Introduction
There has been recent momentum in understanding the role of herding practices in the early exploitation of a range of high-elevation environments, including the Pamir Mountains, the Ethiopian Highlands and the Andes (e.g. Capriles Reference Capriles, Capriles and Tripcevich2016; Gifford-Gonzalez Reference Gifford-Gonzalez, Albarella, Rizzetto, Russ, Vickers and Viner-Daniels2017; Ananyevskaya et al. Reference Ananyevskaya, Buckley, Chowdhury, Tabaldiev and Matuzeviciute2021). The average elevation of the Tibetan Plateau exceeds 4500m above sea level (masl) and much of the area is characterised by alpine tundra environments. The herding of livestock on the plateau has deep historical roots that can be traced back to at least 3500 BP (Zhou Reference Zhou, Renxiang, Huimin and Fang1999; Dong et al. Reference Dong2016; Wang et al. Reference Wang2023). How early herders looked after their flocks in such challenging high-elevation environments, however, remains unknown.
Recent archaeological research has revealed a broad spectrum of subsistence systems across the range of environments on the Tibetan Plateau and adjacent territories that included hunting, herding, foraging, farming and hybrid strategies (Hermes et al. Reference Hermes, Frachetti, Doumani Dupuy, Mar'yashev, Nebel and Makarewicz2019, Reference Hermes, Schmid, Tabaldiev and Matuzeviciute2022; Zhang et al. Reference Zhang2019; d'Alpoim Guedes & Aldenderfer Reference d'Alpoim Guedes and Aldenderfer2020; Song et al. Reference Song2021; Vaiglova et al. Reference Vaiglova2021). During the time between 4000 and 3000 BP, such systems incorporated livestock and cereal crops that originated in east and south-west Asia, forming multiresource agropastoral economies on the plateau (Chen et al. Reference Chen2015; Tang et al. Reference Tang2022, Reference Tang2023; Wang et al. Reference Wang2023). The site of Bangga, in southern Tibet, is one of only a few prehistoric settlements in this part of the world that have been fully excavated, revealing human and animal lifeways at 3750masl during its occupation c. 3000–2200 BP (Figures 1 & 2) (Lü et al. Reference Lü2021). Recent discoveries at Bangga provide us with a glimpse of daily experience at such highland settlements, where the prehistoric community herded sheep, goat, taurine cattle, yak and cattle-yak hybrids and cultivated barley, wheat and likely buckwheat (Lü et al. Reference Lü2021; Tang et al. Reference Tang2021; Zhang et al. Reference Zhang, Xu, Wangdue, Gao, Lü, Liu and Marshall2022; Chen et al. Reference Chen2023).

Figure 1. Locations of Bangga and relevant archaeological sites mentioned in the text: 1) Bangga; 2) Changguogou; 3) Qugong (figure by authors).

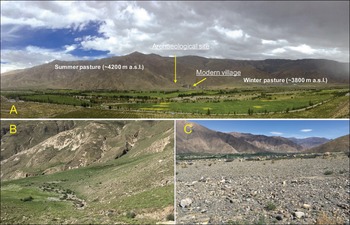
Figure 2. A) Bangga and surrounding environment; B) summer pasture, approximately 4200masl; and C) winter pasture, approximately 3800masl, used by modern day Bangga community (photographs by Z. Zhang).
In contrast to lowland communities, early herders at Bangga would have had to overcome altitude-related difficulties in maintaining their flock size; the birth rate of sheep and goats is lower at high altitudes and lamb mortality is increased owing to extreme weather and disease (Zhang et al. Reference Zhang, Xu, Wangdue, Gao, Lü, Liu and Marshall2022). The challenges faced by modern Tibetan pastoralists were likely also faced by early herders, with severe snowstorms, seasonal nutritional deficiency, droughts and epidemics threatening flocks on the plateau (e.g. Miller Reference Miller2000). A recent zooarchaeological study at Bangga suggests that more than half of sheep did not survive the first year of their life (Zhang et al. Reference Zhang, Xu, Wangdue, Gao, Lü, Liu and Marshall2022). In this study, we focus on the ways in which early herders maintained their domestic animals in the face of such challenges at Bangga.
