Outpatient parenteral antimicrobial therapy (OPAT) was introduced in the 1970s and has been widely used for infectious diagnoses for which long-term intravenous (IV) therapy is needed. However, OPAT has been associated with higher costs, longer length of hospital stay (LOS), and lower patient satisfaction compared to oral therapy. Reference Krah, Bardsley and Nelson1,Reference Markham and Goldman2 Furthermore, OPAT puts patients at risk for catheter-related complications in addition to antibiotic-related complications. Reference Underwood, Marks, Collins, Logan and Pollara3–Reference Keller, Wang, Salinas, Williams, Townsend and Cosgrove6 Up to ∼40% of OPAT is potentially unnecessary. Reference Markham and Goldman2
Bone and joint infections (BJIs), such as osteomyelitis and septic arthritis, have been considered strong indications for OPAT. Although BJIs have traditionally been treated with at least 6 weeks of intravenous therapy, a small randomized control trial and several observational studies have suggested that oral antibiotic combination therapy might be as effective as intravenous (IV) therapy. Reference Euba, Murillo and Fernández-Sabé7–Reference Tornero, Morata and Martínez-Pastor9 In 2019, a randomized control trial (Oral versus Intravenous Antibiotics for Bone and Joint Infection, the OVIVA trial) reported that oral antibiotic therapy was not inferior to IV therapy for BJI. Reference Li, Rombach and Zambellas10 Following the OVIVA trial, a study investigated the economic impact of implementing the concept of the OVIVA trial for patients with BJI treated using OPAT. These researchers found that ∼80% of patients treated by OPAT were eligible for oral antimicrobial therapy and that changing to oral therapy would lead to a reduction of ∼20 days of intravenous therapy and £1,234 ($1,631) cost reduction per patient. Reference Marks, Bell and Jones11 Another study from an orthopedic hospital reported that, following implementation of the OVIVA trial protocol, two-thirds of patients previously treated with IV antibiotics were treated with oral therapy. Reference Azamgarhi, Shah and Warren12 They observed no difference in clinical outcomes as well as decreased LOS, reduced cost of antibiotic treatment, and increased drug-related complications, mainly gastrointestinal intolerance. Although those studies suggested that oral antibiotic therapy for BJI can be acceptable to physicians and has benefits, they were conducted in United Kingdom. Little evidence about implementing the OVIVA trial findings is available in the United States where antimicrobial resistance patterns and the availability of OPAT are different. How those studies affected the clinical practice of ID physicians in the United States remains unclear.
The Iowa City Veterans Affairs Health Care System (ICVAHCS) has an antimicrobial stewardship program with ID providers and an ID pharmacist overseeing all patients on inpatient antibiotic therapy through daily prospective audit and feedback. In this quality improvement initiative led by the antimicrobial stewardship program, we developed a consensus table among ID providers and ID pharmacists and applied the protocol to suggest oral antibiotics for BJI using the consensus table. We aimed to decrease the use of OPAT for patients admitted for BJI by applying the protocol.
Methods
Population and setting or context
We conducted a quality improvement initiative at ICVAHCS as a part of the Veterans Affairs Quality Scholarship program. Our population of interest included all inpatients admitted with BJI (native vertebral osteomyelitis, peripheral osteomyelitis, native joint septic arthritis, prosthetic joint infection and orthopedic hardware-related infection) for whom the ID service recommended treatment with at least 4–6 weeks of either IV or oral antibiotics. We did not include patients who had complete resection of the infected lesion (ie, amputation) because antibiotics were stopped after a short duration. We also excluded patients if the ID service recommended a short duration of therapy for skin and soft-tissue infection, even with evidence of chronic osteomyelitis. Although we limited our study to patients who had inpatient ID consultation, we believe we covered almost all patients with those conditions because ID consultations are almost always made for patients who may require OPAT and because the antimicrobial stewardship team recommends ID consultation if they have not been consulted already.
