Introduction
The Antarctic continent possesses several types of surface waterbodies, including epiglacial and supraglacial lakes. Epiglacial lakes are found close to the oceanic boundary of the continent and at the foot of nunataqs, and supraglacial lakes form in the surface layer of the ice sheet where ‘blue ice‘ exists (e.g. Menzies Reference Menzies1995, Vincent et al. Reference Vincent, Hobbie, Laybourn-Parry, Vincent and Laybourn-Parry2008, Hodgson Reference Hodgson, Bengtsson, Herschy and Fairbridge2012). Epiglacial lakes are characterized as lakes in contact with a glacier and with inflow from glacial meltwater. They are an important habitat of life in the polar world (Kaup Reference Kaup1994, Hawes et al. Reference Hawes, Howard-Williams, Fountain, Vincent and Laybourn-Parry2008). Supraglacial lakes, in turn, are a part of the ice sheet, forming a liquid water layer on top. They are extremely low in biota (Keskitalo et al. Reference Keskitalo, Leppäranta and Arvola2013) and represent one type of extreme habitat. They show high sensitivity to atmospheric conditions and thereby assist in the detection of regional climate change (e.g. Winther et al. Reference Winther, Elvehøy, Bøggild, Sand and Liston1996, Leppäranta et al. Reference Leppäranta, Järvinen and Mattila2013b, Reference Leppäranta, Lindgren and Arvola2016).
The present study region is Vestfjella, Dronning Maud Land, near the grounding line of Riiser-Larsen Ice Shelf. The Finnish Research Station Aboa acted as the field base. In its neighbourhood, epiglacial and supraglacial seasonal lakes and ponds exist in summer at three nunataqs: Basen, Plogen and Fossilryggen. Altogether, these waterbodies have not yet been thoroughly investigated, although they are located in an area with two research stations that have had 30 years of operation. Geological and geochemical studies have focused on the Jurassic flood basalt succession that forms the exposed bedrock in the area (Luttinen et al. Reference Luttinen, Heinonen, Kurhila, Jourdan, Mänttäri, Vuori and Huhma2015). A reconnaissance-type geochemical study on the surface lakes has been reported in Lehtinen & Luttinen (Reference Lehtinen, Luttinen and Ojala2005). The structure of the Vestfjella supraglacial lakes was reported by Leppäranta et al. (Reference Leppäranta, Järvinen and Mattila2013b), and it has been shown that the lakes and ponds in the region support habitats for single-cell and multicellular organisms (Keskitalo et al. Reference Keskitalo, Leppäranta and Arvola2013). These habitats are of major importance for studies regarding the survival of life in extreme environmental conditions.
In Dronning Maud Land, there are perennial ice-covered lakes where the accumulated water storage is deep enough, at more than a few metres, such that wintertime ice growth does not reach the lake bottom. The largest of them is Lake Untersee, with a surface area of 11.4 km2 and a maximum depth of 169 m (e.g. Wand et al. Reference Wand, Schwarz, Brüggemann and Bräuer2004). However, these lakes are located far to the east of the Vestfjella region.
Our field programme on Vestfjella lakes and ponds was initiated in summer 2003–04, and four field seasons were completed by 2014–15. Altogether, we discovered 28 seasonal waterbodies in the study region, with nine of them being mapped in more detail. They represent three different types of seasonal surface lake and pond. The liquid-state period relies on meltwater from the ice sheet or, on top of nunataqs, the meltwater from local small glaciers or seasonal snow patches. The lifetimes of small pools formed from meltwater of seasonal snow can be shorter than the length of the 2 month (December–January) summer (see Leppäranta et al. Reference Leppäranta, Järvinen and Lindgren2013a). In contrast, lakes and ponds with inflows from glacial meltwater grow during the 2 months of summer and possess liquid water into the autumn months, but they are in a solid state for the rest of the year.
The thermodynamics of the proglacial surface waterbodies are governed by the radiation balance and penetration of solar radiation into the ice (Leppäranta et al. Reference Leppäranta, Järvinen and Mattila2013b, Reference Leppäranta, Lindgren and Arvola2016). Ponds on nunataqs are shallow with rock or soil bottoms, which collect radiative heat during the summer. In such environments, the water temperature may vary over a wide range (cf. Quesada et al. Reference Quesada, Goff and Karenz1998), and stratification can be influenced by dissolved substances in addition to temperature. According to Hawes et al. (Reference Hawes, Safi, Sorrel, Webster-Brown and Arscott2011a, Reference Hawes, Safi, Webster-Brown, Sorrel and Arscott2011b), small ponds, which are very common in ice-free areas, can possess extreme chemical conditions with high pH levels and dissolved oxygen (DO) concentrations.
