Introduction
Kush was one of several early states that formed along the Middle Nile, flourishing for centuries until its fall towards the end of the Meroitic period (c. 300 BC–AD 350; Török Reference Török1997; Edwards Reference Edwards1998). Archaeological evidence has been used to delimit the extent of the Meroitic kingdom, extending south of the First Nile Cataract (southern Egypt) and encompassing the confluences of the White Nile, Blue Nile and the Atbara River in Sudan (Figure 1; Eisa Reference Eisa1999; Edwards Reference Edwards2004). While the actual boundaries of the kingdom remain a matter of speculation (Lohwasser Reference Lohwasser, Anderson and Welsby2014), however, its focus was the Nile and its tributaries on which its population was dependent. Agricultural advances—including the adoption of the animal-powered waterwheel (saqia) and the development of a system of water dams and reservoirs (hafirs)—facilitated the expansion of plant cultivation, including the use of fields located further from the Nile and large-scale livestock farming (Ehret Reference Ehret and Adas2001; Fuller Reference Fuller, Stevens, Nixon, Murray and Fuller2014), pushing settlement into new geographical and environmental zones (Edwards Reference Edwards1996, Reference Edwards2004; Edwards & Fuller Reference Edwards and Fuller2005).

Figure 1. Map of Sudan showing approximate northern and southern limits of the Kushite kingdom and sites mentioned in the text (figure by authors).
Factors such as geo-political and economic instability and social unrest have long been considered as prime contributors to the collapse of the Meroitic kingdom in the mid-fourth century AD (Török Reference Török1988). The extent to which external and/or internal factors contributed to the state's disintegration remains the subject of debate and warrants further research, including assessment of the impact of changing regional climate and environment (Fuller Reference Fuller, Stevens, Nixon, Murray and Fuller2014, Reference Fuller and Zach2015). Over millennia in the Nile Valley, climate and environmental changes have influenced patterns of human settlement, mobility and the development of complex cultures and technologies that aided adaptation and sustainability (Nicoll Reference Nicoll2004; Butzer Reference Butzer2012; Honegger & Williams Reference Honegger and Williams2015). Episodes of climate variability and environmental shifts have been linked to episodes of severe drought, famine and state collapse (Touzeau et al. Reference Touzeau2013, Reference Touzeau, Lecuyer and Amiot2017; Welc & Marks Reference Welc and Marks2014; Manning et al. Reference Manning, Ludlow, Stime, Boos, Sigle and Marlon2017).
Stable isotope analysis of bioarchaeological remains can facilitate the identification of environmental and climate change. To date, only a few such studies have provided insights into the environmental conditions during the Meroitic (c. 300 BC–AD 350) and post-Meroitic periods (AD 350–550; e.g. Iacumin et al. Reference Iacumin, Bocherens, Mariotti and Longinelli1996, Reference Iacumin, Matteo, Usai, Salvatori and Venturelli2016; White et al. Reference White, Longstaffe and Law2004). With the aim of determining environmental conditions and their potential contribution to the decline and collapse of the Meroitic state, this article presents new carbon and oxygen isotope data from human and faunal remains from multiple sites in the Middle Nile Valley.
Available proxy records indicate a wetter climate in this region during the first millennium BC and a shift towards increasingly drier conditions thereafter (Machado et al. Reference Machado, Pérez-González and Benito1998; Williams Reference Williams2009: 10–11). We hypothesise that this latter climatic shift should be reflected in higher carbon and oxygen isotope values in the late Meroitic and post-Meroitic samples caused by an increase in the consumption of C4 plants, which are better adapted to drier conditions (δ13C), and a decrease in the amount of precipitation and/or an increase in regional temperatures (δ18O).
