The emergence and spread of the novel human coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has placed significant strain on healthcare resources. Single-use N95 filtering facepiece respirators (FFRs) are essential tools in the prevention of SARS-CoV-2 transmission among those in patient-facing roles. Reference Nguyen, Drew and Graham1 An overwhelming demand for ventilators, laboratory consumables, and personal protective equipment (PPE), including FFR, has created shortages that were further affected by the disruption of global supply chains. Reference Cohen and Rodgers2 In response, many healthcare institutions designed tiered plans for PPE inventory monitoring similar to those recommended by the Centers for Disease Control and Prevention. 3,4 These measures included allowing FFR to be worn for multiple patient contacts, limiting their use to specific clinical situations (eg, aerosol-generating procedures) or reprocessing and reusing N95 FFR beyond their shelf life.
The reprocessing of FFR involves a decontamination procedure to remove bioburden from the FFR surface that may include infectious particles (eg, bacteria, viruses, or fungi). This can be achieved using systems designed for the sterilization of medical devices or those used for postdischarge decontamination of hospital rooms. Reference Russo, Levine and Grady5 Hydrogen peroxide (H2O2), ultraviolet germicidal irradiation, and moist and dry heat methods have been examined for this purpose. Reference Ibáñez-Cervantes, Bravata-Alcántara and Nájera-Cortés6–Reference Zulauf, Green and Nguyen Ba9 To ensure that these treatments do not compromise the FFR, filtration, fit and any chemical residue must be evaluated in accordance with industry standards. 10,Reference Salter, Kinney and Wallace11 Several commercial H2O2-based systems have received emergency authorization for FFR decontamination during the coronavirus disease (COVID-19) pandemic due to their minimal impact on respirator integrity. 12,13 Multiple healthcare facilities in North America have openly discussed the design and implementation of their FFR reprocessing during the COVID-19 pandemic Reference Russo, Levine and Grady5,Reference Beam, Nesbitt, Austin and Ramar14–Reference Jatta, Kiefer and Patoila16 ; however, none have described evaluations of the efficacy of their processes through sterility testing or mechanisms for continuous quality monitoring.
The concept of FFR decontamination and reuse is not unique to the COVID-19 pandemic, Reference Heimbuch, Wallace and Kinney17,Reference Lore, Heimbuch, Brown, Wander and Hinrichs18 but it remains somewhat controversial. The utility of these processes relies on their ability to reduce or eliminate pathogens and other biological substances from porous FFR material. Previously worn N95 FFR can retain high burdens of live microorganisms, which may be present alongside other biological materials that can interfere with the decontamination process. Reference Russo, Levine and Grady5,Reference Rutala, Gergen, Sickbert-Bennett and Weber19 Given the current pandemic, many recent studies focus primarily on the activity of H2O2-based methods against SARS-CoV-2 or surrogate viruses. However, some patients infected with SARS-CoV-2 can have co-occurring or secondary bacterial infections. Reference Langford, So and Raybardhan20 This finding suggests that aerosols or other respiratory fluids may be polymicrobial and could include organisms that are more resistant to H2O2 treatment. The efficacies of H2O2 decontamination processes against nonviral organisms have not been fully evaluated. In this study, we evaluated the efficacy of 4 H2O2-based decontamination methods to reduce gram-positive and gram-negative loads on artificially contaminated N95 FFR.
Materials and methods
Bacterial strains and culture conditions
To represent bacteria that vary in their resistance to hydrogen peroxide, we selected 4 gram-positive bacteria: Staphylococcus aureus ATCC 29213 (methicillin susceptible, MSSA), S. aureus ATCC 43300 (methicillin resistant, MRSA), Bacillus subtilis ATCC 6633, and a vancomycin resistant clinical isolate of Enterococcus faecium. We then selected 2 gram-negative bacteria: Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853. Each organism was freshly subcultured onto Columbia agar with 5% (v/v) sheep blood (Thermo Fisher Scientific, Nepean, ON, Canada) and incubated in 5% CO2 at 35°C for 18–24 hours prior to each experiment.
Decontamination devices, respirator inoculation, and processing
We evaluated 4 H2O2-based decontamination devices that had been used to treat N95 FFRs at 4 clinical centers across the Greater Toronto Area (Ontario, Canada) during the first COVID-19 pandemic wave: STERRAD 100NX (Advanced Sterilization Products, Irvine, CA), Sterizone VP4 (Stryker, Kalamazoo, MI), Clēan Works Clean Flow Mini (Clean Works, St Catherine’s, ON, Canada) and Bioquell Z-2 (EcoLab, St Paul, MN) systems (Table 1). Devices were operated in accordance with recommended N95 FFR decontamination protocols (Table 1).
