Introduction
Clostridioides difficile is the leading infectious cause of healthcare-associated (HA) diarrhea in developed countries. Reference Kelly, Pothoulakis and LaMont1–Reference McFarland3 It is associated with significant morbidity, with clinical manifestations ranging from asymptomatic colonization, severe diarrhea, to toxic megacolon, and death. Reference Kelly, Pothoulakis and LaMont1 In 2003, many hospitals in Québec, Canada, experienced an epidemic of C. difficile infection (CDI) associated with increased severity of disease and recurrences. Reference Valiquette, Low, Pepin and McGeer4–Reference Pepin, Valiquette and Cossette6 A fourfold increase in C. difficile attributable mortality was observed in Canada between 1997 and 2005. Reference Gravel, Miller and Simor7 This epidemic was associated with the dissemination of the hypervirulent North American Pulsed Field Type 1 (NAP1)/ribotype 027 strain. Reference Warny, Pepin and Fang8 Outbreaks associated with this strain have been reported worldwide. Reference He, Miyajima and Roberts9 Recent studies from the United States demonstrate a decreasing trend for HA-CDI in the last decade with a reduced prevalence of the NAP1/027 strain. Reference Giancola, Williams and Gentry10,Reference Guh, Mu and Winston11
Antibiotic use is the major risk factor associated with CDI. Antibiotic classes historically associated with high risk of CDI include cephalosporins and clindamycin. Reference Owens, Donskey, Gaynes, Loo and Muto12 The epidemic dissemination of the NAP1/027 strain was found to be related to fluoroquinolone utilization. Reference Loo, Poirier and Miller13,Reference Pepin, Saheb and Coulombe14 Interventions aimed at restricting the use of these high-risk antibiotics have been associated with a marked decrease in the incidence of CDI in Québec, the United States, and Europe. Reference Valiquette, Cossette, Garant, Diab and Pepin15–Reference Dancer, Kirkpatrick, Corcoran, Christison, Farmer and Robertson18 A UK study combining whole genome sequencing and antimicrobial prescribing data revealed that CDI regression in England was driven by the effect of fluoroquinolone restriction on fluoroquinolone-resistant strains. Reference Dingle, Didelot and Quan19 In the United States, inpatient fluoroquinolone use was found to be a predictor of HA-CDI due to the NAP1/027 strain. Reference Silva, Wilson, Redmond and Donskey20 Furthermore, a recent large-scale 6-year ecological study performed in over 500 US hospitals demonstrated that temporal changes in antibiotic utilization correlated with changes in HA-CDI. Reference Kazakova, Baggs and McDonald21 These studies support antibiotic stewardship as a major factor in reducing HA-CDI incidence. However, reductions in the prevalence of the NAP1/027 strain were observed in absence of nationwide reductions in fluoroquinolones use. Reference Hensgens, Goorhuis, Notermans, van Benthem and Kuijper22 Other studies and experts have advocated that other unmeasured factors (eg, infection control interventions) were likely implicated in the observed decrease in HA-CDI incidence. Reference Fortin, Thirion and Ouakki23,Reference Eyre, Dingle and Didelot24
The C. difficile epidemic in Québec peaked in 2004–2005 with an incidence of 12.6 cases per 10,000 patient-days. 25 In the past 15 years, the incidence of HA-CDI has decreased in Québec, reaching 3.14 cases per 10,000 patient-days in 2019–2020. 26 A series of infection control measures, including an antibiotic stewardship program, 26 were implemented at our institutions over this period in response to the Québec epidemic. The aim of this study was to assess the impact of antibiotic utilization on HA-CDI incidence and the NAP1/027 strain in two major academic centers in Montreal, Québec, Canada.
Methods
Population and surveillance period
We conducted an observational study with antibiotic utilization data to study its effect on both HA-CDI incidence and C. difficile genotypes at the Royal Victoria Hospital (RVH) and Montreal General Hospital (MGH), two adult tertiary care hospitals of the McGill University Health Centre (MUHC), in Montreal, Canada between April 1, 2003, and March 31, 2020.