Stable oxygen (δ18O) and carbon (δ13C) isotope values, serially sampled from dental enamel carbonate, are informative of the dietary and water intake of animals over multiple seasons and have shed light on herding practices in Europe, south-west Asia and, more recently, in central and east Asia (e.g. Balasse et al. Reference Balasse, Boury, Ughetto-Monfrin and Tresset2012a; Makarewicz Reference Makarewicz2017; Hermes et al. Reference Hermes, Frachetti, Doumani Dupuy, Mar'yashev, Nebel and Makarewicz2019, Reference Hermes, Schmid, Tabaldiev and Matuzeviciute2022; Vaiglova et al. Reference Vaiglova, Gardeisen, Buckley, Cavanagh, Renard, Lee-Thorp and Bogaard2020; Ventresca Miller et al. Reference Ventresca Miller, Haruda, Varfolomeev, Goryachev and Makarewicz2020). We conducted sequential isotope analyses on sheep and goat teeth uncovered at Bangga and on modern teeth collected nearby. The results suggest that some sheep/goats were provisioned with water and cultigens (likely barley and/or millet) all year round.
Research background
Bangga
The site of Bangga is situated on an alluvial deposit on the eastern side of the Qonggyai River—a tributary of the Yarlung Zangbo. The site is adjacent to the modern-day Bangga village in Qonggyai county, at approximately 3750masl (Figure 1). The local precipitation is subject to monsoon rainfall and, at this elevation, events such as droughts, flooding and snowstorms can threaten herd production. Excavations at Bangga in 2000–2002 and 2015–2018 uncovered more than 400m2 of the site, revealing archaeological features such as stone-built dwelling structures, hearths, postholes and pits. The 2015–2018 excavation revealed a dwelling complex and adjacent corral area, dating to c. 3000–2200 cal BP (Lü et al. Reference Lü2021).
More than 12 000 fragments of animal bone were recovered during the 2015–2018 excavation. Zooarchaeological study is ongoing but the initial results suggest that sheep (Ovis aries) and goats (Capra hircus) dominate the assemblage (Zhang et al. Reference Zhang, Xu, Wangdue, Gao, Lü, Liu and Marshall2022). Large-sized Bovinae, Equidae and other smaller-sized mammals, including musk deer, gazelle, hare and rodents, are also present but account for a small proportion of the assemblage. Flotation has collected more than 70 000 charred grains (Tang et al. Reference Tang2021). Barley dominates the crop portion of the macrofossils—over 80 per cent of cereal remains—with a small number of wheat and Fagopyrum sp. (cf. buckwheat). A significant number of non-cereal plants was also identified. Notably, Chenopodium (goosefoot) and Amaranthaceae are predominant. Millets were not recovered from Bangga despite being commonly encountered at contemporaneous or older sites nearby (Fu Reference Fu2001; Gao et al. Reference Gao, Yang, Ma, Tong and Yang2021).
Stable isotope analysis and local environmental conditions
The oxygen and carbon isotope compositions of tooth enamel are closely related to dietary and water intake (Longinelli Reference Longinelli1984; Huertas et al. Reference Huertas, Iacumin, Stenni, Chillón and Longinelli1995; Sponheimer & Lee-Thorp Reference Sponheimer and Lee-Thorp1999). Enamel mineralises sequentially along the axis of tooth growth (from the occlusal surface to the root). For sheep, the second molar (M2) mineralises during the first 12 months after birth, and the third molar (M3) mineralises during the first 10–22 months of life (Zazzo et al. Reference Zazzo, Balasse, Passey, Moloney, Monahan and Schmidt2010). Once complete, the bioapatite of the dental crown does not generally remodel. The δ18O and δ13C values of incrementally sampled enamel therefore reflect water and dietary input during these periods, enabling insights into seasonal changes and serving as indicators of grazing behaviour and mobility (e.g. Balasse et al. Reference Balasse, Obein, Ughetto-Monfrin and Mainland2012b; Tornero et al. Reference Tornero, Balasse, Bălăşescu, Chataigner, Gasparyan and Montoya2016).