Based on the field programme of 2003–15, this paper gives a classification of Vestfjella seasonal lakes and ponds, examines their annual cycle of freezing and melting, bathymetry and hydrology and analyses their geochemistry for electrical conductivity (EC), DO, pH and nutrients. The study is largely based on the fieldwork from summer 2014–15, but in order to achieve a broader view it also uses our earlier physical and biological results from 2010–11 (Keskitalo et al. Reference Keskitalo, Leppäranta and Arvola2013, Leppäranta et al. Reference Leppäranta, Järvinen and Mattila2013b) and geochemical and physical results from 2003–04 and 2004–05 (Lehtinen & Luttinen Reference Lehtinen, Luttinen and Ojala2005, Leppäranta et al. Reference Leppäranta, Järvinen and Mattila2013b). These shallow waterbodies are so-called cold environment polar lakes and ponds where thin ice cover exists and the ice surface temperature is below freezing point. Most of them are ponds rather than lakes in the strict sense.
Material and methods
Sites
The present field research was carried out in the Finnish Research Station Aboa in western Dronning Maud Land, located on Basen nunataq at 73°02.5′S, 13°24.4′W, altitude 485 m above sea level (a.s.l.). The data were collected mainly from nine proglacial lakes and ponds in Vestfjella at the nunataqs Basen, Plogen and Fossilryggen (Fig. 1, Table I) during four field seasons: December–January in 2003–04, 2004–05, 2010–11 and 2014–15. Besides the nine study targets, a few extra lakes were mapped. In particular, in summer 2004–05, a pond was mapped in the Swedish station Svea to serve as a significantly colder reference site (74°34.6‘;S, 11°13.5‘W, elevation 1261 m a.s.l.), and in summer 2014–15, we verified 17 ponds with liquid water around and on top of Basen nunataq.
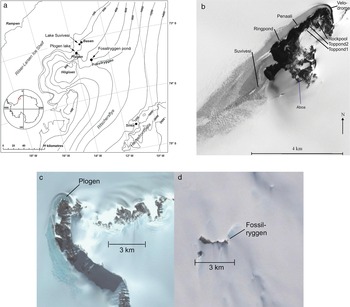
Fig. 1. a. Map of the study area and sites in western Dronning Maud Land. b. Terra/ASTER (Advanced Spaceborne Thermal Emission and Reflection Radiometer) image of 8 November 2001 of Basen nunataq with the local study sites (© NASA). c. Plogen lake next to the nunataq (downloaded from Google Maps). d. Fossilryggen lake on top of the nunataq (downloaded from Google Maps).
Table I. Lake type, location, surface area, maximum depth, altitude and site information of the study lakes and ponds. The types are nunataq ponds (Nqpo), supraglacial lakes (Supra) and epiglacial ponds (Epi).
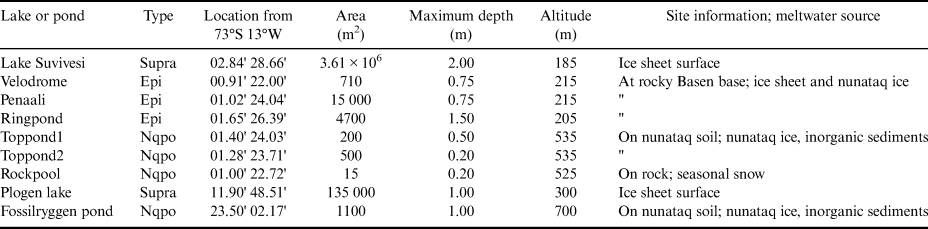
The waterbodies are here called lakes and ponds. There is no generally accepted distinction between them; here, the size distinction is taken, as is often the case, at the area of 50 000 m2 or 5 ha. The nine study lakes and ponds are situated as follows: three ponds (Toppond1, Toppond2 and Rockpool) on the top of Basen, three ponds (Velodrome, Penaali and Ringpond) at the foot of Basen, one lake (Lake Suvivesi) next to Basen, one pond on top of Fossilryggen (Fossilryggen pond, unofficial name) and one lake at the foot of Plogen (Plogen lake, unofficial name). The last two targets are, respectively, at 45 and 30 km distance from Basen. All of the waterbodies are shallow with a maximum depth of ≤ 2 m and only one of them is > 1 km2. Long-term weather statistics by Kärkäs (Reference Kärkäs2004) are used as references for the local climate.