HA-CDI incidence
Patients with a positive assay for toxigenic C. difficile were identified by the MUHC laboratory and reported to the infection prevention and control service. Laboratory detection was performed by cell cytotoxin neutralization assay (CCNA) until June 2009, following which the assay was changed to a commercial nucleic acid amplification test (NAAT). Patients were considered to have HA-CDI if they met one of the following criteria: diarrhea with at least three liquid to semi-liquid stools in 24 hours without any other apparent etiology, or toxic megacolon with a positive C. difficile toxin assay, a change in stool characteristics from baseline without apparent cause in a patient known for chronic diarrhea with a positive C. difficile toxin assay, endoscopic evidence of pseudomembranous colitis, or histopathologic evidence of C. difficile colitis. 25 A case was considered as HA if symptoms occurred more than 72 hours after admission or within 4 weeks of a previous hospitalization. Outpatients with CDI who were not associated with a previous hospitalization in the last 4 weeks, and patients admitted to the obstetrics and psychiatry wards were excluded from the study. HA-CDI incidence was calculated as the number of cases per 10,000 patient-days.
Antibiotic utilization
Antibiotic utilization over the study period was extracted from MUHC pharmacy data. Antibiotic consumption was measured in defined daily doses (DDDs) per 1,000 patient-days. We focused on the following antibiotic classes: first-generation cephalosporins, second-generation cephalosporins, third-generation cephalosporins, narrow spectrum penicillins (penicillin, ampicillin, cloxacillin, amoxicillin), beta-lactam/beta-lactamase inhibitors, carbapenems, fluoroquinolones, aminoglycosides, sulfonamides (trimethoprim-sulfamethoxazole), tetracyclines, macrolides, lincosamides (clindamycin), and glycopeptides (vancomycin). These classes of antibiotics were selected based on the frequency of their use and suspected risk for CDI. Reference Owens, Donskey, Gaynes, Loo and Muto12 We excluded oral vancomycin from total antibiotic utilization given the assumed direct correlation of CDI incidence with its usage, as well as metronidazole due to the inability to determine retrospectively what proportion of its utilization was for the treatment of CDI.
C. difficile isolate genotyping
All stool samples that were positive for C. difficile toxin gene detection by NAAT at our institution were routinely genotyped by the infection control service for outbreak surveillance from 2010 at one institution and from 2014 at the second institution. These isolates were typed by pulsed-field electrophoresis using standard methods from January 2010 to May 2012. Reference Corkill, Graham, Hart and Stubbs27 Ribotyping by standard methods was used from June 2012 to March 2020. Reference Fawley, Knetsch and MacCannell28
Infection control interventions
Infection control interventions implemented at our institution over the study period were reviewed.
Statistical analysis
A dynamic regression model for time series was used to estimate the impact of antibiotic usage (predictor) on CDI incidence (outcome) over time (quarterly data from 2003–2004 to 2019–2020 for both sites) while taking into account the possibility of autocorrelated error terms. Reference Hyndman and Athanasopoulos29 Recognizing that a change in antibiotic usage may not have an immediate effect on CDI incidence, we performed a multiplier analysis by allowing for a two-period lagged effect, with each period representing roughly 3 months. It allowed us to estimate the impact on the CDI incidence we could expect to observe if antibiotic usage was equally increased contemporaneously, 3 months and 6 months ago. We compared antibiotic usage and the proportion of NAP1/027 strains on a biannually basis from 2014–2015 to 2019–2020 for the MGH site and from 2010–2011 to 2019–2020 for the RVH site by calculating the observed change in percentage in both variables between 2014–2015 and 2019–2020 for MGH and between 2010–2011 and 2019–2020 for RVH. A Sieve-bootstrap Mann-Kendall’s trend test was used to test for monotonic trend in antibiotic usage and proportion of NAP1/027 strains. Reference Noguchi, Gel and Duguay30 Analyses were performed using the R statistical software version 4.0.3. 31 Model selection was performed using the stepwise algorithm outlined in Hyndman and Khandakar Reference Hyndman, Bergmeir and Caceres32 and implemented in the forecast R package. Reference Hyndman and Khandakar33
Ethics approval
Institutional review board approval was obtained for this study. All patient information was de-identified.
