Introduction
The domestication of various animal species has marked crucial transitions in human (pre)history. The desire to increase our understanding of the timelines and processes underlying these domestication events drives much archaeological research (Zeder Reference Zeder2012; Larson et al. Reference Larson2014). The domestication of carnivores has received substantial attention (e.g. Benecke Reference Benecke1987; Clutton-Brock Reference Clutton-Brock and Serpell1995; Mech & Janssens Reference Mech and Janssens2021), but relatively little is known of the domestication history of the ferret (Mustela furo or Mustela putorius f. furo). Sparse literary sources (see Owen Reference Owen, Ucko and Dimbleby2009) and genetic studies (Volobeuv et al. Reference Volobuev, Ternovski and Grafodatski1974; Blandford Reference Blandford1987; Davison et al. Reference Davison, Birks, Griffiths, Kitchener, Biggins and Butlin1999; Sato et al. Reference Sato, Hosoda, Wolsan, Tsuchiya, Yamamoto and Suzuki2003) suggest that the domestic ferret is derived from European polecat (Mustela putorius) populations in the Mediterranean region. Its later dispersal across Europe is closely linked with the spread of the rabbit (Oryctolagus cuniculus) from the western Mediterranean, which was facilitated by medieval transportation networks (Van Damme & Ervynck Reference Van Damme and Ervynck1988; Ervynck Reference Ervynck2003; Owen Reference Owen, Ucko and Dimbleby2009). Rabbit breeding, initially practised by the clergy, became a popular pastime of the European nobility in the thirteenth century (Van Damme & Ervynck Reference Van Damme and Ervynck1988; Albarella & Davis Reference Albarella, Davis and Chapman2010). As the rabbits were kept in earthen warrens, the easiest way to remove them was to drive them out with ferrets—whose slender bodies were easily accommodated by the narrow burrows (Van Damme & Ervynck Reference Van Damme and Ervynck1988). This led to the ferret becoming a popular hunting companion for rabbiting (Owen Reference Owen, Ucko and Dimbleby2009).
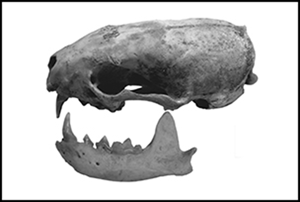
So far, however, it has proven difficult to identify corroborating archaeological evidence (Owen Reference Owen, Ucko and Dimbleby2009). Not only are osteological remains of mustelids scarce, but distinguishing wild polecats from domestic ferrets based on bone morphology alone is challenging (Van Damme & Ervynck Reference Van Damme and Ervynck1988; Albarella & Davis Reference Albarella, Davis and Chapman2010). This issue of identification presented itself in a partial mustelid skeleton, found among the faunal remains from a fourteenth- to sixteenth-century AD cesspit (sp670) from the Scheerstraat site in the Flemish town of Mechelen, Belgium (Gruwier Reference Gruwier2019). Here a skull, two mandibles and several postcranial elements belonging either to M. putorius or M. furo were found (Figure 1) (Gruwier Reference Gruwier2019). A series of fine cutmarks on the zygomatic arch suggests that the animal was skinned for its pelt. Historical sources also indicate that a furrier named Lambrecht Smet was active at the Scheerstraat in the sixteenth century (see archival record Schepenacten Reference Schepenacten1528). It was, nevertheless, unclear whether the bones represented the remains of a skinned ferret or a wild animal that had been hunted for its pelt.

Figure 1. Ferret or polecat remains from the Scheerstraat in Mechelen: A) present elements in dark grey; B) a photo of the mandible and cranium; and C) detail of the zygomatic arch with skinning marks (figure by author; A) after Coutureau Reference Coutureau2021).
This article presents the results of a newly developed method for distinguishing the remains of polecats from those of ferrets, using a geometric morphometric (GMM) approach. Although based on a small sample of extant mandibles, this study aims to enhance the identification of archaeological mustelid remains. As a case study, the method was tested on the archaeological specimen from Mechelen.