Data collected
In each field season, the period of observations was December–January. The data collection consisted of manual sampling and sounding sites and automated stations. Hydrological investigations included the formation, the horizontal and vertical morphology and dimensions and the mass and heat balance of the lakes and ponds. Chemical investigations, in turn, included ice, snow and water samples for a wide set of analyses. Hydrological and chemical measurements were carried out in all of the field seasons. In addition, biological studies were included in 2010–11 and 2014–15, and these results will be published elsewhere.
The first field campaign in December 2003–January 2004 (Lehtinen & Luttinen Reference Lehtinen, Luttinen and Ojala2005) involved the acquisition of water and rock samples and the measurement of the pH and EC of the lake water. Altogether, seven waterbodies were included. For Lake Suvivesi, the changing surface conditions were documented and the thickness of the ice cover and water layer were monitored by drilling approximately every second day. Albedo measurements on Lake Suvivesi (five spots), on adjacent snow-covered areas and on a snow-free slope on Basen were performed using a Middleton EP-16 pyrano-albedometer system (spectral range 0.30–3.00 μm). The positions of five aluminium poles were determined on 8 and 22 December 2003 and on 14 January 2004 with real-time kinematic GPS measurements using the Aboa permanent GPS station as a reference station in order to monitor ice flow.
In the second field season (December 2004–January 2005), Lake Suvivesi was mapped for its cross-sectional structure and heat balance (Leppäranta et al. Reference Leppäranta, Järvinen and Mattila2013b). A radiation station was deployed on the ice cover of the lake. Global solar radiation (range 0.3–3.0 μm) was measured with the Middleton EP-16 pyrano-albedometer system, and the net radiation (range 0.2–100.0 μm) was recorded with a Kipp & Zonen NR Lite net radiometer. Recording underwater sensors for photosynthetically active radiation (PAR; 400–700 nm band), manufactured by MDS-L Alec Electronics Co. Ltd (Japan), were used at several sites, including recordings inside the lake ice and waterbody. Ice and water samples were taken for geochemistry only from Lake Suvivesi. The summer was quite warm, and in large areas over the central lake the ice cover was < 5 cm thick, even showing open spots in places. The thickness field was patchy, and it became increasingly difficult to cross the lake with dry feet. The liquid water production was 1.0–1.5 m vertically in the lake body. Investigations of other lakes were largely limited to visual observations and photography.
The third field season was performed in December 2010–January 2011. Lake Suvivesi was again the main study site with a largely similar field programme to the previous case (Leppäranta et al. Reference Leppäranta, Järvinen and Mattila2013b). A radiation station was deployed as in the previous case. The sampling programme was expanded to eight lakes and ponds (all except Rockpool in Table I) with hydrographic soundings. The mass and energy balances of snow patches on Basen nunataq were also examined, providing valuable information of the liquid water production from these spots (Leppäranta et al. Reference Leppäranta, Järvinen and Lindgren2013a). In situ measurements included water temperature and conductivity measurements using a YSI Professional sounding device. Samples for geochemistry were transported in a frozen state (-20°C) to Finland for analyses in the laboratory of Lammi Biological Station (LBS), University of Helsinki. Plankton samples were taken from several sites and transported in a liquid phase to LBS for microscopic species analyses (Keskitalo et al. Reference Keskitalo, Leppäranta and Arvola2013).
The most extensive field season was December 2014–January 2015. The field programme was performed from 4 December 2014 to 28 January 2015. The seven sampling sites at Basen (Table I) were visited weekly, while Plogen lake and Fossilryggen pond were visited three times during the summer. Therefore, it was possible to obtain the summertime evolution of the waterbodies in detail. In addition, 22 snow samples were collected for basic chemical analyses from the study sites. This summer was the coldest of all, and liquid water production at the sites fell to almost half of that in the warm summers. The automated radiation station was deployed in Lake Suvivesi as before, and manual PAR soundings were made in Suvivesi, Plogen and Fossilryggen with LiCor sensors for spherical and planar irradiance in the water and planar irradiance just above the surface. Water temperature and DO concentration (optical sensors) were mapped using a YSI Professional sounding device, while pH was measured after returning to Aboa with an Orion pH meter (Model 201). In addition, MiniDOT and Optolog data loggers were used in Lake Suvivesi, Toppond1 and Rockpool for water temperature and DO concentration measurements. The loggers were covered with aluminium foil in order to prevent any warming due to sunlight. Plankton samples were analysed in Aboa, and geochemistry samples were transported in a frozen state (-20°C) to the LBS laboratory.
This paper gives a classification of Vestfjella seasonal lakes and ponds, examines their annual cycle of freezing and melting, bathymetry and hydrology and analyses their geochemistry. In later papers, the light transfer and biology will be treated in detail.