Results
Healthcare-associated CDI incidence
The incidence of HA-CDI decreased between April 2003 and March 2020 at both institutions. At the MGH, the incidence decreased from 19.1 cases per 10,000 patient-days in the first quarter of 2003 to 3.0 cases per 10,000 patient-days in the last quarter of 2020. At the RVH, the incidence decreased from 35.7 cases per 10,000 patient-days in the first quarter of 2003 to 4.8 cases per 10,000 patient-days in the last quarter of 2020. At both institutions, a transient rise in the incidence of HA-CDI was observed between 2009 and 2012 after implementation of C. difficile laboratory detection by NAAT.
Antibiotic utilization
Antibiotic utilization at the RVH and MGH between April 2003 and March 2020 is presented in Table 1. A decrease in the incidence of HA-CDI between April 2003 and March 2020 occurred, although total antibiotic utilization was increasing at both hospitals, from 585.97 to 631.75 DDDs per 1,000 patient-days at the MGH and from 705.70 to 900.85 DDDs per 1,000 patient-days at the RVH. At both institutions, we observed an increase in consumption of third-generation cephalosporins (14.79–66.78 DDDs per 1,000 patient-days at the MGH, 13.14–68.90 DDDs per 1,000 patient-days at the RVH), beta-lactam/beta-lactamase inhibitors (80.61–147.03 DDDs per 1,000 patient-days at the MGH, 68.36–192.79 DDDs per 1,000 patient-days at the RVH), and carbapenems (12.20–47.68 DDDs per 1,000 patient-days at the MGH, 30.33–90.80 DDDs per 1,000 patient-days at the RVH). At both hospitals, a decrease in utilization of fluoroquinolones (102.18–47.50 DDDs per 1,000 patient-days at the MGH, 122.63–78.80 DDDs per 1,000 patient-days at the RVH) and clindamycin (10.72–8.53 DDDs per 1,000 patient-days at the MGH, 16.22–7.6 DDDs per 1,000 patient-days at the RVH) was observed over the study period.
Table 1. Antibiotic utilization at the MGH and RVH between 2003 and 2020

Note. MGH, Montreal General Hospital; RVH, Royal Victoria Hospital; DDDs, defined daily doses.
Effect of antibiotic utilization on HA-CDI incidence
Utilization of high-risk antibiotics and HA-CDI incidence at the MGH and RVH are presented in Figures 1 and 2, respectively. At both institutions, a decrease of 10 DDDs in fluoroquinolone utilization was associated with a decrease in HA-CDI incidence (MGH impact −1.40 cases per 10,000 patient-days, 95% CI [−2.44, −0.35]; RVH impact −0.98 cases per 10,000 patient-days, 95% CI [−1.68, −0.28]). A decrease of 10 DDDs in the utilization of first-generation cephalosporins was associated with a decrease in HA-CDI at the MGH (impact −1.87 cases per 10,000 patient-days, 95% CI [−3.62, −0.12]). Such an association was not observed at the RVH. A change in the utilization of second-generation cephalosporins was not associated with a significant change in HA-CDI incidence. A decrease in 10 DDDs of third-generation cephalosporin utilization was associated with an increase in HA-CDI incidence at the MGH (impact 2.30 cases per 10,000 patient-days, 95% CI [0.73, 3.86]). There was no significant change in HA-CDI following the change in third-generation cephalosporin utilization at the RVH. Changes in clindamycin utilization were not associated with a significant change in HA-CDI incidence at either institution.