Stable carbon and oxygen isotope analysis in the Nile valley
Stable carbon and oxygen isotope analysis is commonly used for the investigation of mobility, diet and climate change in the Nile Valley (Iacumin et al. Reference Iacumin, Bocherens, Mariotti and Longinelli1996, Reference Iacumin, Matteo, Usai, Salvatori and Venturelli2016; Dupras & Schwarcz Reference Dupras and Schwarz2001; White et al. Reference White, Longstaffe and Law2004; Buzon & Bowen Reference Buzon and Bowen2010). The analysis relies on the isotopic signatures recorded in human and animal tissues, such as bone and teeth, which may reflect the isotopic characteristics of the water and the types of plants directly or indirectly consumed, thus providing data about prevailing environmental conditions (Iacumin et al. Reference Iacumin, Matteo, Usai, Salvatori and Venturelli2016: 500).
Carbon isotope (δ13C) ratios of tooth enamel carbonate reflect the isotopic composition of the dietary carbon sources consumed by an individual during childhood when teeth are forming. Carbon is assimilated by plants through one of three photosynthetic pathways—C3, C4 and CAM (Crassulacean Acid Metabolism)—resulting in distinct δ13C values for each group of plants typically endemic to different types of environments (Bender Reference Bender1971; Smith & Epstein Reference Smith and Epstein1971). For example, plants from temperate regions that follow the C3 photosynthetic pathway—wheat, barley, most fruits and vegetables—show values of approximately –28‰ to –26‰ (VPDB, Vienna Pee Dee Belemnite international standard for carbon isotopes). C4 plants, typically endemic to hot and arid environments (e.g. sorghum and millet), demonstrate higher values of approximately –14‰ to –12‰ (VPDB). Archaeological evidence indicates that the ancient dietary regime in the Nile Valley was a mix of C3 and C4 sources, with the former being dominant. Sorghum, a C4 plant found sporadically in the third-millennium BC deposits (Brass et al. Reference Brass2019), was widely incorporated into diet by the Meroitic period (Rowley-Conwy Reference Rowley-Conwy1989). The significant increase in the consumption of C4 plants previously observed during the post-Meroitic period (White & Schwarcz Reference White and Schwarcz1994; Fuller & Lucas Reference Fuller, Lucas, Emberling and Williams2021) could reflect changes in the types of cultivated crops dictated by increasing aridity. Previous stable isotope analysis has identified periods where an increase in the consumption of C4 plants corresponds with low Nile water levels and episodes of social unrest (e.g. Iacumin et al. Reference Iacumin, Bocherens, Chaix and Mariotti1998; Thompson et al. Reference Thompson, Richards, Shortland and Zakrzewski2005, Reference Thompson, Chaix and Richards2008; Turner et al. Reference Turner, Edwards, Quinn, Kingston and Van Gerven2007; Touzeau et al. Reference Touzeau2013). For example, Iacumin et al. (Reference Iacumin, Matteo, Usai, Salvatori and Venturelli2016) found changes in subsistence strategies in the Middle Nile Valley dictated by changing environmental conditions between the Pre-Mesolithic (>7000 BC) and Meroitic period (fourth century BC–fourth century AD).
Oxygen isotopes are available to humans and animals from a variety of sources, including the atmosphere, food and drinking water, with the latter being the most significant contributor to oxygen intake (Luz et al. Reference Luz, Kolodny and Horovitz1984). For communities settled along the Nile valley, including all the individuals discussed below, the primary source of water for consumption and irrigation was the river (rather than ground water accessed via wells); the local δ18O values of the river water should therefore be reflected in the oxygen isotope ratios in the mineralised tissues of humans and animals living in the region (White et al. Reference White, Longstaffe and Law2004). Oxygen isotope values in water vary regionally due to a combination of factors—hydrological, geographical and climatological (Gat Reference Gat1996)—which can therefore be used as a proxy for palaeoclimatic conditions and mobility (e.g. Buzon & Bowen Reference Buzon and Bowen2010). In surface water, such as the Nile, these values vary directly with temperature fluctuation and the effects of precipitation and evaporation, providing a reliable record of local climate. Temporal variation of regional δ18O values may represent a shift in climatic conditions, as demonstrated by Touzeau et al. (Reference Touzeau2013, Reference Touzeau, Lecuyer and Amiot2017), with the values increasing as one moves north along the Nile due to the preferential loss of 16O in river water caused by evaporation.
