Published online by Cambridge University Press: 29 July 2021
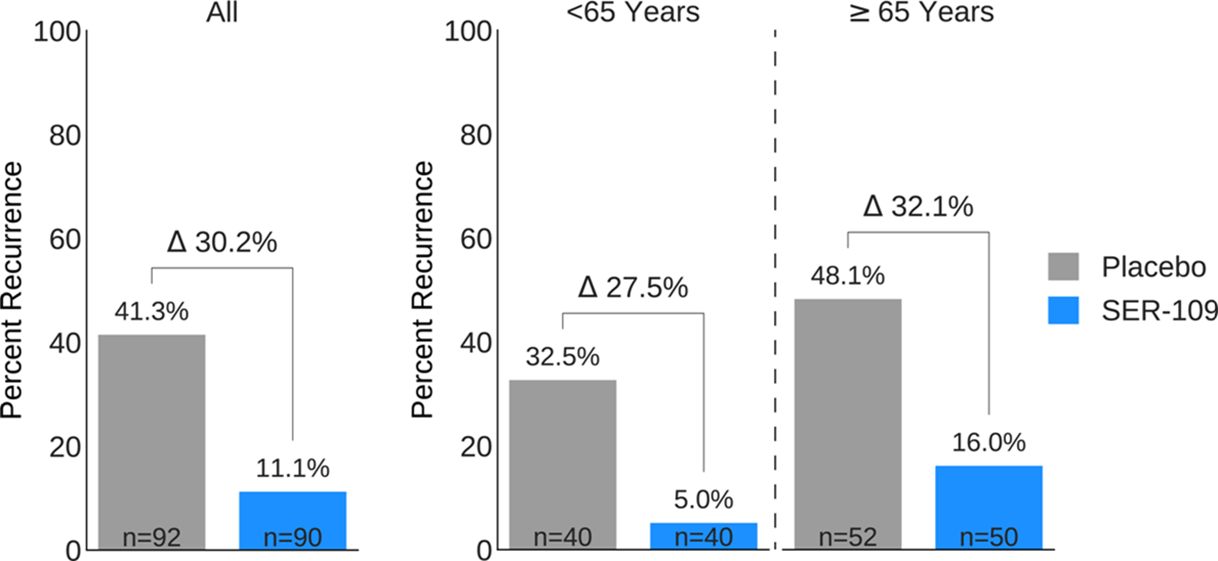
Background: Antibiotics targeted against Clostridioides difficile bacteria are necessary, but insufficient, to achieve a durable clinical response because they have no effect on C. difficile spores that germinate within a disrupted microbiome. ECOSPOR-III evaluated SER-109, an investigational, biologically derived microbiome therapeutic of purified Firmicute spores for treatment of rCDI. Herein, we present the interim analysis in the ITT population at 8 and 12 weeks. Methods: Adults ≥18 years with rCDI (≥3 episodes in 12 months) were screened at 75 US and CAN sites. CDI was defined as ≥3 unformed stools per day for <48 hours with a positive C. difficile assay. After completion of 10–21 days of vancomycin or fidaxomicin, adults with symptom resolution were randomized 1:1 to SER-109 (4 capsules × 3 days) or matching placebo and stratified by age (≥ or <65 years) and antibiotic received. Primary objectives were safety and efficacy at 8 weeks. Primary efficacy endpoint was rCDI (recurrent toxin+ diarrhea requiring treatment); secondary endpoints included efficacy at 12 weeks after dosing. Results: Overall, 287 participants were screened and 182 were randomized (59.9% female; mean age, 65.5 years). The most common reason for screen failure was a negative C. difficile toxin assay. A significantly lower proportion of SER-109 participants had rCDI after dosing compared to placebo at week 8 (11.1% vs 41.3%, respectively; relative risk [RR], 0.27; 95% confidence interval [CI], 0.15–0.51; p-value <0.001). Efficacy rates were significantly higher with SER-109 vs placebo in both stratified age groups (Figure 1). SER-109 was well-tolerated with a safety profile similar to placebo. The most common treatment-emergent adverse events (TEAEs) were gastrointestinal and were mainly mild to moderate. No serious TEAEs, infections, deaths, or drug discontinuations were deemed related to study drug. Conclusions: SER-109, an oral live microbiome therapeutic, achieved high rates of sustained clinical response with a favorable safety profile. By enriching for Firmicute spores, SER-109 achieves high efficacy while mitigating risk of transmitting infectious agents, beyond donor screening alone. SER-109 represents a major paradigm shift in the clinical management of patients with recurrent CDI. Clinicaltrials.gov Identifier NCT03183128. These data were previously presented as a late breaker at American College of Gastroenterology 2020.
Funding: Seres Therapeutics
Disclosures: None
Figure 1.