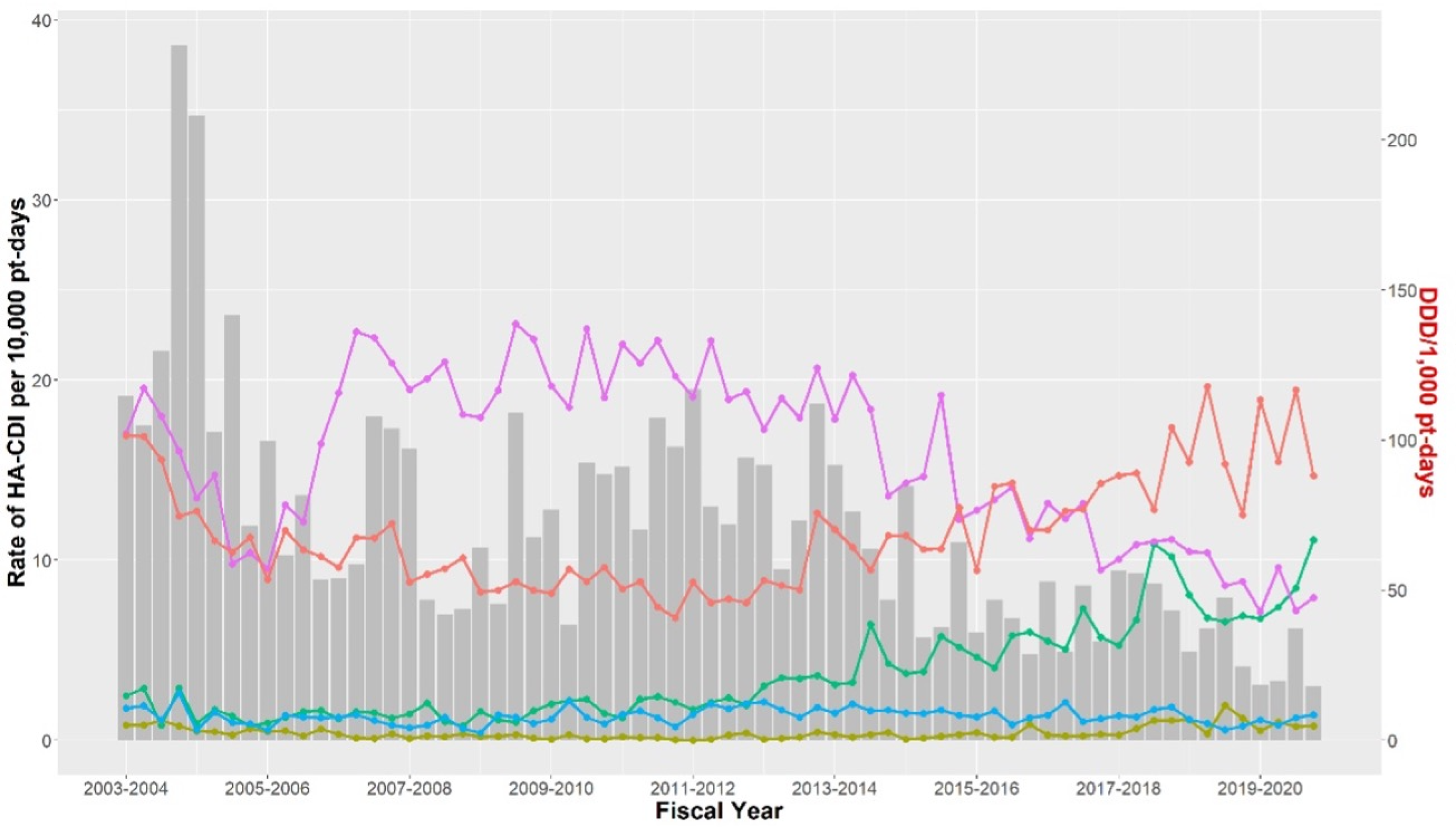
Figure 1. Healthcare-associated C. difficile infection incidence and high-risk antibiotic utilization at the MGH between 2003 and 2020. Antibiotic: ![]() cephalosporin first generation,
cephalosporin first generation, ![]() cephalosporin second generation,
cephalosporin second generation, ![]() cephalosporin third generation,
cephalosporin third generation, ![]() clindamycin,
clindamycin, ![]() fluoroquinolone. Note: C. difficile, Clostridioides difficile; MGH, Montreal General Hospital.