Intervention
We began by developing a consensus table of oral antibiotics for BJI. First, based on available literature, we created a draft table for possible oral antibiotic regimens for BJI caused by specific organisms: Staphylococcus aureus, coagulase-negative Staphylococcus, Streptococcus spp, Enterococcus spp, enteric gram-negative rod organisms, Pseudomonas aeruginosa, or unknown organisms. Reference Euba, Murillo and Fernández-Sabé7,Reference Li, Rombach and Zambellas10,Reference Berbari, Kanj and Kowalski13–Reference Oh, Moon and Chung18 Next, a survey questionnaire was distributed using REDCap among ID physicians and ID pharmacists at the University of Iowa Hospitals and Clinics (UIHC) and ICVAHCS. The questionnaire asked how frequently the provider recommends the specific oral antibiotic (1 “almost never” to 5 “very frequently”) in the setting where there is no contraindication for choosing oral antibiotics and the patient does not have a condition for which IV therapy is preferred, such as an undrained abscess or epidural abscess. According to the average score, antibiotics were classified as first-line or second-line oral options, or they were excluded from the table. In the first round of the survey, 17 or 29 survey recipients responded. In the second round of the survey, 11 or 29 survey recipients responded. After 2 rounds of the survey, we developed a consensus table, which we used for the protocol (Supplementary Fig. 1).

Fig. 1. Monthly proportion of patients who received intravenous antibiotics.
Our protocol had 2 major components. A reminder e-mail was sent to the ID staff clinician on service every week regarding the quality improvement initiative with the consensus table. Real-time, case-based discussions were then held between the project leader (H.S.) and the ID provider on service.
All patients with BJIs were identified through the antimicrobial stewardship team’s prospective audit and feedback process, which is conducted daily on weekdays. It was complemented by the inpatient ID consultation list over the weekend. We limited the case discussion (1) to stable patients with BJI who can take oral antibiotics and (2) to patients for whom there were available oral options based on positive microbiology data (ie, either blood culture or local tissue culture). We did not perform a case discussion for patients with conditions for which OPAT is usually preferred (eg, S. aureus bacteremia, a large undrained abscess, an epidural abscess, meningitis, endovascular infection, or negative culture results). These patients were still included in our outcome analysis.
Measures
Patient data were collected through the computerized patient record system (CPRS). We collected age; comorbidities, which were aggregated as Charlson comorbidity index (CCI); the ID provider who made a final antibiotic recommendation; the type of recommended antibiotics (IV or oral); culture results; LOS after final ID recommendation; disposition at discharge (home or post-acute care facility); surgical treatment; recurrence; and death. Our implementation period was November 1, 2020, to May 31, 2021. As a preimplementation period, we obtained data for patients with a BJI from January 1, 2019, to October 31, 2020. If a patient had >1 BJI over the study period, only the first episode was included. Our primary outcome was the proportion of patients treated with OPAT. We considered the following secondary outcomes: LOS after final ID recommendation, total LOS, the proportion of patients discharged to a facility, and recurrence or death within 6 months from the day of the ID service’s final recommendation. Recurrence was defined as an escalation of antibiotic therapy in the setting of worsening infection or reinitiation of antibiotics after completion of therapy for reasons other than perioperational antibiotics for planned surgery. We did not consider planned surgeries as treatment failure because inpatient surgical debridement for peripheral osteomyelitis tended to be deferred until after hospital discharge due to the lack of an inpatient podiatry service at ICVAHCS.
Analysis
A comparison between the preimplementation period and implementation periods was performed using the Student t test for continuous variables and the χ2 test or the Fisher exact test for categorical variables, as appropriate. The monthly number and proportion of patients discharged on OPAT among all BJI patients were displayed with statistical process control (SPC) charts. Statistical analyses were conducted using R version 3.5.0 software (R Foundation for Statistical Computing, Vienna, Austria) and QI Macros Statistical Software (Denver, CO). This study was reviewed by the University of Iowa/ ICVAHCS Institutional Review Board and was determined to be a quality improvement initiative. A waiver of informed consent was granted.
