Antimicrobial agents are life-saving medications for immunocompromised hosts who rely heavily on these medications. However, antimicrobial resistance (AMR) is a significant threat to these patients and, as such, judicious use of antimicrobials is critical. Antimicrobial stewardship (AMS) programs are essential in creating treatment guidelines as well as promoting and monitoring appropriate antimicrobial treatment in these complex patients. We discuss 5 important, frequently encountered transplant infectious disease stewardship challenges, with suggested strategies to address and improve antimicrobial practices for each clinical syndrome. Although each syndrome has its unique set of challenges, we discuss overarching themes of scarcity of data on stewardship interventions specific to transplant patients, limitations in diagnostic testing, and pharmacotherapy concerns.
1. Asymptomatic bacteriuria in renal transplant recipients
Asymptomatic bacteriuria (ASB), a common condition in renal transplant recipients, is often treated with antibiotics Reference Coussement, Maggiore and Manuel1 based on the theoretical risk of ascending infection leading to pyelonephritis and acute graft loss. However, data against routine treatment of ASB are fairly robust. The Infectious Diseases Society of America (IDSA) and the American Society for Transplantation (AST) updated their clinical care guidelines in 2019, recommending against treating ASB in renal transplant recipients >2 months after transplant. Reference Nicolle, Gupta and Bradley2,Reference Goldman and Julian3 These guidelines reflected results from several limited retrospective studies Reference Kotagiri, Chembolli, Ryan, Hughes and Toussaint4–Reference Lee, Bang and Dadhania6 and a single randomized controlled trial Reference Origüen, López-Medrano and Fernández-Ruiz7 that found no significant differences in outcomes for patients who received antibiotics for ASB compared to those who did not. Following publication of these guidelines, additional randomized controlled trials and 1 meta-analysis have provided additional evidence that treating ASB does not offer benefit Reference Coussement, Kamar and Matignon8–Reference Coussement, Kamar and Abramowicz10 but leads to excessive antibiotic use and increased risk of infection with multidrug-resistant organisms. Reference Coussement, Kamar and Matignon8 Despite strong evidence, ASB continues to be a stewardship challenge in renal transplant recipients.
Data on ASB outcomes in renal transplant recipients within 2 months of transplant or with anatomic genitourinary abnormalities, indwelling catheters, or ureteral stents, remains limited and guidelines do not make strong recommendations for these populations. Reference Nicolle, Gupta and Bradley2,Reference Goldman and Julian3 Many providers may favor treating ASB in these circumstances Reference Coussement, Maggiore and Manuel1 because of concerns that foreign material, intense immunosuppression, or genitourinary tract abnormalities could potentiate the risk of ASB progressing to graft pyelonephritis, although the benefit is unclear. Reference Coussement, Kamar and Abramowicz10
Stewardship efforts are further hindered by the diagnostic complexities of distinguishing ASB from urinary tract infections. In renal transplant recipients with nonspecific signs of infection but no clear urinary symptoms, positive urine cultures are difficult to interpret because denervation from surgery may limit the presence of urinary symptoms. Reference Goldman and Julian3,Reference Coussement, Kamar and Abramowicz10 Pyuria and positive urine cultures in the setting of chronic indwelling catheters or stents often reflect colonization or contamination rather than a UTI. Similarly, acute kidney injury and pyuria can be seen in both graft rejection and UTIs. Diagnostic ambiguities likely contribute to ASB overtreatment despite lack of clinical significance.
The use of other markers of infection (eg, white bold cell count, C- reactive protein level, and erythrocyte sedimentation rate) may be useful in differentiating asymptomatic bacteriuria from urinary tract infection in the setting of nonspecific clinical symptoms, but additional studies are needed. Improved biomarkers and diagnostic testing to discern the relevancy of positive urine cultures and to identify which renal transplant recipients would benefit from antibiotics can hopefully augment stewardship efforts in the future, but these are currently unavailable.