fluoroquinolone. Note: C. difficile, Clostridioides difficile; MGH, Montreal General Hospital.
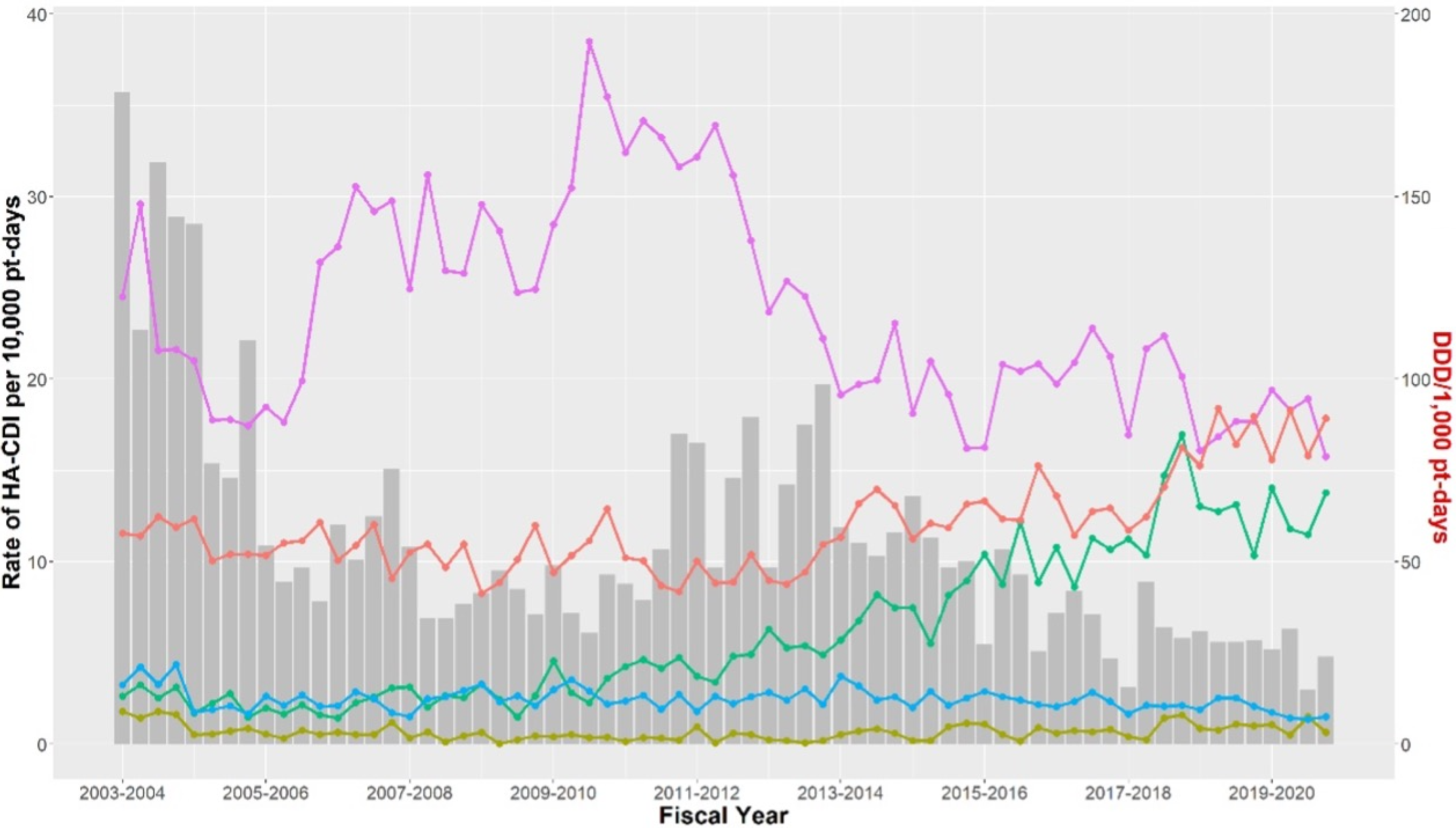
Figure 2. Healthcare-associated C. difficile infection incidence and high-risk antibiotic utilization at the RVH between 2003 and 2020. Antibiotic: ![]() cephalosporin first generation,
cephalosporin first generation, ![]() cephalosporin second generation,
cephalosporin second generation, ![]() cephalosporin third generation,
cephalosporin third generation, ![]() clindamycin,
clindamycin, ![]() fluoroquinolone. Note: C. difficile, Clostridioides difficile; RVH, Royal Victoria Hospital.
fluoroquinolone. Note: C. difficile, Clostridioides difficile; RVH, Royal Victoria Hospital.
Antibiotic utilization and impact on the NAP1/027 strain
Between April 2014 and March 2020 at the MGH, there was a significant decrease in the proportion of the NAP1/027 strain (−85.7%, p = 0.046). During the same period, there was a decrease in fluoroquinolone utilization (−47.79%, p = 0.013), a significant increase in third-generation cephalosporin utilization (160.81%, p = 0.001), and no significant change in clindamycin utilization (−10.97%, p = 0.1) (Figure 3). At the RVH, between April 2010 and March 2020, there was a decrease in the proportion of the NAP1/027 strain (−75%, p = 0). During the same period, there was a trend in decreased fluoroquinolone utilization (−48.13%, p = 0.18), a significant increase in third-generation cephalosporin utilization (182.18%, p = 0), and a significant decrease in clindamycin utilization (−43.21%, p = 0.002) (Figure 4).