Materials and methods
The mandible was selected for GMM analysis due to the morphological changes in this element (e.g. shortening of the facial region) apparent during the domestication of other carnivores (Clutton-Brock Reference Clutton-Brock1999; Kleisner & Stella Reference Kleisner and Stella2009; Janssens et al. Reference Janssens, Perri, Crombe, van Dongen and Lawler2019). Studies on a wide range of mammals have already demonstrated the efficacy of GMM to differentiate between closely related forms (e.g. Cucchi et al. Reference Cucchi, Hulme-Beaman, Yuan and Dobney2011; Gaastra Reference Gaastra2023). Morphometric data on a limited sample of 32 extant ferret and polecat skeletons were collected at the Natural History Museum Rotterdam and the Naturalis Biodiversity Centre in Leiden (the Netherlands). Data from two additional ferrets were shared by the University of Sheffield Zooarchaeology Laboratory. Only adult specimens with all teeth erupted were retained for analysis and pathological specimens were excluded. The total sample included 23 polecats, seven ferrets and two ferret-polecat hybrids (see online supplementary material). A single mandible from the Scheerstraat in Mechelen represented our archaeological case study.
Standardised photographs of the buccal (cheek) side of the left mandible were quantitatively analysed. The photographs, taken with a Nikon D40 camera, were uploaded to the TPSdig 2.16 software (Rohlf Reference Rohlf2004), where six type II landmarks (sensu Baab et al. Reference Baab, McNulty and Rohlf2012) were placed on the outline of each specimen (Figure 2). The Cartesian coordinate data were then exported to the PAST 2.17 software package (Hammer et al. Reference Hammer, Harper and Ryan2001), where a Generalised Procrustes superimposition was conducted to remove differences in landmark configurations due to rotation, scale and orientation. This resulted in a projection of the data in Euclidean space tangent to the Procrustes shape space (Viscosi & Cardini Reference Viscosi and Cardini2011; Baab et al. Reference Baab, McNulty and Rohlf2012).

Figure 2. Position and description of the geometric morphometric landmarks recorded on the outline of the buccal side of the mandible (figure by author).
To assess morphological variation in the dataset, we made use of between groups Principal Component Analysis (bgPCA). As bgPCA separations are based on eigenvectors of a variance-covariance matrix calculated on group means, it has the advantage that differences between groups are emphasised while the original Procrustes distances are retained (Seetah et al. Reference Seetah, Cardini and Miracle2012). Those principal components with eigenvalues cumulatively explaining at least 70 per cent of the variance were retained for further analysis, a frequently used cutoff point in PCA (Jolliffe & Cadima Reference Jolliffe and Cadima2016). To account for overlap between different clusters in the PCA scatterplot, additional testing of the validity of the group separations was deemed necessary. To test the statistical significance (p<0.05) of between group separations, a permutational multivariate analysis of variance (npMANOVA) was conducted on the relevant principal components, followed by Mann–Whitney pairwise comparisons (Polly et al. Reference Polly2013). A non-parametric test was necessary, as some of the assumptions required for parametric testing (e.g. normal distribution) can be violated by GMM data (Cardini et al. Reference Cardini, Seetah and Barker2015; Lopez-Lazaro et al. Reference Lopez-Lazaro, Aleman, Viciono, Irurita and Botella2018). The Bonferroni procedure was additionally used as a multiple correction technique (Dunn Reference Dunn1961).
To account for allometric effects (non-proportional shape differences arising from scaling body size), we conducted a regression of the relevant principal components on the natural log of centroid size (Cucchi et al. Reference Cucchi, Hulme-Beaman, Yuan and Dobney2011). If the resulting correlation between shape and size was significant (p<0.05), an allometric effect was assumed (Zelditch et al. Reference Zelditch, Swiderski, Sheets and Fink2004).