Moreover, whether ASB guidelines are reflected in clinical practice is unclear. In a survey study of European transplant centers, >70% reported routine screening for bacteriuria and treatment was common. Reference Coussement, Maggiore and Manuel1 One solution to limiting the treatment of ASB is avoiding routine surveillance cultures in the absence of symptoms or laboratory abnormalities because providers may be inclined to treat known positive results. Updating institutional treatment guidelines to include avoiding treatment and providing prescriber feedback are AMS tools that can help decrease the treatment of ASB.
2. Febrile neutropenia in stem-cell transplant recipients
Hematopoietic stem cell transplant (HSCT) recipients are vulnerable to infectious complications, especially during the pre-engraftment period in which significant neutropenia and mucosal damage increase the risk of bacteremia. Reference Dhakal, Giri and Levin11–Reference Lind, Mooney and Carone13 HSCT recipients often receive several weeks of broad-spectrum antimicrobials to mitigate this risk. However, this extensive antimicrobial exposure, combined with prior antimicrobial therapy and conditioning chemotherapy, can contribute to gut dysbiosis and poor outcomes. Reference Shallis, Terry and Lim14,Reference Taplitz, Kennedy and Flowers15 AMR has been recognized as a leading cause of death globally, with carbapenem-resistant Enterobacterales (CRE) and vancomycin-resistant enterococci (VRE) emerging as major threats to HSCT recipients. Reference Murray, Ikuta and Sharara16–Reference Kamboj, Cohen and Huang19 Although AMS has been advocated for patients with hematological malignancies and HSCT, clinicians have to balance maintaining adequate antimicrobial coverage against minimizing unnecessary antimicrobial exposure at the individual and population level. Reference Averbuch, Orasch and Cordonnier20–Reference Gyssens, Kern and Livermore23 Strategies to guide antimicrobial use in the HSCT population are urgently needed.
One area of stewardship interest is antimicrobial use for febrile neutropenia in HSCT recipients. Routinely, broad-spectrum coverage is maintained until absolute neutrophil count recovers to >500 cells/µL and the patient is afebrile, irrespective of the presence of a documented infection or fever of unknown origin. Reference Freifeld, Bow and Sepkowitz24 Antimicrobial prescribing practice in HSCT patients with febrile neutropenia varies widely; it informs and is informed by regional epidemiology. Reference Verlinden, Mikulska and Knelange25
Recent data suggest that a shorter duration of antibiotic therapy is safe and effective for febrile neutropenia when coupled with close monitoring. The “How Long” study compared the conventional approach to high-risk febrile neutropenia with early discontinuation of broad-spectrum antimicrobials based on resolution of fever after 72 hours and clinical recovery. Reference Aguilar-Guisado, Espigado and Martín-Peña26 Patients in the experimental arm received a significantly shorter duration of antimicrobials with numerically fewer adverse events, indicating that the symptom-driven approach avoided unnecessary antimicrobial exposure. Reference Aguilar-Guisado, Espigado and Martín-Peña26 Although this study included HSCT patients, allogeneic recipients accounted for only 9% of the study population. Reference Aguilar-Guisado, Espigado and Martín-Peña26 Nevertheless, these findings show that in fever of unknown origin, shortening duration of empirical antimicrobial therapy with close monitoring can be safe and feasible. Two recent studies with a similar intervention and a larger representation of HSCT patients reported congruous findings, but well-designed, prospective studies are imperative to supporting practice changes in the era of AMR. Reference Le Clech, Talarmin and Couturier27–Reference Stern, Carrara, Bitterman, Yahav, Leibovici and Paul29