Figure 3. Proportion of NAP1/027 C. difficile genotype and utilization of high-risk antibiotics at the MGH between 2014 and 2020. Antibiotic: ![]() cephalosporin third generation,
cephalosporin third generation, ![]() clindamycin,
clindamycin, ![]() fluoroquinolone. Note: C. difficile, Clostridioides difficile; MGH, Montreal General Hospital.
fluoroquinolone. Note: C. difficile, Clostridioides difficile; MGH, Montreal General Hospital.

Figure 4. Proportion of NAP1/027 C. difficile genotype and utilization of high-risk antibiotics at the RVH between 2010 and 2020. Antibiotic: ![]() cephalosporin third generation,
cephalosporin third generation, ![]() clindamycin,
clindamycin, ![]() fluoroquinolone. Note: C. difficile, Clostridioides difficile; RVH, Royal Victoria Hospital.
fluoroquinolone. Note: C. difficile, Clostridioides difficile; RVH, Royal Victoria Hospital.
Infection control interventions
Infection control interventions that were implemented during the study period are presented in Table 2.
Table 2. Infection control interventions implemented at the MUHC between April 1, 2003, and March 31, 2020

Note. MUHC, McGill University Health Centre; NAAT, nucleic acid amplification test; VRE, vancomycin-resistant Enterococcus; CDI, Clostridioides difficile infection.
* Transforming care at the bedside: weekly huddles where infection control would meet with the nurse manager, attending physician, senior resident, and other allied health professionals to review CDI rates and address interventions that could be improved.