Stable oxygen isotope values of enamel bioapatite are controlled by body water and reflect local meteoric water, which is itself driven by the hydrological cycle and geographic factors (e.g. Gat Reference Gat, Fritz and Fontes1980; Bowen & Wilkinson Reference Bowen and Wilkinson2002). On the southern Tibetan Plateau, three processes are critical to the variation of δ18O values in surface water: altitude, temperature and the ‘amount effect’. Higher atmospheric temperatures increase rates of evaporation, leading to an enrichment of the heavier 18O isotope in surface water (as the lighter 16O isotope evaporates preferentially), resulting in higher δ18O values in the summer and lower values in the winter (Gat Reference Gat, Fritz and Fontes1980). The corollary of this effect is that summer rains brought to the plateau during the Indian Ocean monsoon are 18O depleted, in contrast to the 18O enriched surface water. Additionally, at high elevations, meltwater from surface snow and glacial ice (accumulated over colder seasons/periods with considerably lower δ18O values) contributes to further 18O depletion of meteoric water during the warm season (Yao et al. Reference Yao, Zhou and Yang2009). These effects combine to various degrees. Modern data from local meteorological stations on the Tibetan Plateau suggest that the ambient temperature effect is outweighed by the monsoon amount effect and snow melt, resulting in the lowest δ18O values for surface water in the summer (approximately –20‰) and the highest values in the spring (approximately –5‰) (Yao et al. Reference Yao, Zhou and Yang2009) (see online supplementary material (OSM) Figure S1). Though ultimately different, this pattern resonates with the bimodal seasonality of 18O depletion during summer (the rainy season) observed in the East Asian monsoon region (Dai et al. Reference Dai2016). It is, however, significantly different from the system in the continental interior where surface temperature is the main driver (Makarewicz & Pederzani Reference Makarewicz and Pederzani2017). It is also worth noting that the large seasonal variation in water δ18O values on the plateau (over 15‰) has the potential to overshadow any interpretation of altitude mobility (precipitation δ18O values decrease along the altitude gradient on an order of 1–3‰/km). In theory, vertical transhumance can be detected using sequential enamel δ18O values with well thought-out models (e.g. Hermes et al. Reference Hermes, Pederzani, Makarewicz, Miller and Makarewicz2017). On the southern Tibetan Plateau, however, the large seasonal variation in meteoric water δ18O values (over 15‰) and its bimodal nature have the potential to offset this altitude effect, making such interpretation challenging.
Stable carbon isotope values in enamel reflect δ13C values of food plants (Ambrose & Norr Reference Ambrose, Norr, Lambert and Grupe1993; Lee-Thorp & Sponheimer Reference Lee-Thorp and Sponheimer2003). Plants utilising C3 and C4 photosynthetic pathways differ significantly in δ13C values—averaging approximately –26.5‰ and –12.5‰, respectively—allowing assessment of the relative proportion of C3 and C4 plants consumed. Theoretically, C4 plants are maladapted to high altitudes, where they exhibit low productivity due to frequent chilling injuries and inferior quantum yields (Sage et al. Reference Sage, de Melo Peixoto, Friesen and Deen2015). This results in a substantial drop in the C4 contribution to biomass and local vegetation in high elevation locations like Bangga. On a global scale, environments above 3000masl are considered C3-plant dominant, but cold-tolerant C4 plants—including grasses, sedges and dicots—may occur, particularly in saline environments during warm seasons (Sage et al. Reference Sage, de Melo Peixoto, Friesen and Deen2015). In addition, C4 cultivars (broomcorn and foxtail millet) have been reported from several archaeological sites in the southern part of the Tibetan Plateau (Fu Reference Fu2001; Gao et al. Reference Gao, Yang, Ma, Tong and Yang2021; Tang et al. Reference Tang2021) and could have been used for foddering. In modern Tibetan herbivores, the cut-off δ13C value (bioapatite) for a pure C3 diet is –7.3 to –8‰ (subjected to a fossil fuel effect of +1.5‰; Wang et al. Reference Wang, Kromhout, Zhang, Xu, Parker, Deng and Qiu2008). Above this threshold, the dietary contribution of C4 plants can be detected.