In patients with documented infections, the decision to tailor therapy targeting the pathogen versus continuing with broad-spectrum antimicrobials has not been fully elucidated. One guideline recommended a patient-specific approach, which may lead to wide variability in antimicrobial prescribing. Reference Baden, Bensinger and Angarone30 A multinational, prospective, longitudinal study of patients with high-risk febrile neutropenia, including acute leukemia and HSCT (autologous and allogeneic) recipients, evaluated the association between bacteremia and mortality at 7 days and 30 days. Reference Weisser, Theilacker and Tschudin Sutter31 P. aeruginosa bacteremia was associated with the highest 7-day and 30-day mortality at 16.7% and 26.7%, respectively, compared to coagulase-negative staphylococci (2%) or streptococci (<1%), whereas enterococci were associated with an unexpected increase in mortality. Reference Kern, Roth and Bertz32 Candidemia and gram-negative bacteremia were independently associated with intensive care unit admission. Reference Kern, Roth and Bertz32 In a related study, predictors of bacteremia due to multidrug-resistant P. aeruginosa included prior exposure to piperacillin-tazobactam, antipseudomonal carbapenem, fluoroquinolone prophylaxis, underlying hematological disease, and presence of a urinary catheter. Reference Gudiol, Albasanz-Puig and Laporte-Amargós33 These data suggest that a pathogen-specific approach combined with judicious use of broad-spectrum antimicrobials may be optimal. Although prophylactic fluoroquinolones have been routinely recommended for patients with acute leukemia and HSCT to reduce the rates of bacteremia, a more thoughtful, risk-stratified approach should be considered given its implications for institutional epidemiology. Reference Taplitz, Kennedy and Flowers15,Reference Egan, Robinson and Martinez34–Reference Mikulska, Averbuch and Tissot36
Implementing an institution-specific guideline for management of neutropenic fever in hematology-oncology patients that accounts for local susceptibility patterns is a recommended AMS intervention. Reference Barlam, Cosgrove and Abbo37,Reference Barreto, Aitken and Krantz38 To ensure sustained practice change, evaluating the quality of antimicrobial prescribing through auditing and provision of feedback to prescribers will help AMS programs identify areas for improvement and provide ongoing support for guideline-adherent practices in hematology patients and HSCT recipients. Reference Douglas, Hall and James39 In the management of high-risk febrile neutropenia, data supporting judicious prescribing with close monitoring of patients with fever of unknown origin are encouraging. A multipronged approach with risk stratification, implementation of local guidelines, and evaluation of quality of antimicrobial prescribing offers a potential solution that may overcome the challenges of AMS in this vulnerable patient population.
3. Antifungal prophylaxis in liver and lung transplant recipients
Antifungal prophylaxis has been advocated for SOT recipients because diagnostic limitations for invasive fungal infections can translate into treatment delays that confer significant morbidity and mortality. Reference Hosseini-Moghaddam, Ouédraogo and Naylor40 Stewardship challenges involving antifungal prophylaxis in liver transplant recipients include pharmacokinetic considerations and the availability of local epidemiological patterns of fungal infections. In lung transplant recipients, inhaled amphotericin or systemic azole therapy are used because invasive aspergillosis is a significant concern. However, stewardship efforts are hindered by the absence of strong evidence, and a better understanding of risk is needed.
Targeted rather than universal antifungal prophylaxis based on a risk stratification approach outlined by the AST is preferred for liver transplant recipients, but prospective studies to guide treatment duration are lacking. Reference Aslam and Rotstein41 Withholding antifungal prophylaxis in low-risk liver transplant recipients has been shown to be safe, to reduce unnecessary exposure, and to avoid potential drug–drug interactions with immunosuppressants. Reference Aslam and Rotstein41,Reference Lavezzo, Stratta and Ballaris42 In a 2008 study, 28% and 72% of North American transplant centers surveyed used universal and targeted prophylaxis, respectively. Reference Singh, Wagener, Cacciarelli and Levitsky43 More recent data on prescribing trends are lacking, but regular institutional review of prescribing patterns in low-risk recipients is a realistic AMS tool to ensure that evidence-based practice is followed and that antifungal overuse is limited.