Modern δ18O values of Nile River water vary between –5.7‰ in rainy season flows in the Blue Nile and +2‰ to +4‰ (VSMOW, Vienna Standard Mean Ocean Water) at the Nile delta (Buzon & Bowen Reference Buzon and Bowen2010). Precipitation in the areas north of 18°N (Nubia) is negligible but increases gradually to the south; in Khartoum (15°N), modern records (1961–2009) indicate average annual rainfall of 155.7±69.6mm during the summer months (June to October), with rainwater averaging annual δ18O values of –0.93‰ (IAEA/WMO n.d).
Materials and methods
This study reports isotopic analyses on 64 dental enamel samples collected from 56 human individuals recovered from 13 cemeteries (Figure 1 & Table 1), including 10 recently excavated sites. Eight Meroitic and post-Meroitic cemeteries—all near the Nile—are located between the Third and Fourth Cataracts and a further two are downstream from the Sixth Cataract. The main study assemblage (n=58) includes 11 early Meroitic (300 BC–AD 90), 10 Meroitic (300 BC–AD 350), 14 late Meroitic (AD 90–350) and 23 post-Meroitic (AD 350–500) samples; below, these are treated as four temporal subsets. For comparative purposes, we also analyse additional samples: two inhumations from Toshka associated with the Bronze Age C-Group culture (c. 2400–1550 BC) and one contemporaneous inhumation from Jebel Moya, plus a further three inhumation burials from medieval (AD 550–1500) and post-medieval (AD 1500–1900) contexts at Er-Roseire and Hamadab, respectively. In addition to human samples, isotope ratios in samples (n=15) of bovids, ovicaprids and one canid associated with the post-Meroitic burials at El-Zuma and El-Detti are also analysed, primarily for context (online supplementary material (OSM) Table S1).
Table 1. Summary of sources of human and faunal samples.

Nh – number of human samples; Na – number of faunal samples.
* Approximate location – submerged by Lake Nasser.
To avoid the effects caused by δ18O enrichment through breast-milk consumption (Roberts et al. Reference Roberts, Coward, Ewing, Savage, Cole and Lucas1988), enamel samples were collected primarily from post-weaning teeth (M2 and M3), with canines and premolars as substitutes where necessary; in the latter case, samples were taken from the lower parts of the crown that form after weaning at approximately five years and over.
Powdered samples were flushed with helium (He), then five drops of water-free orthophosphoric acid (H3PO4) were added. Following reaction with H3PO4 and chromatographic isolation of carbon dioxide (CO2) on a Gasbench II automated preparation device, the carbon and oxygen composition of hydroxyapatite carbonate was analysed using a Thermo-Fisher MAT253 Isotope Ratio Mass Spectrometer. Both δ13C and δ18O values are reported relative to the VPDB standard (standard deviations of ≤0.05‰ and ≤0.02‰, respectively). The datasets were analysed using non-parametric tests with STATISTICA software package (StatSoft, USA version 13). The cultural chronologies of the 11 cemeteries from which the samples were obtained are verified with radiocarbon dating (see Table S2).
Results
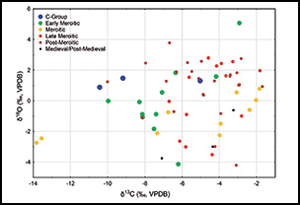
The δ13C and δ18O measurements (Table S1) are pooled according to discrete time periods (Table 2) to determine temporal trends in the isotopic values. Figure 2 shows the temporal distribution of the δ13C and δ18O values in human tooth enamel samples. When the outliers are removed (two individuals from El-Kurru with exceptionally low δ13C values and one individual from Tabo with the highest δ18O value), the differences in δ13C values between the four Meroitic subsets are statistically significant (Kruskal-Wallis test, N=55, H=15.10, p=0.0017) and the post-hoc pairwise comparison indicates a statistically significant difference between the early Meroitic (the lowest δ13C values) and the subsequent subsets (Figure 3).