Results
In total, 77 patients during the preimplementation period and 22 patients during the implementation period were identified to have a BJI (Table 1). We detected no significant differences in age, CCI, diagnosis, or culture results between the 2 periods. In both periods combined, peripheral osteomyelitis accounted for 62.6% of all cases, including 46 (58.4%) in the preimplementation period and 16 (72.7%) in the implementation period. Furthermore, 70.1% of patients during the preimplementation period received OPAT, whereas only 31.8% of patients received OPAT during the implementation period (P = .003). The median LOS after the final ID recommendation was significantly shorter during the implementation period (median 3 days vs 1 day; P < .001), whereas the difference was not significant for total LOS (median 7 days vs 6 days; P = .06). The proportion of patients discharged home was higher in the implementation period, although the difference was not statistically significant (66.2% vs 86.4%; P = .07). We detected no significant difference in the 6-month rate of recurrence (24.7% vs 31.8%; P = .46) or mortality (9.1% vs 9.1%; P = 1.00). The monthly proportion of patients who had OPAT among all eligible patients with BJI is shown in Figure 1. Compared to the preimplementation period, there was a significant decrease in the proportion of patients who were started on OPAT during the implementation period.
Table 1. Comparison of Patient’s Characteristic Between the Preimplementation and Implementation Periods

Among the 38 patients who were treated with oral antibiotics, the most commonly selected antibiotic was amoxicillin–clavulanate (20 patients) followed by fluoroquinolones (16 patients), doxycycline (13 patients), metronidazole (13 patients), linezolid (2 patients) and trimethoprim–sulfamethoxazole (2 patients). Also, >2 oral antibiotics were used in 16 patients. In patients for whom fluoroquinolones were used for treatment, they were used to treat GNR in 10 of 16 cases. In the 6 remaining cases, fluoroquinolones were used as an empiric therapy because no organism was identified.
During the implementation period, a case discussion was held for 8 patients (36.4%). After these discussions, 3 cases were treated with OPAT and 5 cases were treated with oral therapy. The following reasons were given for not holding a case discussion: no culture result to guide therapy (9 patients), final recommendation from the ID service had already been made (3 patients), and S. aureus bacteremia (2 patients).
In a sensitivity analysis limited to patients with peripheral osteomyelitis, the proportion of patients who had OPAT was 57.8% in the preimplementation period and 25.0% in the implementation period, respectively (P = .05). The median LOS was still significantly shorter in the implementation period: 2 days versus 1 day (P = .01). Surgical debridement was performed during treatment in 37 (60.7%) cases, and recurrence rates did not differ between the 2 periods (Supplementary Table 1 online).
The comparison of patients who received OPAT and with those who received oral antibiotics is shown in Table 2. A significantly higher proportion of patients who received oral therapy had peripheral osteomyelitis: 49.2% in the OPAT group versus 81.6% in oral antibiotics group (P = .003). The 6-month rate of recurrence was higher in the oral antibiotic group although the difference was not statistically significant: 21.3% in the OPAT group versus 34.2% in the oral antibiotics group (P = .24). Details of patients who had recurrence or death within 6 months are listed in Table 3.
Table 2. Comparison of Patient’s Characteristics Between Those Who Received Outpatient Parenteral Antimicrobial Therapy (OPAT) and Those Who Received Oral Antibiotics
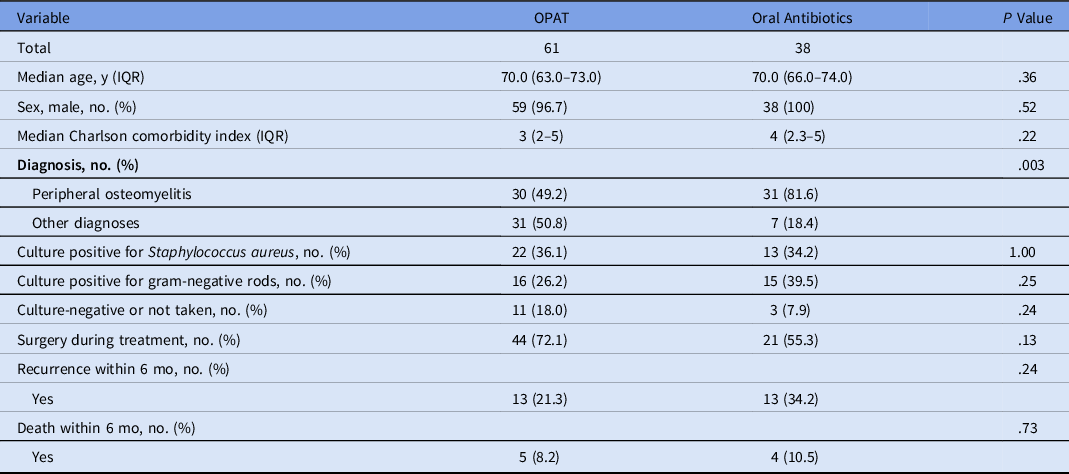
Table 3. Description of 31 Patients Who Experienced Recurrence or Death Within 6 Months

Note. M, male; F, female; ID, infectious diseases; MSSA, methicillin-susceptible Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus; OPAT, outpatient parenteral antimicrobial therapy; IV, intravenous; PJI, prosthetic joint infection; BKA, below-knee amputation; AKA, above-knee amputation.