The various antifungal agents available for targeted prophylaxis in high-risk liver transplant recipients have notable limitations. Liposomal amphotericin B is effective, Reference Fortún, Martín-Davila and Moreno44 but it offers unnecessarily broad coverage, it is costly, and it is limited to intravenous administration. Fluconazole, the preferred agent based on expert opinion, Reference Aslam and Rotstein41 is faced with rising resistance, increasing rates of non-albicans Candida spp infections, Reference Fortún, Muriel and Martín-Dávila45 and known interactions with calcineurin inhibitors. A single-center study demonstrated that fixed fluconazole dosing was effective, and no invasive fungal infections with reduced fluconazole-susceptible strains occurred. Reference Jorgenson, Descourouez and Marka46 However, applicability across institutions with different local epidemiologies and among critically ill patients with renal dysfunction (in whom fluconazole pharmacokinetics are variable) are concerns. Reference Muilwijk, de Lange and Schouten47 Echinocandins are associated with fewer toxicities and drug–drug interactions, but there are significant pharmacokinetic–pharmacodynamic limitations. Echinocandins achieve limited therapeutic concentrations intra-abdominally because of their molecular characteristics, Reference Gatti, Rinaldi and Ferraro48 predisposing patients to the emergence of echinocandin resistance. Reference Shields, Nguyen, Press and Clancy49 An 8% acquired resistance rate Reference Prigent, Aït-Ammar and Levesque50 and breakthrough invasive fungal infections Reference Gatti, Rinaldi and Ferraro48 while on echinocandin therapy have been noted. To help prevent the emergence of fluconazole and echinocandin resistance, AMS programs can assist in optimizing dosing for patients who are critically ill, who require renal replacement therapy, or who have infections at sites of known poor drug penetration. Reference Muilwijk, de Lange and Schouten47,Reference Gatti, Rinaldi and Ferraro48 The risk of invasive aspergillosis in high-risk liver recipients is another crucial consideration. Reference Lavezzo, Romagnoli, Balagna and De Rosa51 The decision to administer antimold coverage involves weighing the local incidence of and a recipient’s risk for invasive aspergillosis Reference Lum, Lee, Vu, Strasser and Davis52 against potential toxicities, drug–drug interactions, and emergence of azole-resistant Aspergillus. This challenging situation underscores the importance of updated local epidemiological data and of longitudinal monitoring of fungal susceptibilities, outcomes, and adverse events to form AMS strategies that can be feasibly adopted in clinical practice. Reference Johnson, Lewis and Dodds Ashley53,Reference Khanina, Urbancic and Haeusler54
For lung transplant recipients, the preferred choice between universal or pre-emptive antifungal prophylaxis against Aspergillus (the latter involving routine surveillance with broncho-alveolar lavage culture and galactomannan) is undefined, but either approach is recommended over no prophylaxis. Reference Husain and Camargo55,Reference De Mol, Bos and Beeckmans56 Although 90% of US transplant centers had previously reported routine universal antifungal prophylaxis for lung transplant recipients, a review of administrative claims data showed that only 41.5% of patients received antifungal prophylaxis. Reference Pennington, Baqir, Erwin, Razonable, Murad and Kennedy57 The reasons for this incongruence is unknown, but further analysis could potentially provide insights to stewardship areas of interest. An understanding of the current practices would help direct AMS efforts toward high-yield measures. Data on universal and pre-emptive therapies are mixed because studies are limited by small sample size, variable study design, and heterogeneous immunosuppression and antifungal agents included. 58 Prospective studies comparing universal and pre-emptive prophylaxis are needed not only to evaluate efficacy but also to characterize potential stewardship benefits of pre-emptive prophylaxis.
If universal prophylactic therapy for lung transplant recipients is employed, the recommended duration of prophylaxis is 4–6 months. Nevertheless, 22.2% of transplant centers in one survey continued universal prophylaxis for >12 months. Reference Pennington, Yost, Escalante, Razonable and Kennedy59 The use of long-term or lifelong azole prophylaxis has not been shown to alter the incidence of invasive fungal infection in lung transplant recipients, even in the setting of therapeutic azole levels, and it is associated with medication toxicities, healthcare costs, and potential resistance. Reference Chong, Kennedy, Hathcock, Kremers and Razonable60 Another area of stewardship concern is whether routine prophylaxis is driving the emergence of delayed aspergillosis and invasive fungal infections in lung transplant recipients after prophylaxis is discontinued, beyond the traditional risk period. Reference Vazquez, Vazquez-Guillamet, Suarez, Mooney, Montoya and Dhillon61,Reference Bae, Lee and Jo62 One center reported the median time of onset for invasive aspergillosis in lung transplant recipients to be 363 days. Reference Vazquez, Vazquez-Guillamet, Suarez, Mooney, Montoya and Dhillon61 The incidence and consequences of invasive aspergillosis occurring beyond the first year have not been clearly established, even though immunosuppression may be less intensive and the risk of anastomotic fungal infection or ulcerative tracheobronchitis may be lower.