An altitude effect on C3 plants may apply due to changes in atmospheric pressure and/or high carboxylation efficiency along the elevation gradient (e.g. Körner et al. Reference Körner, Farquhar and Wong1991; Szpak et al. Reference Szpak, White, Longstaffe, Millaire and Vásquez Sánchez2013). On the Tibetan Plateau, a linear regression may be observed between elevation and plant δ13C values with a 0.7–2.6‰/km decreasing rate (Deng & Li Reference Deng and Yumei2005). A relatively moderate seasonal variation (1–2‰) in δ13C values may also occur, with the higher values in dry seasons and lower values in wet seasons (Tornero et al. Reference Tornero, Balasse, Bălăşescu, Chataigner, Gasparyan and Montoya2016). An increase in δ13C values would therefore be expected if animals were moving vertically towards higher pastures in the summer. However, the extent to which this pattern would be counterbalanced by the accessibility of C4 plant food (via foddering or abundance of C4 in rare locations) depends on the context.
Materials and methods
Fourteen modern mandibular molars, from four sheep and three goats, were sampled to help interpret the archaeological measurements. They were collected at the village of Bangga in the summer of 2017 (Table S1). The archaeological samples consist of 10 Caprinae mandibular molars that likely represent five individuals (Table S2).
We followed approaches established in previous studies (e.g. Balasse et al. Reference Balasse, Obein, Ughetto-Monfrin and Mainland2012b, Reference Balasse, Bălăşescu, Janzen, Ughetto-Monfrin, Mirea and Andreescu2013; Tornero et al. Reference Tornero, Bălăşescu, Ughetto-Monfrin, Voinea and Balasse2013), which suggest that localised seasonal movements in both wild and domestic herding strategies of bovids can be identified through sequential analysis of oxygen and carbon stable isotopes from hypsodont crowned herbivore dental enamel (see OSM for detailed explanation of method). We fit a cosine function to the δ18O values from each analysed tooth to visualise the data and estimate seasonality. Such an approach was initially implemented by Balasse and colleagues (Reference Balasse, Obein, Ughetto-Monfrin and Mainland2012b) using Excel and subsequently widely used. We follow the method and R scripts described by Hermes and colleagues (Reference Hermes, Frachetti, Doumani Dupuy, Mar'yashev, Nebel and Makarewicz2019, Reference Hermes, Schmid, Tabaldiev and Matuzeviciute2022) to generate 95% confidence intervals for the curve fitting. Unfitted plots are in Figures S2 & S3.
Results
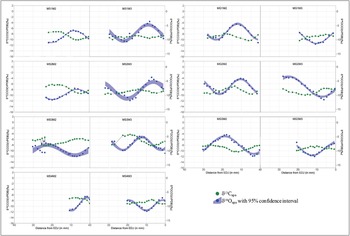
The isotopic values of all modern and archaeological samples are presented in Table 1 and Figures 3, 4 and 5, S2 and S4. The intra-tooth δ18O and δ13C values of most modern teeth are inversely proportional (Figure 3), with two exceptions (MG2M2 and MS3M3, see Table S3 for statistical tests). For both modern sheep and goats, intra-tooth variations in δ13C series for M2 are slightly greater than M3. Mean δ13C values of modern sheep are consistently slightly higher than those of modern goats at Bangga, a trend also observed in other regions of the world (Janzen et al. Reference Janzen, Balasse and Ambrose2020).