Geochemical analyses
The geochemical analyses of water samples from summer 2003–04 were performed at the Natural Resources Institute Finland, Rovaniemi. The cations were analysed using a Dionex AS40 automated sampler and Dionex ICS-1000 with IonPack CG12A and IonPack CS12A columns. The anions were analysed using Dionex DX-120 with IonPack AG 9-HC and IonPack AS 9-NC columns. The pH and EC measurements were carried out using a Mettler Toledo pH meter at Aboa station within 12 h of sampling.
In the LBS laboratory, 2010–11 and 2014–15 geochemistry samples (meltwater) were analysed for their absorption spectra, EC, pH, total nitrogen, total phosphorus and cation concentrations. All chemical determinations were completed within 1 month of them having been received at LBS in the middle of April. The pH was measured with an Orion 3 Star pH meter, EC with a YSI 3200 pH meter and cation concentrations with a Varian SpectrAA 220/FS atomic absorbance spectrophotometer. Total phosphorus and total nitrogen concentrations were determined after wet oxidation with a Gallery Plus Thermo Scientific analyser (Koroleff 1983). Absorption spectra were measured with a Shimadzu Spectrophotometer (UV-2100) across the 200–750 nm band with a 2 nm spectral resolution.
Most statistical analyses for geochemistry were performed using SigmaPlot12.5 and Real Statistics software. The normalized chemical variables (xi – X)/s, where X is the mean and s is the standard deviation, were included in the K-means cluster analysis. The water variables included were pH, EC, colour (beam absorption of the filtered sample at 440 nm wavelength), total phosphorus, total nitrogen, potassium, sodium, calcium, magnesium, iron and manganese.
Results
Classification of lakes and ponds
The hydrological classification of Antarctic surface waterbodies (Table I) is made based on the timescale (seasonal/perennial), bottom type (ice/ground) and water source (glacial/seasonal snow). The study lakes and ponds are all seasonal and belong to the following groups:
(1) Nunataq group. Ponds on nunataqs, ground bottom of inorganic sediment and/or rock. Sub-classification is based on the source of water: 1a) glacial meltwater (Toppond1 and Toppond2, Fossilryggen pond), 1b) meltwater of seasonal snow patches (Rockpool).
(2) Supraglacial group. On top of ice sheet, inflow mainly glacial meltwater (Lake Suvivesi, Plogen lake).
(3) Epiglacial group. At edge of ice sheet, fed by meltwater from ice sheet and drainage from land (nunataq; Ringpond, Penaali, Velodrome).
Another way of expressing the classification is that, in addition to atmosphere, nunataq ponds interact with the ground, supraglacial lakes interact with the ice sheet and epiglacial ponds interact with both the ice sheet and ground. Lakes Suvivesi and Plogen are also close to a nunataq on one of their sides; they are large and predominantly form from the local ice sheet, but close to the nunataq the influence of inflow from the nunataq is significant. Thus, ‘near-supraglacial' would be a more precise classification of these lakes. In the study area, blue ice spots in the ice sheet were only found at the sides of nunataqs created by dominant wind conditions. It will be shown later that the cluster analysis of the geochemistry of the study lakes supports the chosen classification.
Annual cycle of the proglacial waterbodies
Consider heating of the ice sheet prior to the beginning of the ice melting. Using the heat diffusion equation with the solar source term, the temperature distribution can be solved from the quasi-stationary balance between absorption of radiation and heat conduction until the ice starts to melt (see e.g. Leppäranta Reference Leppäranta2015). The heat gained inside the ice is partly conducted back to the atmosphere and partly used to warm the ice. The maximum temperature is obtained at the depth
where κ ≈ 0.6 m−1 is the light attenuation coefficient, Q 0 is the surface heat balance and Qs + is the net solar radiation penetrating into the ice. For a solution zm > 0, we must have 0 < -Q 0 < Qs + for the heat fluxes (i.e. there is heat loss at the surface but the total flux is positive). If Q 0 > 0, the maximum temperature is located at the surface zm = 0. Ice melting can start when the maximum temperature has reached the melting point.
Our field data from the four summers fit well with this simple theoretical reasoning. According to the observations, Q 0 < 0 and melting started at ~0.5 m depth and continued from this depth vertically upwards and downwards, increasing the size of the liquid water body. This start-up was observed to take place close to 1 December in the Basen sites, where the minimum mean monthly air temperature is -21.9°C in August. The liquid waterbody formed and grew only during December and January due to the intensive solar radiation. The body of ice changed to a liquid state in a continuous manner and more or less monotonously depending on the weather (Fig. 2). The structure is described by the porosity υ = υ (x, y, z; t), or the relative amount of liquid water. When the porosity reached the critical ‘no strength’ level υ = υ0 ≈ 0.5, the ice lost its integrity and the remaining ice pieces rose up due to their buoyancy. As a result, a liquid water layer was developed, and the deeper ice was less and less porous with depth.