Discussion
Our 17-year analysis of the epidemiology of HA-CDI in two tertiary care hospitals in Montreal, Québec, demonstrated an approximate 80% decrease in the incidence of HA-CDI at both institutions. This decline occurred while total antibiotic consumption was increasing, but use of certain high-risk antibiotics—fluoroquinolones, second-generation cephalosporins, and clindamycin—was decreasing. A decrease in fluoroquinolone utilization was associated with a significant decrease in HA-CDI incidence at the 6-month interval at both hospitals. A decrease in first-generation cephalosporins was associated with a decrease in HA-CDI incidence at one of the two centers. In the later years of our study, we observed a significant decrease over time in the C. difficile NAP1/027 strain which is inherently resistant to fluoroquinolones. There was a parallel significant decrease in fluoroquinolone utilization at one of the two hospitals.
Our findings are consistent with previous studies reporting an association between HA-CDI and the use of high-risk antibiotic classes, particularly fluoroquinolones and cephalosporins. Reference Silva, Wilson, Redmond and Donskey20,Reference Kazakova, Baggs and McDonald21,Reference Pereira, Farragher, Tully and Jonathan Cooke34 Interventions targeting the restriction in the utilization of these antibiotics have been successful at driving a decline in HA-CDI in North America and Europe. Reference Kallen, Thompson and Ristaino16,Reference Schönherr, Ranft, Lippmann and Lübbert17,Reference Dingle, Didelot and Quan19,Reference Pereira, Farragher, Tully and Jonathan Cooke34–Reference Lawes, Lopez-Lozano and Nebot36 In Québec, at the height of the HA-CDI epidemic between 2003 and 2006, implementation of antibiotic stewardship led to a decrease in the utilization of high-risk antibiotics and a decrease in HA-CDI incidence. Reference Valiquette, Cossette, Garant, Diab and Pepin15
The effect of fluoroquinolone utilization is of particular interest as the HA-CDI epidemic in Québec was associated with dissemination of the NAP1/027 strain which is known to be resistant to this antibiotic class. Reference Loo, Poirier and Miller13 Our findings demonstrated an association between a decrease in fluoroquinolone utilization and a decrease in total C. difficile incidence. We were further able to demonstrate that a significant decrease in the incidence of the NAP1/027 strain was concomitant with decrease in fluoroquinolone utilization at one of our two hospital sites in the final years of the study. There was a trend in decreased fluoroquinolone utilization over time with the decrease in proportion of isolates of the NAP1/027 strain at our second study hospital, but this did not reach statistical significance. Our findings are similar to the results of a study conducted in the United Kingdom between 2006 and 2013. This study demonstrated that the decline in C. difficile incidence was primarily driven by the elimination of fluoroquinolone-resistant strains. Reference Dingle, Didelot and Quan19
In our study, the association between the utilization of cephalosporins and HA-CDI incidence is equivocal. The change in the utilization of the different cephalosporin classes had inconsistent effects across the two hospitals. We found that an increase in third-generation cephalosporin use was associated with a decrease in HA-CDI incidence at the MGH. This contrasts with previous literature that supported the restriction of third-generation cephalosporins to control HA-CDI. Reference Kazakova, Baggs and McDonald21,Reference Pereira, Farragher, Tully and Jonathan Cooke34 One hypothesis is that third-generation cephalosporins replaced fluoroquinolones in the treatment of urinary and pulmonary pathogens at our institution. Since we did not determine the specific effect of an antibiotic independent of changes in other antibiotic classes, it is possible that the potential effect of the increase in utilization of third-generation cephalosporins on HA-CDI was offset by the concomitant effect of decreasing fluoroquinolones on the predominant NAP1/027 strain.