Figure 3. Sequential δ13C and δ18O values of modern sheep and goat enamel bioapatite (CO3) from Bangga. First M stands for modern sample; S or G indicate sheep or goat; M2: second molar; M3: third molar; EDJ: enamel-dentine junction (figure by authors).

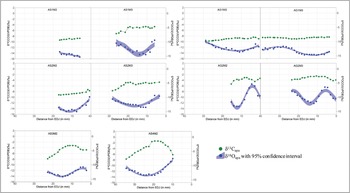
Figure 4. Sequential δ13C and δ18O values of archaeological samples from Bangga. A stands for archaeological sample; S or G indicate sheep or goat; M2: second molar; M3: third molar; EDJ: enamel-dentine junction (figure by authors).

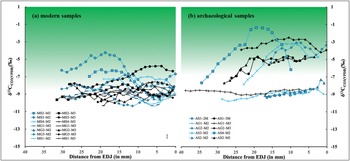
Figure 5. The δ13C values of modern (a) and archaeological (b) samples analysed in this study. The shaded areas indicate ranges of bioapatite δ13C values for herbivores not purely fed on C3 plants (> –8‰ for modern high-elevation animals, > –6.5‰ for archaeological specimens due to the Suess effect). Measurement error is shown in the bottom-right of (a); EDJ: enamel-dentine junction (figure by authors).
Table 1. Stable carbon and oxygen isotope values of tooth enamel carbonate analysed in this study.

The δ13C and δ18O values of enamel carbonate from archaeological sheep and goats also show inverse cyclicity (except for samples AS2M2 and AS3M2, see Table S3). Only AS3M2, AG2M2 and AG2M3 display patterns close to the sinusoidal variation in δ13C and δ18O values (Figure 5). The δ18O and δ13C values of the remaining teeth show either shallow curves or curves that are too short to record an entire annual cycle. The mean δ18O values from archaeological samples are significantly lower than those of modern samples.
Discussion
Crop and water provisioning at Bangga
The majority of modern sheep and goats show inverse cyclicity in intra-tooth isotopic sequences (i.e. lower δ18O values correspond to higher δ13C values and vice versa). This is consistent with what would be expected for seasonal mobility to higher pastures in the summer, reflecting modern herding practice (e.g. Yao et al. Reference Yao, Zhou and Yang2009; Balasse et al. Reference Balasse, Obein, Ughetto-Monfrin and Mainland2012b; Tornero et al. Reference Tornero, Balasse, Bălăşescu, Chataigner, Gasparyan and Montoya2016). The lower δ18O values reflect summer conditions when meteoric water is most 18O depleted, while higher δ13C values are due to the altitude effect on C3 plants. As expected for an elevation above 3750masl, most modern animals display enamel δ13C values consistent with pure C3-plant diets (below the current –8‰ threshold). But there are exceptions. Sheep MS3, for example, shows clear C4 (or CAM) plant dietary input during summer. No C4 crops (e.g. maize, millet, sorghum) are cultivated at Bangga today, and there is no ethnographic record for the use of free-standing C4 plants as fodder (Jiang Reference Jiang2011). Maize is, however, cultivated in the broader region today and may sometimes be used for provisioning livestock. The C4 signals observed in MS3 may therefore result either from grazing on free-living C4 plants in the summer or from being provisioned with maize fodder. Our results also show a relatively small amplitude in intra-tooth δ18O variation (ranging between 4 and 8‰), which is much lower than reported in other mid-latitude environments (e.g. Tornero et al. Reference Tornero, Balasse, Bălăşescu, Chataigner, Gasparyan and Montoya2016; Hermes et al. Reference Hermes, Frachetti, Doumani Dupuy, Mar'yashev, Nebel and Makarewicz2019). This is likely due to the counterbalance between temperature and amount effects in surface water δ18O values.
