as daytime dens in an old-growth riparian forest in California. However, in the tall-tussock grassland on Macquarie Island, Ship Rats excavated burrows and constructed nesting chambers at the base of tussock shoots (Pye et al. Reference Pye, Swain and Seppelt1999). In a Hawaiian Macadamia Macadamia integrifolia orchard, cultivated on a lava rock substrate with subterranean cracks and crevices, most Ship Rats remained in burrows during the day and ascended into the canopies of the macadamia trees during the night to feed on nuts (Tobin et al. Reference Tobin, Sugihara, Koehler and Ueunten1996). Arboreal open-cup bird nests would be valuable to Ship Rats for resting or nesting in the agricultural landscape of Minami-daito Island, because Alexandrian Laurel and Garcinia trees without cavities are dominant in the hedgerows, and because potential predators of rats - introduced Japanese Weasels and feral cats Felis catus - search for food on the ground.
There are several possible structural or ecological reasons why Ship Rats use shrike nests to breed: (1) shrike nests have more structural stability than rat nests; (2) shrike nests are of a suitable size for rats; and (3) Ship Rats and Bull-headed Shrikes overlap considerably in nesting height preference. We did not observe any bulbul nests remodelled by Ship Rats. It might be difficult for rats to enlarge bulbul nests because only a few dead coniferous leaves were found in the nest-cups (Figure 1b), and the bottoms of bulbul nests were significantly thinner than those of shrike nests (Table 2). Moreover, there would be little overlap of nest-site characteristics between bulbuls and rats because nesting height of bulbuls was somewhat higher than that of rats; the difference was not significant, however, perhaps because of the small sample size.
The overlap of nesting height between Ship Rats and Bull-headed Shrikes was larger than between the rats and Brown-eared Bulbuls. This similarity of nest sites between rats and shrikes is inferred to reflect the overlapping of their ecological niches. The overlap of nesting sites would have the following negative effects on shrikes: (1) the risk of nest predation by Ship Rats would increase, which is supported by the evidence that Ship Rats caused at least 70% of egg predation and 20% of nestling predation of Bull-headed Shrikes on Minami-daito Island (S.M. and M.T., unpublished data); (2) the risk of nest usurpation by Ship Rats would increase, because several researchers have shown that Ship Rats use between two and nine dens during the daytime and some individuals change den sites frequently (Dowding and Murphy Reference Dowding and Murphy1994, Whisson et al. Reference Whisson, Quinn and Collins2007); and (3) competition for food resources between Ship Rats and Bull-headed Shrikes, both of which feed on invertebrates, e.g. grasshoppers, locusts, cockroaches and spiders, would be intensified (Innes Reference Innes2001).
Introduced species may reduce the number of native birds through interspecific competition for nest sites (Kerpez and Smith Reference Kerpez and Smith1990, Ingold Reference Ingold1994). Birds and mammals seeking nest sites interact through exploitation competition and kleptoparasitism (Sarà et al. Reference Sarà, Milazzo, Falletta and Bellia2005, Lambrechts et al. Reference Lambrechts, Bourgault, Mennerat, Galan, Cartan-Son, Perret, Doutrelant and Charmantier2007, Kappes and Davis Reference Kappes and Davis2008). An example of the effect of exploitation competition is the occupation of nest boxes by Ship Rats, which decreased the use of boxes by Great Tits (Barba and Gil-Delgado Reference Barba and Gil-Delgado1990, Lambrechts et al. Reference Lambrechts, Bourgault, Mennerat, Galan, Cartan-Son, Perret, Doutrelant and Charmantier2007). Kappes (Reference Kappes1997) argues that heterospecific usurpation of cavities excavated by woodpeckers is appropriately described as cavity kleptoparasitism, because the interaction is negative for woodpeckers and beneficial for cavity usurpers. In North America, Southern Flying-squirrels Glaucomys volans are seen as major predators or cavity kleptoparasites of nesting Red-cockaded Woodpeckers Picoides borealis (Ligon Reference Ligon1970, Rudolph et al. Reference Rudolph, Conner and Turner1990, Kappes and Davis Reference Kappes and Davis2008). We found that temporal nest utilization by Ship Rats forced Bull-headed Shrikes to abandon their nests before egg-laying. We suggest that kleptoparasitism applies to the interaction between Bull-headed Shrikes and Ship Rats, which usurp their arboreal open-cup nests, because the interaction is negative for Bull-headed Shrikes and beneficial for Ship Rats. Invasive Common Starling Sturnus vulgaris usurped the nesting cavities of native birds and reduced their fecundity (Ingold Reference Ingold1989, Reference Ingold1994). Nest usurpation by Ship Rats, as well as by introduced Common Starlings, has the potential to reduce the fitness of shrikes by increasing the cost of re-nesting, delaying breeding and decreasing the number of broods reared. With regard to the conservation of birds on an island with Ship Rats, which are able to use burrows, nest boxes, tree holes and open-cup bird nests to rest or breed in a wide range of habitats, it is necessary to consider the potential effects of introduced rats on avian communities not only through predation but also through nest-site competition and nest kleptoparasitism.
Acknowledgements
We are deeply indebted to Kiyoshi Asanuma, Kazuaki Higashi, Mitsunori Okuyama and members of the board of education of Minami-daito village for kindly supporting our fieldwork. We thank Kana Akatani for making the sketch of rat and bird nests in Figure 1. We are grateful to Kazuhiro Eguchi and Naoki Tomita for valuable comments on the drafts of the manuscript. We appreciate the improvements in English usage made by Caitlin Stern through the Association of Field Ornithologists’ program of editorial assistance. This manuscript was greatly enhanced by constructive comments from Lance Woolaver and an anonymous reviewer.