Our findings regarding the association between the reduction of use of high-risk antibiotics and incidence of HA-CDI contrast with a coincident study conducted in 12 Québec hospitals between 2012 and 2017. That study demonstrated that the change in high-risk antibiotics did not entirely account for the decrease of HA-CDI in the province. Reference Fortin, Thirion and Ouakki23 These different results may be explained by the shorter study duration of 5 years in the Fortin et al publication. Our study was of longer duration and included more time points for analyses.
We reviewed infection control interventions that were implemented at our institution during the study period and identified measures other than antibiotic stewardship that were potentially at play in the changing epidemiology of HA-CDI. Both of our hospital sites experienced an increase in HA-CDI incidence between 2009 and 2012. This temporally correlates with the change to the NAAT test from CCNA for the detection of C. difficile in 2009. US data from the Center for Disease Control and Prevention suggested that such a change in testing method could account for a rise in HA-CDI ranging from 43% to 67%. Reference Gould, Edwards and Cohen37 In Québec, 35 institutions modified their testing algorithm between 2010 and 2014 and those detecting toxigenic C. difficile instead of C. difficile toxin had higher rates of HA-CDI. Reference Bogaty, Lévesque and Garenc38 Despite the initial increase in HA-CDI incidence with the change in testing, HA-CDI incidence in our institutions continued to decline after 2012.
Another possible contributor to the decline in HA-CDI incidence was the move to a single-room facility for the RVH site in 2015. A previous time-series analysis conducted at our institution did not demonstrate an association of HA-CDI incidence and housing patients in single rooms. Reference McDonald, Dendukuri, Frenette and Lee39
There are several limitations to this investigation. Firstly, only two hospitals were included in this analysis. More data is needed from multiple institutions to clarify whether the reduction in fluoroquinolone use has been a major driving factor to the decrease in HA-CDI incidence and the NAP1/027 strain in North America and Europe. Secondly, there were multifaceted infection control interventions that were implemented over the study period. Hence, the effect of changes in antibiotic utilization and HA-CDI incidence are subject to potential confounders that are difficult to measure. Previous epidemiological studies have also demonstrated that there are shifts in epidemic C. difficile strains in the absence of substantial changes in antibiotic use. Reference Belmares, Johnson and Parada40 Furthermore, the change in laboratory diagnostic method for C. difficile detection to NAAT which is a more sensitive test than CCNA likely led to a transient increase in incidence during the study period and may have blunted certain associations between antibiotic utilization and HA-CDI. In addition, we did not evaluate for associations between antibiotic classes and the impact of the change of an antibiotic class independent of the change of other classes. Lastly, the retrospective nature of the study led to limited accessibility to C. difficile strain genotyping from the earlier years of the study. Due to this significant amount of unavailable data, we could not evaluate the effect of changes in antibiotic utilization on the NAP1/027 strain during the entire study period. Despite this, we were still able to demonstrate an association of the decrease in the proportion of C. difficile isolates of the NAP1/027 genotype with the decrease in fluoroquinolone utilization in one hospital and a trend in the second hospital.
In conclusion, the decline in HA-CDI following the epidemic of the early 2000s at two hospitals in Montreal, Canada, was accompanied by a reduction in the utilization of high-risk antibiotics. Specifically, a decrease in the use of fluoroquinolones was associated with a significant decrease in HA-CDI incidence. Moreover, at one of our study sites, the decrease in fluoroquinolone utilization was also associated with a parallel decrease in the proportion of C. difficile isolates of the NAP1/027 genotype. Our study demonstrates that antibiotic stewardship is an essential infection control strategy to decrease HA-CDI incidence and can effectively suppress hypervirulent strains at a facility level.
Acknowledgments
None.
Author contributions
No one other than the named authors had a role in the gathering or preparation of data or in the writing of the manuscript.
Financial support
There was no funding received for this study.
Competing interests
YL received salary support from the Fonds de Recherche du Québec outside of this work. VGL received consulting fees from Ferring Inc., Merck Inc., and Xediton Pharmaceuticals Inc. outside of this work. All other authors report no competing interests.