Fig. 2. Structural profile of the supraglacial lakes and nunataq ponds in Vestfjella.
The amount of liquid water in supraglacial and epiglacial lakes and ponds varied inter-annually by a factor of two. Diurnal variations of liquid water content could be relatively large in small ponds, and ponds where the inflow was from seasonal snow patches could dry up in summer due to a lack of water. In December, several small pools formed at the top of Basen, but later, in January, all of the water had dried up. The water volume was not measured and only two photographs are available in addition to the visual observations at the site.
In Lake Suvivesi in January 2004 and 2005, open water areas formed in places and the water layer was 60–130 cm deep. In the peripheral areas, the ice cover was 5–15 cm thick and the water layer was 40–100 cm thick. The extent of the liquid layer appeared to reach its maximum in mid-January. In January 2011, the situation was almost the same, but in summer 2014–15, the lake growth ceased at the end of December, water volume was less than in earlier years and surface ice cover was 25–30 cm thick.
Refreezing may last for several months. According to Stefan's formula, a new ice layer grows with accumulated freezing degree-days (FDD, in °C⋅d), as a⋅FDD0.5, a ≈ 3 cm (°C⋅d)−1/2 is a coefficient depending on the thermal properties of ice. Thus, it requires FDD ≈ 1000°C⋅d or 2 months with normal autumn temperatures of approximately -15°C to freeze a 1 m water layer completely. Thus, each year for 4–5 months there is liquid water. The lake bottom is a heat sink because the glacier or ground beneath the ice is colder than the freezing point of water. It is well known that at 10 m depth the temperature is equal to the annual average air temperature, which is -15°C in the study region. The heat loss to the deeper ice sheet can be estimated, assuming a linear temperature profile below the waterbody, as ~5 W m−2, which corresponds to an ice growth rate of 1.4 mm d−1. The heat loss to the ground depends on its thermal quality, but it is not expected to be much different from the heat loss to the deeper ice.
Bathymetry
All of the studied liquid waterbodies were seasonal and shallow regardless of their location on the top of the ice sheet or on the top of a nunataq (Table I). No perennial lakes or ponds were found in the Vestfjella region. Equation (1) gives the depth where melting of the ice starts on the top layer of an ice sheet. Then the part of the solar radiation that penetrates through the surface, Qs +, increases the waterbody vertically up to 1–2 m in depth over one summer. The maximum depth can be estimated using the heat balance. The heating power Qs + is distributed vertically as κe− κz, and to become part of the waterbody, melting must bring the porosity to a critical level υ* during the melt season of length tm. The scale of the lake depth then becomes
where ρ is the ice density and L is the latent heat of freezing. The first factor in the logarithm is the rate of ice melting, and the second factor distributes the melting to an active layer. In our study region, tm ≈ 60 days and κ ≈ 0.6 m−1. For Qs + ≈ 50 W m−2 and υ* ≈ 0.3, the melt rate is 1.4 cm d−1, the active layer thickness is 2 m and H ≈ 0.9 m. This corresponds to the dominantly clear sky summers at Aboa. If the summer is cold and cloudy weather dominates, the depth may be restricted down to 0.5 m, such as in the summer of 2014–15. The atmospheric warming rate is ~5°C per month in the Vestfjella spring, and therefore at Svea station, which is at 1 km higher altitude than the foot of Basen, the melt season would be 1 month shorter with H ≈ 0.3 m, as was observed in January 2005.
In nunataq snow and ice spots, melting may reach the ground (Fig. 3). When the ice sheet thickness is small (≤ κ−1), heating of the ground plays a major role in the formation, growth and freeze-up of the liquid waterbody. Bottom heating brings the liquid waterbody to the ground and warms the bottom water. A shallow lake acts as a greenhouse cover above the bottom, and the water temperature becomes notably higher. The concentration of dissolved matter may also be high, such that it influences the water density and, consequently, stratification and circulation. Due to the combined influence of solar heating and salinity stratification, temperatures as high as 9°C were observed in the bottom layers of very shallow nunataq ponds (Table II). In Fossilryggen pond, the warm bottom layer was persistent, although the surface ice cover was 20 cm thick in the 2014–15 summer. In our epiglacial and supraglacial lakes, the temperature did not get much above 1°C.

Fig. 3. Toppond2 on Basen, 17 January 2015.