Discussion
Infectious disease healthcare providers used significantly less OPAT for BJIs after implementation of our protocol, which included weekly e-mail reminders and case-based discussions. The LOS after the final ID recommendation was significantly shorter during the implementation period. Clinical outcomes, defined as recurrence or death during the 6 months after final ID recommendations, were not significantly different between the preimplementation period and the implementation period.
Increased use of oral antibiotics for BJI in this study may imply that ID providers are changing their practice and moving away from the traditional dogma “IV therapy is required for BJI” and accepting oral antibiotics for BJI. Although we saw a rapid decrease in OPAT use after application of our protocol, we do not think our protocol changed ID provider practice solely by itself. The protocol was implemented nearly 2 years after the OVIVA trial was published, and providers may have already been starting to change their practice. Another important factor that could have affected the shift to oral therapy was the coronavirus disease 2019 (COVID-19) pandemic. The COVID-19 pandemic could have made ID providers more likely to choose oral antibiotics to avoid physical, in-person visits or nursing home placement to administer OPAT. Nevertheless, we believe that the process of developing the protocol and its implementation augmented ID providers’ acceptance for oral antibiotics through presenting evidence for the utility of oral antibiotics. Our experience suggests that developing hospital-specific guidance for BJIs, which draws input from multiple stakeholders, may be an effective implementation strategy for incorporating the OVIVA findings into routine medical care. Reference Waltz, Powell and Matthieu19
The most common oral antibiotics used for treatment of BJI in our study was amoxicillin–clavulanate. Because most of our cases were peripheral osteomyelitis (eg, diabetic foot infections, which are frequently polymicrobial), it is reasonable that amoxicillin–clavulanate, which covers gram-positive pathogens, gram-negatives pathogens, and anaerobes, was frequently used. The second most used antibiotic was fluoroquinolones. Interestingly, this class was used mainly to treat gram-negative infection or a part of empiric therapy but did not seem to be used for Staphylococcus infection. This finding is somewhat contrary to the OVIVA study in which fluoroquinolones were used for >40% of cases, including BJIs caused by S. aureus. Reference Li, Rombach and Zambellas10 The difference could be at least partially explained by the reluctance of ID providers to use fluoroquinolones for S. aureus due to the US Food and Drug Administration (FDA) warnings about the side effects of fluoroquinolones, 20,21 the unnecessarily broad spectrum these agents provide, and the increasing resistance of S. aureus to fluoroquinolones in the United States. Reference Sader, Castanheira, Arends, Goossens and Flamm22 In addition, a previous study in the United States reported far more unintended drug discontinuation with fluoroquinolone-based regimens for prosthetic joint infections compared to non–fluoroquinolone-based regimens. It is possible that ID providers are not comfortable in selecting fluoroquinolone as a drug of choice for S. aureus BJI in the United States. Reference Vollmer, Rivera and Stevens23
Our 6-month recurrence rates were 21.3% in patients treated with OPAT and 34.2% in patients treated with oral antibiotics. These recurrence rates were higher than those reported in the OVIVA trial, which reported 1-year recurrence rates of 14.6% in OPAT group and 13.2% in the oral antibiotic group. Reference Li, Rombach and Zambellas10 The difference can be at least partially explained by the different types of BJI between the 2 cohorts and the lower rate of timely surgical treatment in our study compared to the OVIVA trial. Although most of our patients had peripheral osteomyelitis, ∼60% of patients in the OVIVA trial had prosthetic joint infections or orthopedic device–related infections, all of whom underwent some form of debridement. In addition, only ∼60% of patients with peripheral osteomyelitis in our cohort received timely surgical treatment; this was much lower than that of the OVIVA trial, which reported that 85.6% of patients with chronic osteomyelitis underwent debridement. Reference Li, Rombach and Zambellas10 It is possible that peripheral osteomyelitis carries a higher recurrence rate without timely surgical treatment. Based on these findings, the ID providers at the ICVAHCS are engaging our local orthopedic and podiatry services to discuss how patients with peripheral osteomyelitis, namely due to diabetic foot infections, can receive more timely surgical interventions.