Table 1. Differences in N95 Decontamination Parameters Across the 4 Devices Used

Note. HPV, hydrogen peroxide vapor; UV-C, ultraviolet C light.
a Reflects the total capacity of a device suggested by the manufacturer under the assumption that no additional factors (eg, staffing) are limiting factors.
b As recommended by the respective manufacturer.
Devices were challenged with up to 5 different 3M N95 FFR models (3M 1860, 1860S, Aura 1870+, 8210, 8110S, or VFlex 9105S or 1805) that were cut in half to conserve FFR supplies. Briefly, 3 cell suspensions of each test organism equivalent to a 0.5 McFarland standard (˜1.5×108 cells/mL) were prepared by emulsifying 4–5 colonies from fresh subculture plates into sterile 0.45% (v/v) saline. Three 1-cm × 1-cm squares were drawn onto the patient-facing side of each FFR half using a permanent marker and inoculated with 10 µL of the corresponding 0.5 McFarland suspension. Five FFR halves were inoculated for each device, which included a positive (untreated) and negative (inoculated with 0.45% (v/v) saline) control. Following inoculations, FFRs were air dried (20–30 minutes) and placed into a sealed Tyvek pouch for distribution to the testing sites. Decontamination was performed according to the device manufacturer’s recommendations (Table 1). Successful cycle completion was confirmed using biological monitors or indicators where appropriate. Respirators treated by the Bioquell Z-2 and Clean Works instruments were removed from their packaging and repackaged after treatment. All FFRs were returned to the University Health Network/Sinai Health Microbiology Laboratory (Toronto, ON) within 24 hours. Additional procedural information can be found in the Supplementary Materials (online).
Determination of decontamination efficacy
Inoculated segments (1-cm Reference Cohen and Rodgers2 squares) were excised from treated FFR using sterile scissors and placed into 10 mL brain heart infusion broth (Thermo Fisher). Colony counts were obtained as follows: inoculated tubes were placed in an orbital shaker (4°C for 5 minutes at 250 rpm), mixed with a vortexer (5 seconds), placed in 1-mL aliquots on a Columbia sheep blood agar plate, and incubated for 18–24 hours at 37°C in 5% CO2. The remaining broth was incubated (48 hours at 37°C) and subcultured onto Columbia sheep blood agar if turbid. Colonies were identified using the VITEK matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF MS) system (bioMérieux, Marcy-l'Étoile, France). Decontamination efficacy, defined as the percentage reduction in colony-forming units (CFU), was determined by comparing colony counts cultured from treated and untreated FFR segments. The effectiveness of sterilization was determined using Health Canada guidance (e.g. minimum 6-log reduction) (FDA 2020). 21
Results
Bacterial recovery was not influenced by FFR model because all test organisms could be cultured from excised FFR segments that had been inoculated, dried, and placed into a Tyvek pouch but not sterilized in initial pilot experiments (data not shown). All decontamination cycles in each instrument were completed successfully, as noted by biological monitors used at each testing site. All decontamination devices exceeded FDA recommendations (≥6-log reduction in the inoculated microbial burden for all 6 organisms based on an average inoculum (CFU/mL) of the bacterial suspension of 5.9×108 for E. coli ATCC 25922, 6.5×108 for P. aeruginosa ATCC 27853, 8.1×108 for S. aureus ATCC 25923, 7.9×108 for S. aureus ATCC 43300, 5.0×108 for B. subtilis ATCC 6633, and 5.9×108 for the clinical VRE isolate. However, complete sterilization of the N95 FFRs of all gram-positive organisms was not universally achieved.
All 4 systems performed equally well with gram-negative organisms. Neither E. coli ATCC 25922 nor P. aeruginosa ATCC 27853 were recovered on any of the inoculated segments from treated FFRs, corresponding to a decontamination efficacy of 100.0% and complete sterilization being achieved. The most effective system for decontamination in our hands was the Bioquell Z-2, which demonstrated 100% effectiveness and complete sterilization of all 6 test organisms on the five 3M FFR models tested (Table 2). Similar performance characteristics were observed with the Clēan Flow Mini system with no viable organisms being recovered for 5 of the 6 pathogens following decontamination. Nevertheless, this system had difficulty eliminating VRE, which was recovered on a single coupon from an 1805 model FFR.