Table 2. δ13C and δ18Oca (ca=tooth carbonate) values in human enamel carbonates and animal samples.


Figure 2. Scatter plot of δ13C and δ18Oca (ca=tooth carbonate) values for samples from the six temporal subsets (figure by authors).

Figure 3. Boxplot showing the temporal pattern in δ13C values (figure by authors).
The distributions of the δ18O values in the early Meroitic and Meroitic subsets roughly follow a normal distribution with the mode close to –1‰, but in the late Meroitic and post- Meroitic subsets it is left-skewed with the mode close to 2‰ (Figure 4). As the distributions of the δ18O values in the early Meroitic and Meroitic subsets are similar, these two subsets are combined. The temporal differences in the δ18O values between the three resulting periods—early Meroitic/Meroitic, late Meroitic and post-Meroitic—are statistically significant (Kruskal-Wallis test, N=55, H=8.24, p=0.016), mainly due to the difference between the Early Meroitic/Meroitic and post-Meroitic subsets (post-hoc pairwise comparison p=0.014). The difference in the δ18O values between the early Meroitic/Meroitic and late Meroitic subsets is not statistically significant (p=0.095) due to the small sample size; nevertheless, the distribution of the δ18O values in the late Meroitic subset follows the same pattern as in the post-Meroitic subset (Figure 4). The C-Group and medieval/post-medieval subsets are too small to be included in the statistical analysis; however, they demonstrate a pattern of continuity (particularly in the δ13C values) between the Bronze Age and the early Meroitic period, with a decrease in the δ18O values between the post-Meroitic and medieval/post-medieval periods (see Figure 2).

Figure 4. Gaussian kernel density distribution of δ18Oca (ca=tooth carbonate) in three temporal (figure by authors).
Due to the small sample size, the differences in the δ13C and δ18O values between males and females can only be tested for the combined late Meroitic and post-Meroitic subsets. The differences in the δ18O values of males and females is not significant (Mann Whitney U-test, Z=−1.15); however, the average δ13C value of females is significantly higher than in males (Mann Whitney U-test, n(f)=16, N(m)=24, Z=2.28, p=0.023) and the variability is lower in females (median –4.25‰, mean –4.23‰, SD=0.99‰) than in males (median –5.47‰, mean –5.45‰, SD=1.92‰).
Next, the post-Meroitic human dataset is compared with contemporaneous faunal data from bovids and ovicaprids (Figure 5). When one outlier (an ovicaprid) is excluded, the average δ13C and δ18O values are higher in animals than in humans. In bovids, two relatively distinct clusters can be observed: one overlapping the distribution of the δ13C and δ18O values in ovicaprids and one with distinctly higher δ13C and δ18O values. The difference in the δ13C values between humans and bovids is statistically significant (Kruskal-Wallis test, n=36, H=10.61, p=0.005; post-hoc pairwise comparison p=0.0034). In the δ18O values, humans differ significantly from both bovids and ovicaprids (Kruskal-Wallis test, n=36, H=20.17, p<0.0001; post-hoc pairwise comparisons p=0.00031 and p=0.0069, respectively).

Figure 5. Scatterplot of δ13C and δ18Oca (ca=tooth carbonate) values in humans, bovids and ovicaprids in the post-Meroitic period (figure by authors).
Discussion
The isotopic analysis of human enamel samples from sites in Upper Nubia (Third to Sixth Cataracts) reveals an upward shift in the δ13C values following the early Meroitic period. This result indicates a progressive dietary shift towards greater consumption of C4 plants (sorghum and millet) and reduced consumption of C3 crops (wheat and barley). These findings corroborate previous isotopic studies conducted on human hair samples from Lower Nubia (First to Second Cataract) that found a marked shift towards a greater consumption of C4 plants and agricultural bi-seasonality in the post-Meroitic Period (Schwarcz & White Reference Schwarcz and White2004; Fuller & Lucas Reference Fuller, Lucas, Emberling and Williams2021). The data presented here for Upper Nubia are not directly comparable with those from Lower Nubia because they are based on different tissues (tooth enamel versus hair, White & Schwarcz Reference White and Schwarcz1994). Notwithstanding, data from the present study strongly suggest that the dietary shift from C3 to C4 plants occurred over a significantly larger geographical region than previously believed.