Results
To explore shape variance in the dataset, a PCA was conducted on the Procrustes residuals (Figure 3). As the cumulative variance of the first two axes amounted to 98.6 per cent, only principal components (PCs) 1 and 2 were retained for further analysis. Thin plate spline deformation grids associated with PC1 (see Figure 3: x-axis) indicate that the principal shape changes along this axis were (A) a difference in length of the horizontal ramus relative to the height of the vertical ramus, and (B) a difference in antero-posterior length of the canine and carnassial (first molar) alveoli. Specimens with a lower score for PC1 have smaller alveoli and a comparatively short horizontal ramus. Specimens with a higher score have a longer horizontal ramus and larger canine and carnassial alveoli. PC2 mainly describes a difference in (A) the position of the condyle process relative to the coronion, and (B) the orientation of the canine and carnassial alveoli. Specimens with a high score for PC2 have a more posteriorly projecting condyle process and alveoli oriented parallel to the horizontal ramus. Specimens with a low score on PC2 have a less posteriorly projecting condyle process and alveoli oriented more towards the anterior side of the mandible.

Figure 3. Scatterplot of PC1 and PC2 of a bgPCA on all mustelid mandibulae, with eigenvalues in parentheses and thin plate spline deformation grids for both axes showing the variation of morphological changes represented by each PC (figure by author).
Although the scatterplot reveals overlap between the different groups, a separation between the wild and the domesticated forms is visible. All ferret specimens give low scores on PC1 and high scores on PC2, while the polecat specimens generally score higher on PC1 and lower on PC2. The hybrid specimens give intermediate scores. Pairwise comparisons following an npMANOVA (p<0.01) on the relevant components confirm that the polecat group is significantly different from the ferret group (p<0.01). Separations between the hybrids and ferrets (p=1) or polecat (p=0.34) are not significant, however.
Overall, the results imply that ferrets have mandibles with a relatively short horizontal ramus, short alveoli oriented parallel to the horizontal ramus, and a more posteriorly projecting condyle process. In contrast, polecats tend to have a long horizontal ramus, with larger alveoli, often oriented more perpendicular to the ramus, and a less projecting condyle process. Hybrids have an intermediate shape that overlaps with both forms.
The archaeological specimen from the Scheerstraat gives intermediate scores on PC2, indicating the orientation of its alveoli and condyle process overlaps with those of the wild, domestic and hybrid forms. On PC1, however, it gives a very high score, demonstrating the presence of a comparatively long horizontal ramus and large canine and carnassial alveoli, typical of polecat.
To assess the dataset for allometric effects, we conducted a regression of the shape variables on centroid size (Figure 4). Our regression of PC1 against the size variable indicates a significant, but relatively weak correlation (R2=0.2, p=0.01), implying that a small part of the variance summarised by PC1 can be attributed to allometry. No significant correlation is found between PC2 and centroid size (R2=0.02, p=0.4), suggesting the absence of an allometric effect in this component.

Figure 4. Results of a regression of PC1 and PC2 against natural log of centroid size (figure by author).
Discussion
Keeping in mind the limited size of the dataset, the results presented here indicate that the method successfully differentiates ferrets from polecats. Although there is some overlap between the two forms, in general the mandibles of the domesticates have a morphotype different from the wild form. A possible explanation for the overlap is that some of the wild specimens in the dataset may not be of pure Mustela putorius ancestry. As introgression between ferrets and polecats occurs throughout Europe and hybrid specimens may be easily confused with polecats (Davison et al. Reference Davison, Birks, Griffiths, Kitchener, Biggins and Butlin1999; Croose et al. Reference Croose, Duckworth, Ruette, Skumatov, Kolesnikov, Vyacheslav and Saveljev2018), it is possible that some of the observed overlap indicates the presence of additional hybrid specimens within the dataset. Such a hypothesis is supported by the fact that the two confirmed hybrid specimens gave intermediate scores.