Table II. Electrical conductivity at 25°C (EC), maximum (T max) and mean (T mean) temperature, oxygen (O2) saturation and coloured dissolved organic matter (CDOM) in beam absorption at 420 nm during the three summer seasons. Temperature and oxygen measurements indicate those made using a YSI device in daytime. The number of measurements varied from 3 (Plogen and Fossilryggen) up to 11 (Suvivesi).

Morphology
The state of the lake surface depends on the surface heat balance. When the air temperature is close to freezing point, both liquid and solid surface states are possible in equilibrium. Continuous surface heat gain from above means an open water surface, but otherwise we have ice cover with surface heat loss compensated for by conduction from the liquid waterbody. Albedo is a stabilizing factor: the high albedo of the ice surface keeps the solar heating low, which tends to conserve the ice cover, while, correspondingly, the low albedo of the open water surface tends to conserve the open water state. In our study lakes, an ice surface is the predominant situation. Thus
where k is the thermal conductivity of ice, T is the temperature and z is the vertical coordinate. This gives the surface ice layer thickness as ![]() $h\approx {{k\lpar {T_0-T_f} \rpar } \over {Q_0}}$, where T 0 is the surface temperature and Tf is the freezing point temperature (e.g. for Q 0 ≈ -10 W m−2 and T 0 – Tf ≈ -1°C, we have h ≈ 20 cm). The surface ice thickness decreases in this way, approaching the seasonal equilibrium. According to our field results, this equilibrium has typically been 10–30 cm in the study region, with patches down to 0 cm thickness or open water state in warm summers.
$h\approx {{k\lpar {T_0-T_f} \rpar } \over {Q_0}}$, where T 0 is the surface temperature and Tf is the freezing point temperature (e.g. for Q 0 ≈ -10 W m−2 and T 0 – Tf ≈ -1°C, we have h ≈ 20 cm). The surface ice thickness decreases in this way, approaching the seasonal equilibrium. According to our field results, this equilibrium has typically been 10–30 cm in the study region, with patches down to 0 cm thickness or open water state in warm summers.
The birth and growth of a proglacial surface lake depend strongly on the albedo. According to our measurements, the mean summer albedo is 0.55–0.60 over lake surfaces, with significant spatial variability. As horizontal transfer of heat is very slow, the result is a patchy appearance of the lake body. There are deep spots with high porosity and more solid spots, depending on the albedo. Due to the positive albedo feedback, the patchy appearance is persistent. The overall structure is comparable to wetland soil. Albedo over snow is much higher than over lakes. Our albedo measurements over the snow-covered ice sheet averaged 0.85 and over nunataq snow patches they averaged 0.70.
In supraglacial lakes, in the main body υ > υ0, and deeper down there is a 1 m soft bottom layer with a sub-structure of hard layers and slush layers with sediment particles. The slush layers form due to absorption maximum by the sediments, which have originated from the nearby nunataq in the past years. Similar stratification was also seen in Plogen lake, as well as in a supraglacial lake at Fossilryggen in summer 2004–05. Nunataq ponds are like normal ponds, with the waterbody possibly covered by an ice layer.
Water balance
Blue ice refers to spots in the accumulation zone where the atmospheric water balance is negative and therefore the surface appears as blue ice in contrast to the normal white snow-covered surface. In blue ice patches, snowfall is compensated for by sublimation and snow drift, a necessary condition to keep the blue surface. To compensate for the mass loss, the ice velocity is upward near the surface. Blue ice has a much lower albedo and a much higher transparency than snow and therefore can absorb several times more solar radiation and allow it to reach deeper. In a warming climate, blue ice spots may grow and locally provide strong positive feedback.
According to the observations, the local production of meltwater in blue ice in December–January corresponds to a 0.5–1.0 m liquid water layer, which is distributed in the top 1.0–2.0 m of the ice sheet. At the foot of nunataqs, runoff from the mountain adds to the water volume of neighbouring lakes. In Lake Suvivesi, the flow was clearly seen in warm summers. In 2003, minor streams were observed to flow from Basen to Lake Suvivesi by 10 December. Melting on nunataqs was efficient, and the inflow streams were strong. Nevertheless, parts of Lake Suvivesi also lacked a water layer during this time, and parts of the lake surface layer dried up, with large subsurface cavities. Sublimation accounts on average for 1 mm of ice per day and reaches 50–100 mm over the whole summer, but in extreme storm situations sublimation rates of 10–15 mm per day have been observed.