Perhaps a more tailored approach to antifungal prophylaxis in lung transplant recipients is necessary. Evidence-based risk stratification models to identify recipients who would benefit from a short course versus a standard course or from lifelong antifungal prophylaxis, relative to local incidence of invasive fungal infection, would be valuable for AMS programs. We also suggest that AMS programs monitor closely for a potential risk in delayed invasive aspergillosis and that they analyze any occurrences because such cases will have significant local and population-level stewardship implications.
4. Left ventricular assist device infections
Infections, a leading complication of left ventricular assist devices (LVADs), are estimated to occur in nearly 40% of recipients. Reference Zinoviev, Lippincott, Keller and Gilotra63 Despite the high incidence, management guidelines for LVAD infections are based on observational data and expert opinion due to the absence of randomized controlled trials. Reference Kusne, Mooney and Danziger-Isakov64 The lack of strong evidence, along with diagnostic complexities and uncertain effects of LVADs on antimicrobial pharmacokinetics, contribute to the challenges facing AMS in LVAD-specific and related infections. Compounding these issues are the nature of the infections, which are potentially incurable without source control through transplantation, and the growing proportion of LVADs implanted for destination therapy. Reference Molina, Shah, Kiernan and Cornwell65 In 2019, 73.1% of LVADs implanted were for destination therapy. Reference Molina, Shah, Kiernan and Cornwell65 Because LVADS are increasingly used for destination therapy, these infectious complications will be a growing challenge for AMS.
Device driveline infections, which account for 12%–35% of all LVAD-specific or related infections Reference Zinoviev, Lippincott, Keller and Gilotra63 , occur most frequently but diagnosing and distinguishing superficial from deep infection is problematic. Clinical and physical exam features of driveline or endovascular infections can be subtle, nonspecific, or absent. Reference Blanco-Guzman, Wang, Vader, Olsen and Dubberke66,Reference Koval and Stosor67 Imaging to assist in diagnosis is not standardized and has limitations: Computed tomography has variable performance and is affected by device artifact, ultrasound detects only superficial fluid collections, and access to FDG-PET may be a barrier, Reference Koval and Stosor67 although gallium single-photon emission computed tomography (SPECT) appears to be a promising imaging modality. Reference Puius, Parkar and Tlamsa68
Even if the extent of infection is successfully diagnosed, uncertainties remain regarding duration of therapy and the role of chronic antimicrobial suppression (CAS). The evidence for the current treatment duration recommendations for superficial and deep driveline infections is limited Reference Kusne, Mooney and Danziger-Isakov64,Reference Koval and Stosor67 and, in clinical practice, widely variable. Reference Koval and Stosor67 Whether superficial driveline infections progress to deeper infections or if CAS for driveline infections significantly reduces recurrence remains to be determined. Conflicting data are likely driven by the various LVAD-specific infections included in each study. Several studies estimate a 30% failure rate of CAS, Reference Radcliffe, Doilicho, Niu and Grant69–Reference Hamad, Blanco-Guzman and Olsen71 with recurrence even in superficial driveline infections. Reference Jennings, Chopra, Chambers and Morgan70 One study has suggested that CAS resulted in no significant difference in the proportion of patients with relapse. Reference Hamad, Blanco-Guzman and Olsen71 For an infection associated with foreign material that may not be reasonably removed and that can occur in LVAD recipients who have altered immune responses, Reference Koval and Stosor67 the implications of these retrospective data on clinical practice are unclear.