Overall differences in morphology between the wild and domestic form are primarily expressed in variation in the relative length of the horizontal ramus and in the size of the alveoli (PC1). Although this can, to a small extent, be explained by an allometric effect, it is conceivable that these morphological changes are mainly an effect of domestication. Reduction in the length of the mandible is identified as characteristic of domestication in wolves (e.g. Benecke Reference Benecke1987; Clutton-Brock Reference Clutton-Brock and Serpell1995; Germonpré et al. Reference Germonpré, Lázničková-Galetová, Losey, Räikkönen and Sablin2015) and wild boar (Evin et al. Reference Evin, Owen, Larson, Debiais-Thibaud, Cucchi, Vidarsdottir and Dobney2017). Although the mechanisms behind these changes (e.g. paedomorphism, dietary adaptation, or changes in ontogenetic trajectory—the retention of, or alteration to, the development from a juvenile morphology) are still a matter of debate (Evin et al. Reference Evin, Owen, Larson, Debiais-Thibaud, Cucchi, Vidarsdottir and Dobney2017; Janssens et al. Reference Janssens, Perri, Crombe, van Dongen and Lawler2019; Neaux et al. Reference Neaux, Louail, Ferchaud, Surault and Merceron2022), a shortening of the facial region may also have taken place during the domestication of the ferret. The trend towards smaller alveoli, mirroring the size of the carnassial and canine teeth, is reminiscent of tooth size reductions seen in other domesticates. Carnassial size reduction is noted in domestic dogs (Clutton-Brock Reference Clutton-Brock and Serpell1995; Janssens et al. Reference Janssens, Perri, Crombe, van Dongen and Lawler2019) and diminished canine and carnassial length have been proposed as an indicator of domestication in cats (Krüger et al. Reference Krüger, Hertwig, Jetschke and Fischer2009). Smaller alveoli may, therefore, again be a marker of domestication in the ferret. It should, nevertheless, be stressed that the findings presented here are limited by a small sample size and that further testing on additional specimens is required to confirm the validity of the observed trends with more confidence.
Based on this limited dataset, however, the fourteenth- to sixteenth-century specimen from Mechelen can be tentatively identified as a wild polecat. With its strongly elongated horizontal ramus and large canine and carnassial alveoli, this specimen gives very different values (on PC1) from those of the ferret and hybrid specimens. This extreme PC1 value could indicate a lack of any ferret introgression within the specimen's genetic lineage. The animal from the Scheerstraat should therefore be considered a hunted animal, procured from outside of the city for the purpose of fur processing.
Conclusions
The results of this study show that the application of a GMM approach to the mandible can aid in the differentiation of M. furo and M. putorius remains. Although the comparative sample is small, further testing of this method will allow for more robust results. When expanding this study to include more specimens, it will also be useful to consider additional analyses on the dataset, including methods to further explore the within and between group variation in the two forms (see e.g. Gruwier et al. Reference Gruwier, De Vos, Wirkner, Hertler, Kovarovic, Louys, Albers and van der Geerin press).
The new methods presented here provide (zoo)archaeologists with a useful new tool for the distinction between ferrets and polecats. The implications of this distinction are not only of zoological relevance but more broadly affect the interpretation of archaeological faunal assemblages that contain mustelid remains. The presence of hunted fauna, such as the wild polecat, within assemblages conveys a very different message regarding human-animal relationships than the presence of a domesticated animal kept as a pet (Jones O'Day et al. Reference Jones O'Day, van Neer and Ervynck2004). Moreover, the wider application of this method will likely lead to a better understanding of the different pathways and mechanisms that led to the domestication of the ferret, and how this species was integrated in the social fabric of human societies (Zeder Reference Zeder2012).
Acknowledgements
We thank the curators of Naturalis, the Natural History Museum Rotterdam and the city of Mechelen for allowing us to study their collections. We are grateful to Lenny Salvagno and Umberto Albarella for sharing data on Mustela furo. Dana Piessens and Frank Kinnaer are thanked for their advice on the history and archaeology of Mechelen. The data that support the findings in this article are openly available at: https://doi.org/10.5281/zenodo.7938116.
Funding statement
This work was funded by a grant from the city of Mechelen.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.15184/aqy.2024.4.