Although some epiglacial ponds around the Basen nunataq are hydraulically interconnected, large differences in water tables in many of them indicate that this is not the case for all of them. The ice sheet tilts down towards the nunataq and the ponds are formed in this valley at the nunataq foot (Fig. 4). In summer 2014–15, Penaali pond (unofficial name) received plenty of meltwater from the surrounding ice sheet, while in other ponds no visible hydrological interconnectivity with the ice sheet was found. This means that solar radiation was the primary factor causing the subsurface melting. However, in addition, it was observed that some meltwater drained down from the nunataq, where patches of snow and ice melted over the course of summer. In particular, the northern and north-eastern side of Basen, where most of these valley ponds are situated, is exposed to sunlight. Based on site photographs, it was seen that the water level of Ringpond was 2 m higher in 2004–05 than 6 years later. In addition, old cyanobacterial mats on the cliff in 2014–15 indicated that the water table had been at least 2 m higher in the recent past.

Fig. 4. The valley of ponds on the north side of Basen.
Heat balance
The heat budget is governed by solar and terrestrial radiation. The surface radiation balance is close to zero, with turbulent heat losses resulting in a surface ice layer, while solar radiation penetrating beneath the surface takes care of the warming up and melting of ice (Fig. 5). In Lake Suvivesi, the mean net solar radiation in December–January was 115–148 W m−2 in the three last field summers, while the net terrestrial radiation and turbulent losses were 33–78 and 25–53 W m−2, respectively. The mean December–January heat flux for internal ice melting in the lake was 10–59 W m−2, which corresponds to melting by 0.3–1.7 cm d−1.

Fig. 5. Heat balance of supraglacial lakes (left) and nunataq ponds (right). The numbers provide the scales (in W m−2) of net solar radiation, net longwave radiation, turbulent fluxes, conductive flux through surface ice and heat flux to bottom. The circled numbers give the total heat gain by the waterbody.
In supraglacial and epiglacial waterbodies, the daytime water temperature may rise a little above the melting point in finite water pockets due to absorption of sunlight and slow molecular diffusion. While conduction is slow, convection may take place during the day, driven by solar heating. Over short periods, the increased temperature of interior water pockets leads to density-driven convection with sinking warm water. This contributes to increasing the liquid water layer. Between 29 December 2014 and 13 January 2015, the highest water temperature at 50 cm depth in Lake Suvivesi was 1.0°C in the afternoon. After mid-night, the temperature fell back to 0°C.
The patchy melting brings about pressure differences, which, as observed, force horizontal movement of liquid water in the porous, slushy lake body. The velocity depends on the hydraulic conductivity, which is highly sensitive to porosity. According to our measurements, the hydraulic conductivity increased from 0.1 to 10.0 cm s−1 when porosity increased from a low level to the order of 0.5, where the solid ice lattice breaks down. In slush, a mixture of free ice pieces and water with a porosity within 0.3–0.5, the conductivity was measured at 6 cm s−1. The conductivity measurements were made by water-pumping experiments applying the Dupuit hydraulic well formula and using tube tests.
In shallow nunataq ponds, bottom heating by solar radiation can raise the water temperature to much greater than the melting point in the lower layers. What exactly happens then is also dependent on water salinity. The density of freshwater in the temperature interval of 0–8°C varies by 0.132 kg m−3, which corresponds to the variation by 0.16 μg l−1 in the concentration of dissolved solids. Thus, even weak salinity stratification can overcome the influence of temperature on density in cold water. The thermal stratification in nunataq ponds such as Fossilryggen pond can therefore be influenced by the salinity gradient. When a lake forms from ice, the water temperature remains near freezing point until the liquid water level reaches the ground, where bottom heating begins and stratification forms (Table II). The temperature of the bottom water has been observed to be as high as 9°C in our nunataq ponds. In a case of unstable stratification, turnover would occur, but no such event was observed.
Figure 5 illustrates the heat balance of supraglacial lakes and nunataq ponds. Solar heating of the interior well overcomes the boundary losses and produces liquid water of 1.1–1.3 cm per day on average, corresponding to a heat gain of 40–45 W m−2. The surface radiation balance is below zero due to terrestrial radiation and turbulent losses, and this is seen in the existence of a surface ice layer. The turbulent loss is dominated by sublimation. Over three summers, the average net solar radiation was 140 W m−2, of which half contributed to the surface balance and half penetrated into the lake. Over thick ice (>> κ−1), the loss from the lake body to the deeper ice sheet is estimated at ~5 W m−2. Over thin ice (≤ κ−1), solar heating is returned back to the waterbody from the bottom.
At extremes, an open surface is possible in the daytime, but at night a very thin ice layer forms. As the air temperature remains below freezing point, in practice it is not possible for an open surface to gain heat from the atmosphere. On the one hand open surfaces absorb more solar radiation, but on the other hand terrestrial radiation and turbulent losses are larger, more than compensating for this increased solar radiation absorption. Thus, in the Vestfjella climate, an open surface is not a stable situation.