In addition to ongoing questions regarding duration of therapy for LVAD infections, 2 studies have suggested altered intravenous vancomycin pharmacokinetics from LVADs, which may further complicate AMS efforts in these patients. Those with LVADs had a significantly higher incidence of supra-therapeutic trough levels, potentially due to an overestimated volume of distribution and rate of elimination. Reference Hall, Athans, Wanek, Wang, Estep and Williams72,Reference Jennings, Makowski, Chambers and Lanfear73 Further characterization of this finding and its implications are important because S. aureus and coagulase-negative Staphylococcus are the predominant etiological agents of LVAD infections.
The challenges facing AMS in LVAD infections are driven by the need for improved diagnostics and well-designed studies on treatment. In the absence of heart transplantation, determining the therapy end point is complex and requires careful consideration of a patient’s clinical, microbiological, radiographic, and surgical factors. The role for CAS remains ambiguous due to the limited evidence on efficacy and adverse effects. As more individuals receive LVADs for destination therapy, studies describing long-term outcomes of CAS categorized by each type of LVAD infection and pathogen involved are needed to assist in optimizing antimicrobial use.
5. Clostridioides difficile infection
Clostridioides difficile infection (CDI) is a major cause of morbidity and mortality that disproportionally affects HSCT and SOT recipients. Compared to the general inpatient population, HSCT and SOT patients have a higher incidence of CDI, are more likely to have severe infection, and are at greater risk of recurrence. Reference Revolinski and Munoz-Price74 For these reasons, reducing CDI rates is a priority of AMS programs. Interventions aimed at restricting antimicrobial exposure and providing provider education and feedback have been highly successful. Reference Mullane and Dubberke75,Reference Pouch and Friedman-Moraco76 Effective CDI antimicrobial stewardship practices are an interdisciplinary effort engaging diagnostic stewardship and infection prevention and control. Reference Revolinski and Munoz-Price74,Reference Pouch and Friedman-Moraco76 Active adaptation of these practices to the dynamic and unique factors of each transplant center is crucial for sustained progress in reducing CDI burden in this population. Stewardship areas of uncertainty include appropriate patient selection for testing, implications of asymptomatic screening, and the role of anti-CDI therapeutic prophylaxis.
The use of multistep testing algorithms has improved the analytic diagnostic stage, but CDI is a clinical diagnosis that depends on preanalytic decisions. Reference Pouch and Friedman-Moraco76 Differentiating asymptomatic colonization from CDI is a long-standing diagnostic conundrum that is particularly problematic in the transplant population. Transplant patients are at risk of overdiagnosis because of increased risk of toxigenic C. difficile carriage Reference Boly, Reske and Kwon77 and multiple confounding factors that cause diarrhea, including antibiotic use, immunosuppressive medications, mucositis, and graft-versus-host disease. Reference Revolinski and Munoz-Price78 Comprehensive review for other etiologies of diarrhea relative to a patient’s clinical symptoms such as degree of diarrhea, should be a priority prior to testing. Reference Alonso, Maron and Kamboj79
Screening transplant patients at admission for C. difficile colonization facilitates early implementation of infection prevention measures and may result in decreased horizontal transmission. Reference Revolinski and Munoz-Price78,Reference Barker, Krasity, Musuuza and Safdar80 In one study of patients admitted to an inpatient hematological unit, colonization with C. difficile conferred an 11.6 times higher odds of progression to CDI compared to those without colonization. Reference Cannon, Musuuza and Barker81 Identification of these asymptomatic carriers is an opportunity for targeted risk-reduction measures, such as antimicrobial review, to reduce the risk not only for symptomatic infection but for vertical transmission as well. However, the role of prophylactic, pharmacological measures to prevent the progression of colonization to infection is unknown. It is unclear how to optimally use asymptomatic screening for AMS efforts without unintentionally causing inappropriate treatment from misinterpretation of tests. Reference Barker, Krasity, Musuuza and Safdar80,Reference McCort, Oehler and Enriquez82
Oral vancomycin prophylaxis is an attractive option to prevent CDI. Retrospective reviews suggest that primary prophylaxis in allogenic HSCT recipients is associated with significantly lower rates of CDI Reference Ganetsky, Han and Hughes83,Reference Altemeier and Konrardy84 and that secondary prophylaxis is effective in reducing CDI recurrence in kidney transplant patients. Reference Splinter, Kerstenetzky and Jorgenson85 Although these studies found no instances of vancomycin-resistant Enterococcus (VRE) colonization or bacteremia, Reference Ganetsky, Han and Hughes83,Reference Altemeier and Konrardy84 several other studies have found that the use of oral vancomycin increased the risk of VRE overgrowth and infection Reference Lee, Plechot, Gohil and Le86,Reference Zacharioudakis, Zervou, Dubrovskaya and Phillips87 and can alter gut microbiome, which is linked to poor outcomes. Reference Alonso, Maron and Kamboj79 Despite the suggested benefit, a number of questions on the potential role for prophylaxis remain, including optimal duration, cost implications, effects on the intestinal microbiome, potential to drive the emergence of vancomycin-resistant C. difficile strains, and the benefit of secondary prophylaxis if fidaxomicin was used as initial therapy. Additional research can help characterize factors that may portend increased risk of CDI in HSCT and SOT recipients to build a risk stratification approach to prophylaxis.