Archaeobotanical evidence from Meroitic settlements attests to the presence of sorghum and the practice of double-cropping—a system combining the cultivation of winter (wheat and barley) and summer (sorghum, cowpea, hyacinth bean) crops (Fuller Reference Fuller2004, Reference Fuller, Stevens, Nixon, Murray and Fuller2014). The latter are sown during the Nile low water season; the introduction of irrigation systems permitted the expansion of the cultivation of these crops northwards beyond the savannah zone and on to land further away from the Nile floodplain. In contrast to the Meroitic heartland, the cultivation of summer crops in Lower Nubia was insignificant until the third and fourth centuries AD when saqia irrigation was introduced. This development created more arable land and supported the cultivation of such crops during annual low Nile water season and during periods of low flood levels, likely probably caused by fluctuation in temperature and reduced precipitation.
This agricultural shift, characterised by intensification of the cultivation of summer crops, may be attributed to contemporaneous climate change, reduced precipitation and increasing aridity in the region (Machado et al. Reference Machado, Pérez-González and Benito1998). The isotopic data presented here show a clear temporal shift in the δ18O values between the early Meroitic, late Meroitic and post-Meroitic periods, correlating with the palaeoclimatic data, which suggest lower rainfall in Ethiopia—and by extension in Sudan—during the first centuries AD. The data collected in this study come from individuals who lived near the Nile and its water would have been their main source for irrigation, cultivation and consumption. This is supported by the presence of hafirs—water-catchment basins—identified at several Meroitic sites, for example Meroë-Begrawiya, Musawwarat es-Sufra and Naga (Berking et al. Reference Berking, Beckers and Schütt2010; Berking & Schütt Reference Berking and Schütt2018). This water-harvesting technique, introduced during the Meroitic period, allowed for the capture and long-term retention of water following the rainy season and/or seasonal flooding of the Nile, and it remains in use in modern-day Sudan.
The different sources of water consumed by humans have implications for all isotopic studies because the ratios of oxygen isotopes differ between surface and ground water and may be affected by methods of water storage and food preparation. In the present context, however, there is broad evidence for the use of surface water for irrigation and consumption; therefore, the temporal differences in the δ18O values most probably reflect a progressive decline in precipitation due to climate change.
The expansion of agricultural areas and intensification of crop cultivation were instrumental in supporting permanent settlements and stimulating population growth; in turn, these developments may have caused the overexploitation of the limited arable land available and conflict with pastoralists over access to land. The higher δ13C and δ18O values in the post-Meroitic faunal samples compared with the contemporaneous human isotope values suggest that animals were kept at a distance from the Nile settlements and grazed on primarily C4 grasses and shrubs typically found in semi-desert or dry savannah pastures. The isotopic diversity observed for the two bovid groups—one with values similar to the ovicaprids, the other with distinctively higher values—could be interpreted as evidence of distinct herding strategies and landscape exploitation during the post-Meroitic period, possibly in response to environmental pressures. The narrow strip of arable land along the Nile would have been reserved for the cultivation of crops for human consumption and crop waste used as fodder for the animals kept nearby (the ovicaprids and bovids with similar isotopic values), whereas mobile herding would have taken place in drier environments away from the Nile (as suggested by the bovids with higher isotopic values).