Most archaeological animals display some degree of sinusoidal variation in δ18O values, although the amplitudes of such variations are much lower than in modern samples. At least two individuals (AS2 and AG1) display extremely low intra-tooth variation (Figure 4); year-round access to groundwater, in which the δ18O value does not typically vary seasonally (Fricke et al. Reference Fricke, Clyde and O'Neil1998; Darling Reference Darling2004), could explain this observation. In general, unlike surface water, the δ18O value of groundwater reflects mean oxygen isotope composition in long-term precipitation accounting for a recharge rate according to the local hydrological system (e.g. Ge et al. Reference Ge, Wu, Lu, Jiang and Ball2008; Kong et al. Reference Kong, Wang, Pu and Shi2019). In other words, some animals have been provided with groundwater all year round.
Foddering with crops and the source of C4 dietary input
Archaeological specimens fall into two groups for carbon isotope values: 1) teeth that display shallow curves and low δ13C values (AG1M2, AG1M3, AS1M2 and AS2M2), and 2) teeth that show sinusoidal variations and high δ13C values (AS1M3, AG2M2, AG2M3, AS3M2, AS4M2 and AS2M3) (Figures 3, 4 & 5). The second and third molars for some individuals (e.g. AS1 and AS2) fall into the different groups, indicating shifting dietary conditions during the first two years of life. The group 1 teeth probably reflect human provisioning with C3 plants; grazing on pasture is unlikely to produce such shallow curves but show more varied intra-tooth δ13C values. Considering the archaeobotanical evidence at Bangga, it is possible that livestock were provisioned with barley by-products (Tang et al. Reference Tang2021).
Group 2 teeth, with sinusoidal curves, show clear dietary input of C4 plants (Figure 5b). Most data points in this group are above the –6.5‰ pure-C3 diet threshold (Friedli et al. Reference Friedli, Lötscher, Oeschger, Siegenthaler and Stauffer1986; Wang et al. Reference Wang, Kromhout, Zhang, Xu, Parker, Deng and Qiu2008). Considering that the enamel-diet offset for ruminant sheep and goats (+11‰) is likely lower than the average enamel-diet δ13C enrichment factor (+14‰), the actual pure C3 diet threshold could be lower than the current –6.5‰ model (Cerling et al. Reference Cerling, Harris, MacFadden, Leakey, Quade, Eisenmann and Ehleringer1997). Therefore, our data unambiguously show that the group 2 teeth come from animals that consumed a considerable amount of C4 plants.
Given that C4 plants are generally maladapted to high elevations, the elevated δ13C values in archaeological caprine teeth at Bangga are striking. There are two possible explanations. Some animals could have been taken to saline-alkali or marsh environments in the summer (most lakes are brackish water in the region), where C4 plants tolerate such conditions more than C3 plants (Drake Reference Drake1989). Support for this explanation is found in the ubiquity of salt-tolerating taxa, such as Chenopodium sp. and Salsola collina—the latter a C4 plant—in the flotation assemblage at Bangga, possibly incorporated through dung burning (Mao Zhou, pers. comm.). Yet the seasonal use of saline environments cannot explain those teeth (AS1M3, AS2M3, AG2M2 and AG2M3) that show C4 dietary input and at the same time have very low intra-tooth δ13C variations. AG2, for example, displays almost flat curves in both M2 and M3. This indicates that those animals grazed on C4 plants across seasons, even in the winter when C4 plants are extremely scarce at high elevations and pasturing is highly risky. This hints at human provisioning as an alternate explanation. C4 crops such as broomcorn and foxtail millet were not recovered from Bangga but are documented at nearby sites, such as Changguogou, Bangtangbu and Qugong and were thus known to these communities during the period in which Bangga was first occupied (Fu Reference Fu2001; Gao et al. Reference Gao, Yang, Ma, Tong and Yang2021).