In conclusion, the challenges facing AMS in transplant infectious diseases illustrate the difficulties in integrating the available evidence and diagnostic uncertainties with host-specific and local epidemiological factors to implement measures catered to both individuals and larger populations. The challenges, which are summarized in Table 1, have shared features, but specific solutions vary and should be personalized to institutional epidemiological patterns. In these uniquely vulnerable hosts, there is no “one size fits all” approach to AMS. For AMS challenges supported by strong evidence, we suggest that the implementation of practices in context of local epidemiology and dynamic evaluation over time to develop sustained, targeted measures. For challenges driven by knowledge gaps, recognizing the limitations of current evidence and engaging interdisciplinary teams to help risk-stratify patients are important to inform clinical practice. Employing thoughtful strategies is crucial for this population, which is disproportionally affected by infections and at risk for adverse effects of antimicrobial misuse and overuse.
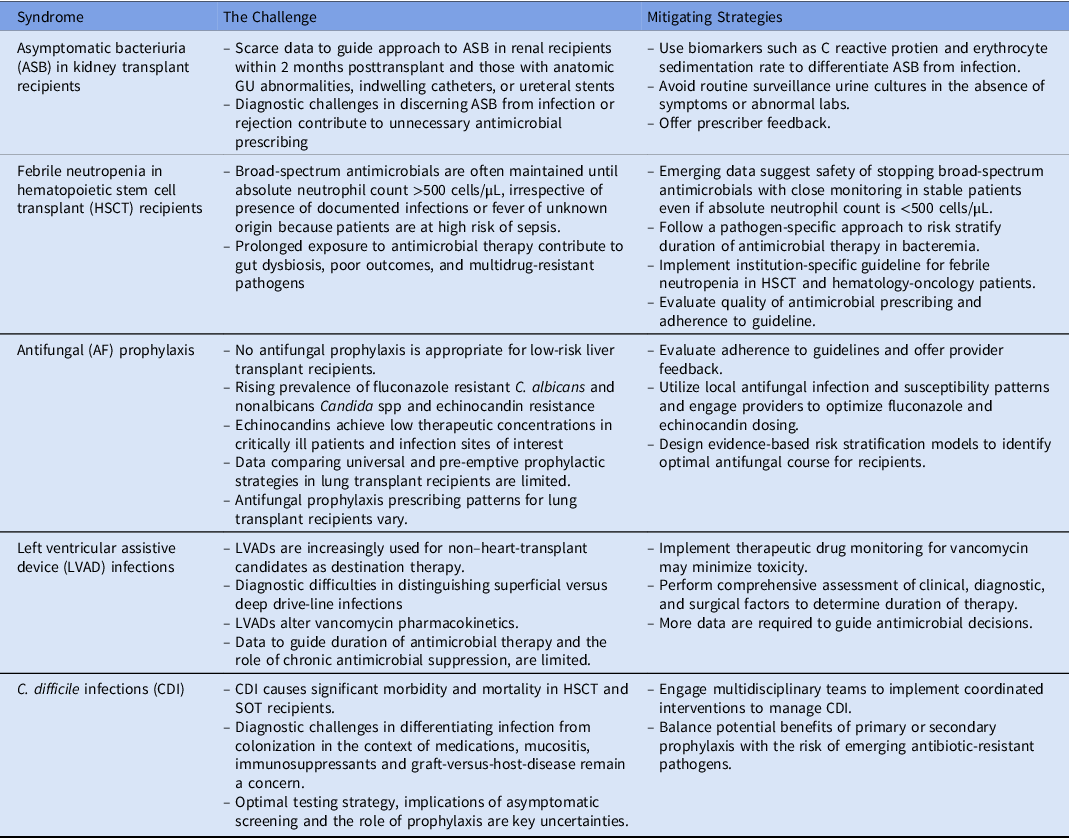
Table 1. Summary of the Top 5 Antimicrobial Stewardship Challenges and Potential Mitigating Strategies
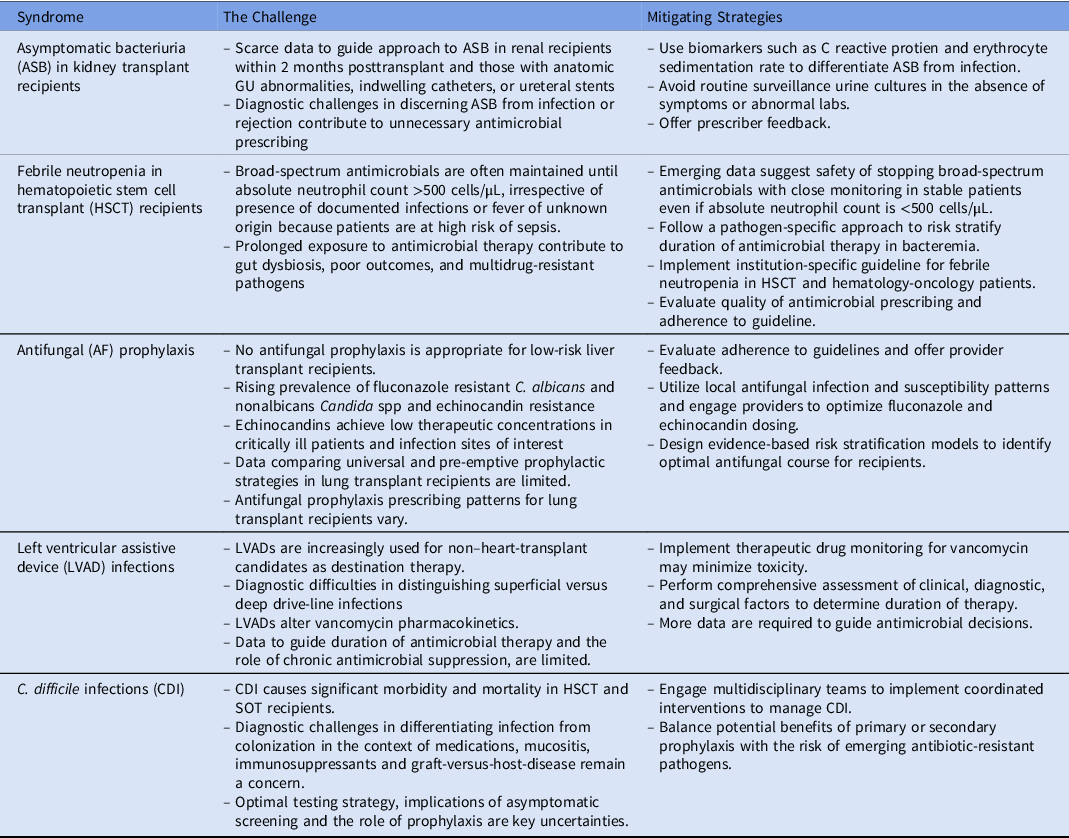
Note. HSCT, hematopoietic stem cell transplant.
Acknowledgments
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.