The (progressively) higher δ13C values in humans towards the post-Meroitic period probably reflect a dietary shift towards C4 plants and cereals and/or the products of C4-plant-eating animals (Iacumin et al. Reference Iacumin, Bocherens, Chaix and Mariotti1998). The data reveal that the post-Meroitic sub-sample shows a great range of δ13C values in comparison to the Meroitic sub-samples (Figure 3). This is due to a small number (4/23, 17.4%) of outliers—all males—with either higher (–1.68) or lower (–7.96, –8.14, –10.00) δ13C values than the standard range (–6.85 to –2.82) observed for the period (Table S1). Overall, the late/post-Meroitic sub-sample demonstrates a difference in average δ13C values between males and females, with the former lower than the latter. Considering local δ18O values for the post-Meroitic male outliers, the observed difference in average δ13C values between males and females could suggest a gender-based difference in diet among sub-adults (when teeth are forming), with higher C4-plants intake in females. Further insights into individual dietary practices can be observed by comparing δ13C values obtained from teeth that form at different life stages. For instance, differences in δ13C values recorded in the canine and third molar in two of the male outliers (El-Zuma T.9 & T.27; Table S1) may indicate age-based (early childhood versus late childhood/early adolescence) differences in diet; alternatively, they could indicate periods of environmental stress that led to a temporary shortage of one plant group and a reliance on the other (e.g. stored grain), leading to a downward or upward shift in δ13C values (diet either enriched or depleted in C4 plants). These observations warrant further investigation into the dietary practices of the period.
Conclusions
In this article, we have presented new isotopic data sourced from 13 cemeteries along the Nile in Upper Nubia finding evidence for higher δ13C and δ18O values during the post-Meroitic period that indicate greater consumption of C4 plants and an increase in aridity. These findings reinforce the existing palaeoclimatic and proxy data on climate and environmental changes that affected Nubia and the wider region in the first centuries AD (e.g. McCormick et al. Reference Mccormick2012). Palaeopathological data from the region (Davies-Barrett et al. Reference Davies-Barrett, Roberts and Antoine2021) reflect the impact of progressive aridification on humans and support the hypothesis of an environmental shift. The interplay of environmental, political, socio-cultural and demographic factors, including intensification of agricultural practices, population growth and urbanisation, was instrumental in the decentralisation and dissolution of the Meroitic kingdom. Changing climate and environment likely acted as a catalyst for the socio-political transformation that culminated in the cessation of centralised power in favour of smaller splinter states (Fuller Reference Fuller1997, Reference Fuller, O'Connor and Reid2003, Reference Fuller, Stevens, Nixon, Murray and Fuller2014). A series of such successor states formed during the post-Meroitic period, later replaced by Christian kingdoms that dominated the territory of the former Meroitic kingdom (Török Reference Török1988; Edwards Reference Edwards2004).
More broadly, by adding to the body of isotopic data from Meroitic and post-Meroitic Nubia, this study contributes to the wider debate on past climate–society interactions (e.g. Degroot et al. Reference Degroot2021; Biagetti et al. Reference Biagetti2022). Such insights into the complexity of climate–society interactions inform studies of human resilience and of adaptation strategies to changing environmental conditions, past and present.
Acknowledgements
We thank Abdelrahman Ali Mohamed and Abdelhay Abdelsawi, National Corporation for Antiquities and Museums, Sudan, for access to skeletal remains (Dabba Dam Archaeological Survey Project—DDASP), export permission, and their continued support. Thanks also to Mahmoud El-Tayeb, Pawel Wolf, Michael Brass and Adam Ahmed for access and sampling permission of skeletal remains from El-Zuma/El-Detti, Tanqasi, Hamadab/Meroë and Jebel Moya; to Denise Doxey for dental samples from El-Kurru; to Nohlanhla Dlamini-Stoll and Sabine Eggers for access and sampling permission of skeletal remains from Tabo and Toshka. Samples were analysed at the Stable Isotope Laboratory, CAGE, the Arctic University of Norway. AMS dating was conducted by Poznan Radiocarbon Laboratory and Gliwice Radiocarbon Laboratory, Silesian University of Technology. We thank the anonymous reviewers for their valuable comments and suggestions.
Funding statement
This study was conducted as part of the research project ‘Environmental changes and the collapse of the kingdom of Meroë, Sudan’ funded from the European Union Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 665778, and the National Science Centre, Poland, under the POLONEZ grant agreement no. 2016/21/P/HS3/00893.
Supplementary information
To view supplementary information for this article, please visit https://doi.org/10.15184/aqy.2023.162.