Table 2. Comparative Evaluation of Bacterial Recovery From N95 FFRs Following Decontamination
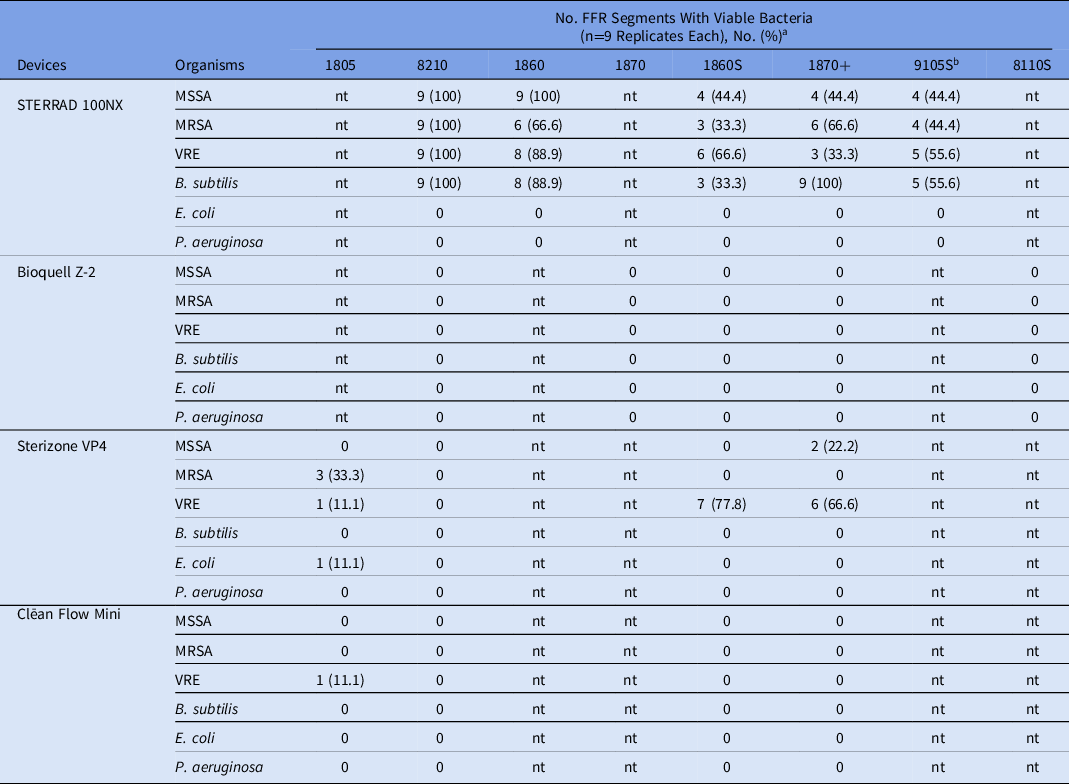
a nt, not tested.
b Cellulose containing respirator and should not be decontaminated with H2O2.
Although acceptable CFU reductions based on FDA decontamination standards, the H2O2 gas plasma STERRAD 100NX and H2O2/ozone Sterizone VP4 systems were unable to completely sterilize FFR inoculated with gram-positive bacteria (Table 2). Both MSSA and MRSA strains remained viable on up to 100.0% of FFR segments following decontamination by the STERRAD 100NX, with mean percentage reductions of 99.97% (range, 99.84%–100.0%) and 99.98% (range, 99.91%–100%), respectively. The B. subtilis strain was completely sterilized in only 11 of the 45 total excised segments (24.4%) from STERRAD 100NX–treated FFR, with an average of 11.2 CFU (range, 2–46 CFUs) recovered from the remaining inoculated segments posttreatment (data not shown). Similar to the S. aureus strains, VRE remained viable on up to 100.0% of the inoculated segments (range, 33.3%–100.0%), with mean percentage reductions in CFU of 99.96% to 99.99%. The 3M 8210 model FFR performed the poorest with the STERRAD 100NX treatment, and all 4 gram-positive organisms were recovered from all 36 segments inoculated (100.0%). In contrast, the Sterizone VP4 system performed well with MSSA and B. subtilis (100.0% reduction). An average of 64 CFU of MRSA were recovered from 4 of 36 inoculated segments, most of which (75.0%) were extracted from the 1805 FFR model. The VRE strain was 100.0% killed following sterilization in 21 of 36 inoculated segments tested, and the percentage reduction ranged from 99.86% to 99.99% in the remaining segments. In contrast to the performance in the STERRAD 100NX, no viable organisms were recovered from the 3M 8210 FFR following treatment with the Sterizone VP4 device.
Discussion
The implementation of FFR decontamination and reuse strategies can assist healthcare facilities with restoring depleted supplies to protect those in patient-facing roles from pathogens in droplets or aerosols. The 4 H2O2-based methods evaluated here differed in their ability to decontaminate artificially soiled FFR and were less likely to completely sterilize FFR carrying a high inoculum of gram-positive pathogens.
