No CrossRef data available.
Published online by Cambridge University Press: 29 July 2021
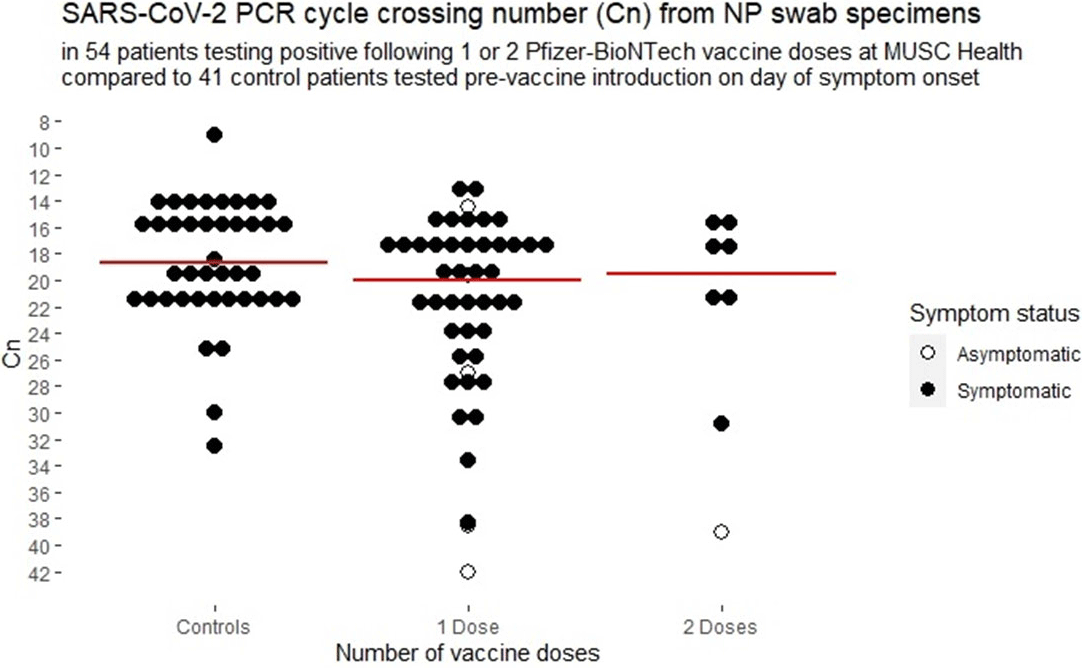
Background: Medical University of South Carolina Health began vaccinating care team members December 15, 2020, with the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine. We report surveillance data for cases diagnosed following vaccination. Methods: Care team members (CTMs) diagnosed with COVID-19 following SARS-CoV-2 vaccination were self-identified during online electronic contact-tracing surveys. Demographic data, symptoms, and dates of symptoms were recorded. CTMs testing positive at MUSC were linked to viral burden data from nasopharyngeal swabs tested on Abbott PCR platforms. Results: As of January 31, 2021, 111 CTMs tested positive for SARS-CoV-2 following vaccination: 99 and 12 after 1 and 2 doses, respectively, at medians of 10 days (range, 1–22) and 5 days (range, 1–16), respectively, from vaccination to testing. Of 2 cases that tested positive >14 days from dose 2, CTMs had symptom onset at 4 and 12 days from dose 2. Among CTMs reporting symptoms, 104 did so after a median of 7 days (mean 6.3, range −23 to +22) from vaccination to symptom onset, with 8 reporting symptoms before vaccination, 9 on the day of vaccination, and 3 CTMs at 1 day after vaccination, 6 CTMs at 2 days after vaccination, and 11 CTMs at 3 days after vaccination. Overall, 86 (78%) of 111 were female and 95 (86%) of 111 were white. The median age was 44 years (range, 22–71). Clinical job roles were most frequently nurses (n = 31, 28%), physicians or physician extenders (n = 19, 17%), and CTMs with no patient contact (n = 21, 19%). Assessment by the contact-tracing team assigned sources as household clusters (n = 22, 23%), local transmission (n = 21, 22%), occupational acquisition from coworkers (n = 11, 12%), travel related (n = 9, 10%), and unknown (n = 32, 34%). Only 32 (32%) CTMs were compliant with physical distancing. Among 104 CTMs reporting symptoms, cough (75%), headache (71%), rhinorrhea (63%), myalgia (60%), sore throat (48%), anosmia (44%), and subjective fever (40%) were the most commonly reported symptoms. Among 54 symptomatic CTMs with available viral-load data, the median and mean cycle numbers (Cn) were 19.98 and 21.91, respectively, for samples tested a median 3 days from symptom onset. Asymptomatic and symptomatic CTMs had a median Cn of 30.1 vs 20.9, respectively (p <0.001) and overall 50% of vaccinated CTMs had Cn >20, with no significant effect seen by vaccine dose (Figure 1). Conclusions: Most COVID-19 cases following vaccination occurred in CTMs with infection incubating prior to vaccination. No significant attenuation of viral load is apparent among vaccinated CTMs with COVID-19, but asymptomatic CTMs diagnosed with COVID-19 following vaccination appear to have resolved infections. Our data reinforce the need to adhere to public health measures by people who have been vaccinated.
Funding: No
Disclosures: None
Figure 1.