This study had several limitations. It was a single-center study within the VA healthcare system, and the number of patients included in this quality improvement initiative was relatively small. We were not able to continue the implementation longer because of the lack of available resources. Therefore, it is possible that we could not detect a true difference in clinical outcomes due to type II error. In fact, the 6-month rate of recurrence was higher in the oral antibiotic group compared to the OPAT group, although the difference was not statistically significant. The difference might have been confounded by the fact that oral antibiotics were used more often for peripheral osteomyelitis and that many of these patients did not have timely surgical debridement. We were unable to perform a more robust analysis (eg, an interrupted time-series analysis) due to the small number of patients. Our local practice might have affected the results. For example, because there is not an inpatient podiatry service at the ICVAHCS, many patients with diabetic foot osteomyelitis could not have timely surgical debridement while hospitalized. This factor might have affected some decisions by ID provider to administer IV therapy because source control was not complete, and this could have increased the chance for recurrence in those patients with peripheral osteomyelitis. We used recurrence or death within 6 months from the ID service’s final recommendation as the definition of clinical failure. This time frame was shorter than that of some previous studies that followed patient outcomes for 1 year. BJIs are sometimes caused by indolent organisms and recur after months of antibiotic therapy. Furthermore, we could not detect a recurrence if the patient received care outside the VA system. For those reasons, we may have underestimated the occurrence of clinical failure. We were unable to assess whether unmeasured factors, such as the COVID-19 pandemic, affected the practice change we observed. Lastly, we did not collect other relevant information such as adverse reactions associated with IV and oral antibiotics, cost associated with antibiotic treatment, or patient satisfaction.
In conclusion, more patients admitted with BJIs were treated with oral antibiotics during the implementation phase of our quality improvement initiative. The presence of an antibiotic stewardship team provided a structure to identify and encourage oral treatment for eligible patients. Although the use of more oral antibiotics led to shorter LOS and likely led to more patients discharged home, the overall recurrence rate on oral therapy was higher than we anticipated, which may reflect the high proportion of cases with peripheral osteomyelitis and the inconsistent performance of surgical debridement. BJI is heterogenous, and in some situations oral therapy can be safely used as an alternative to IV therapy, but in other situations IV therapy is still better. Larger studies will be needed to validate our findings regarding clinical outcomes and to further investigate the best situations in which oral antibiotics can be used for BJI.
Acknowledgments
We thank members of VA Quality Scholarship Program Iowa City (Drs Nicole Loew, Tamara Tasseff, Amy Brown, Ashley Stillwell, Karla Miller, Sherry Brewer, Jamie Wilson, and Amany Farag) for their constructive feedback regarding this project. The content is solely the responsibility of the authors and does not necessarily represent the official views of the VA Quality Scholars or the Department of Veterans Affairs.
Financial support
This project was supported by the VA Quality Scholars fellowship through the VA Office of Academic Affiliations Advanced Fellowships Program.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ash.2021.250