This observation enables complementary insights into earlier suggestions of a transition towards a barley-dominant economy during the second millennium BC (Tang et al. Reference Tang2021), grounded in the establishment of a mono-cropping (i.e. barley) system that was possibly associated with the diminished roles of other cereals (e.g. millet) in human diets. Millet could have been used for animal provisioning in some context, a scenario that resonates with the Dzhungar Mountains in the third millennium BC, where isotopic analysis reveals that sheep and goats were foddered with millet without any corresponding archaeobotanical evidence (Hermes et al. Reference Hermes, Frachetti, Doumani Dupuy, Mar'yashev, Nebel and Makarewicz2019). Currently, available data do not allow us to distinguish between scenarios where livestock consumed C4 plants from marsh environments and where they were provisioned with millet, although the latter possibility is, in our view, more likely, given the evidence of C4 consumption in winter. Nonetheless, millet cultivation at Bangga remains an open inquiry. Isotopic analyses of human skeletal remains or sequentially sampled tooth enamel of other domestic taxa will, in future, enable us to clarify the role of millet in human and animal diets.
Overall, our results show that provisioning was a key strategy for ensuring the success of herding at 3750masl, c. 3000–2200 BP. Faced with extreme seasonal and hypoxic conditions and high lamb mortality rates (Zhang et al. Reference Zhang, Xu, Wangdue, Gao, Lü, Liu and Marshall2022), Bangga herders provisioned their flocks with water and fodder from agricultural products, in some cases all year round. Such a labour-intensive strategy constitutes a departure from—rather than a continuity of—the Bronze Age pastoral systems that have been well documented across the Asian interior at lower elevations. These lower elevation communities relied on extensive approaches in which productivity was driven by the size of herds, by the quality and expansiveness of the pastures, and by mobility (e.g. Makarewicz Reference Makarewicz2017; Lazzerini et al. Reference Lazzerini2021). This differs substantially from the less mobile and more labour-intensive provisioning strategies inferred at Bangga, which probably included foddering, corralling and water provisioning alongside heavy investment in barley cultivation to survive in a high elevation environment.
Conclusion
This article presents one of the first applications of sequential isotope analysis of archaeological animal teeth from the Tibetan Plateau, adding to pioneering work in similar environments (cf. Hermes et al. Reference Hermes, Schmid, Tabaldiev and Matuzeviciute2022). Our results enable three principal inferences. First, some sheep and goats at Bangga were provisioned with groundwater all year round. Second, herders likely foddered their animals with agricultural products, seasonally or year round. And third, some animals show considerable dietary input from C4 plants. The isotopic evidence presented here is unambiguous regarding water and fodder provisioning but it is unable to identify the source of the C4 dietary component documented in the enamel δ13C values. Either millet foddering or pasturing in saline environments are possible scenarios. Future research can clarify the situation. Together with zooarchaeological and archaeobotanical evidence (Tang et al. Reference Tang2021; Zhang et al. Reference Zhang, Xu, Wangdue, Gao, Lü, Liu and Marshall2022), our results indicate that the ancient Bangga community engaged in a labour-intensive agropastoralism on the Tibetan Plateau that has not previously been documented.
Acknowledgements
We are grateful to Xuepeng Wei and Lunzhu Qunpei for their assistance in collecting the modern sheep and goat teeth analysed in this study and to Mao Zhou for providing archaeobotanical information from Bangga. We acknowledge Zujun Chen for material access and conducting excavation at Bangga and Rachel Reid, Mica Jones and Mekhi Airhart for assistance with laboratory work at Washington University.
Funding statement
We are grateful to the National Science Foundation (grant nos. 2017247 and 1826727) for support of specialist work. The Bangga excavation and post-excavation research are supported by National Key R&D Programs of China (2021YFC1523600), the Ministry of Education of China (grant no. 16JJD78011), the Cultural Relics Bureau of the Tibetan Autonomous Region and Sichuan University.
Online supplementary materials (OSM)
To view supplementary material for this article, please visit https://doi.org/10.15184/aqy.2024.137 and select the supplementary materials tab.