We challenged the 4 devices with bacteria that are more tolerant to H2O2 than other organisms such as enveloped viruses (eg, SARS-CoV-2). Reference Wigginton, Arts and Clack7,Reference Kumar, Kasloff and Leung22 The properties of the 4 systems, the organisms tested, and FFR type can influence performance. Viable gram-positive organisms were recovered from FFR segments treated by the STERRAD 100NX and Sterizone VP4 systems. Differences in the physiologic properties of our test bacteria including cell-wall composition, organism size, and the production of environment-modulating enzymes, may contribute greatly to these distinct reactions. We inoculated FFRs with a high bacterial burden to simulate clinical situations such as pneumonia, in which secretions may contain upwards of 107 CFU per gram of sputum. Reference Sibila, Lasema and Shoemark23 At high cell densities, H2O2 degradation can occur via catalase enzymes produced by S. aureus soon after exposure and can contribute to their persistence. Reference Otter and French24 Recently, Ibáñez-Cervantes et al Reference Ibáñez-Cervantes, Bravata-Alcántara and Nájera-Cortés6 reported that S. aureus was not recoverable from 3M 8210 model FFRs when inoculated at concentrations of 102–106 CFU and treated by the STERRAD NX system, which is discordant with our study. This incongruity may be explained by differences in cycling parameters between the STERRAD NX and 100NX systems, which also complicated our ability to compare results between studies. 25
The Bioquell Z-2 device performed the best of the 4 devices in our study. This system was operated in a specially designed room dedicated to contactless sterilization, and stringent parameters were applied to prevent the dissipation of sterilant and ensure optimal distribution and contact (eg, sterilization-resistant surfaces and barriers). Wigginton et al Reference Wigginton, Arts and Clack7 noted that S. aureus ATCC 29213 could be recovered with high frequency from inoculated 3M 1860 FFR segments following treatment with a similar Bioquell Q10 system, which also performed poorly with several viruses. Reference Wigginton, Arts and Clack7 Whether differences in experimental design played a role in these variable results is unclear. Challenges with FFR decontamination by low-temperature sterilization approaches are perhaps not surprising; recent reports have noted sterilization failures with S. aureus, VRE, and other organisms on other nonporous surfaces such as stainless steel. Reference Rutala, Gergen, Sickbert-Bennett and Weber19,Reference Smith, Hanseler and Welle26 Thus, additional sterility monitoring (alongside any biological test standards) should be used to ensure the safety and efficacy of these processes among decontaminated FFR batches, particularly when FFR have been previously treated and reused.
Our study has several limitations. FFR resources were limited, so only a small number were processed in this study and all were cut in half for testing. We did not assess variation in decontamination efficacy across different parts of the FFRs (eg, the head straps or metal nosepiece) or the impact of the 4 sterilization modalities on fit, filtration efficiency, and any potential posttreatment chemical exposures to healthcare providers. Our test organisms were limited to several common bacterial pathogens with varying levels of H2O2 resistance due to the enhanced biosafety requirements needed to propagate SARS-CoV-2 and other viruses. Because FFRs were inoculated directly from cell suspensions rather than in the presence of a biological sample matrix that may impair decontamination efficacy (eg, saliva, sputum, or pus), the results of our evaluation may not translate directly to the clinical setting. Regardless, we demonstrated that gram-positive organisms were difficult to decontaminate the H2O2 treated FFRs.
Reductions in CFU equivalent to 6-log was noted for all test organisms, which may be insufficient in heavily contaminated FFR where the starting bacterial load may be much higher than that used here. The persistence of epidemiologically significant organisms, such as MRSA and VRE, in the hospital environment is of concern due to the possible spread, colonization, and safety risk this may pose to both patients and healthcare providers. There is some debate regarding whether our test organisms could be found on N95 FFR prior to decontamination. Hospital air sampling studies have identified MRSA and carbapenemase-producing P. aeruginosa among others, which suggests that these organisms could unknowingly contaminate FFR surfaces. Reference Creamer, Shore and Deasy27,Reference Hopman, Meijer and Kenters28 Facilities must recognize the limitations associated with decontaminating and reusing FFRs that may have been soiled with clinically significant pathogens other than SARS-CoV-2.
In conclusion, prior to initiating FFR decontamination practices, institutions should ensure that quality assurance plans include assessments of FFR bioburden reduction and routine sterility monitoring to verify the effectiveness of decontamination and safety of reprocessed FFRs.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/ash.2021.183
Acknowledgments
We thank the technical staff at University Health Network (Toronto, Canada), Sinai Health System (Toronto, Canada), The Centre for Phenogenomics (Toronto, Canada), The Hospital for Sick Children (Toronto, Canada) and St. Joseph’s Healthcare (Hamilton, Canada) for assisting with decontamination experiments. This study was supported by Ontario Health. The authors have no conflicts to disclose.