Electrical conductivity and colour of the water
Table II shows the EC of the lake water samples. In very low salinity, as here, the density of water is proportional to the EC. When EC ≤ 10 μS cm−1, the influence of salinity on the density can be ignored here. The supraglacial lakes form a specific group with a very low EC, being 1.5–5.3 μS cm−1 in Lake Suvivesi, with an average of 3.7 μS cm−1. In summer 2004–05, only Lake Suvivesi was included, and the EC result was then 5.0 μS cm−1. In the epiglacial ponds at the foot of Basen, the EC magnitude was 10–100 μS cm−1, and higher values were recorded in nunataq ponds of up to 1000 μS cm−1, which corresponds to a concentration of dissolved solids of 0.7 g l−1. The EC showed a decreasing trend through the summer. In summer 2014–15, the mean EC of snow was 3.2 μS cm−1 in the study region, which is very close to the value in supraglacial lakes.
The transparency of the lakes depends on the concentrations of optically active substances (coloured dissolved organic matter (CDOM), suspended matter, chlorophyll a and iron), as well as the distribution and proportion of ice with its gas bubbles. The CDOM is proportional to the limnological colour index of the water. This was more closely examined in our expedition in 2014–15. In Lake Suvivesi, the light attenuation coefficient ranged from 0.5 to 2.0 m−1. In the mixture of ice pieces and water, scattering of light was very strong, and this was a major factor in the light attenuation in the main bodies of supraglacial and epiglacial waterbodies. Particularly in supraglacial lakes, the ice and meltwater were very clean, with low absorption coefficients. The CDOM values were extremely low in all of the study lakes and ponds, except for in the nunataq ponds on Basen nunataq (Table II). The highest CDOM values were measured in Rockpool and the second highest in Toppond1, with both lakes lying above the bedrock and mineral sediment.
Geochemistry
Water pH varied between lakes and between years. However, there was a clear distinction in that pH was mostly within 6.0–7.5 in supraglacial and epiglacial lakes and ponds, while higher values were found in nunataq ponds (Table III). In 2014–15, the highest pH value (10.1) was found in Rockpool, followed by Toppond1 and Fossilryggen, where pH was above 9. The lowest pH (5.9) was measured in the supraglacial Lake Suvivesi in the same summer. It was only 0.5 units higher than the pH of snow. The pH of the frozen samples transported to Finland was generally 0.5–1.0 units lower than those measured from the fresh samples. It was found that the water pH and EC were strongly correlated (r > 0.9, P < 0.0001). In summer 2010–11, very similar chemical results were obtained to those in 2014–15, as water pH varied mostly between 6.0 and 8.5, with a few higher values being found in Velodrome pond and Fossilryggen.
Table III. Geochemistry of the study lakes and ponds. Seasonal averages of sodium (Na), potassium (K), magnesium (Mg), calcium (Ca), manganese (Mn), chloride (Cl), iron (Fe), pH, sulphate (SO4), phosphate (PO4), total phosphorus (TP), nitrate nitrogen (NO3-N) and total nitrogen (TN) based on 3 (Plogen and Fossilryggen) up to 11 (Suvivesi) samples (± SD).

In summer 2003–04, different sampling sites showed highly variable ion concentrations, with Rockpool having the highest seasonal average values and Lake Suvivesi having the lowest values, with sodium concentrations of, respectively, 72.00 and 0.12 mg l−1 (Table III). The epiglacial sampling sites all recorded over 10 times higher average ion concentrations than in Lake Suvivesi. The water samples collected from Lake Suvivesi indicated an increase of salinity with time. For example, the concentrations of sodium (0.04 mg l−1 on 14 December, 0.10 mg l−1 on 3 January and 0.21 mg l−1 on 21 January), magnesium, chloride and sulphate showed a relatively strong enrichment. These values are averages of three samples from individual drill holes in area of ~104 m2. Comparison of the chemical and EC data obtained from different sites shows pronounced spatial variation, possibly associated with localized inflow from Basen water into Lake Suvivesi. Consequently, the apparent increase in salinity may be an artefact and, instead, may reveal a minor epiglacial component in Lake Suvivesi. High salinity in nunataq meltwater ponds and streams may at least partly result from the formation of salt deposits in response to repeated evaporation cycles. X-ray diffraction measurements indicated that the light-coloured salt deposits on basaltic bedrock and soil are mainly calcite (aragonite; CaCO3) and thenardite (Na2SO4).
In summer 2014–15, the lowest difference between minimum and maximum element concentrations was recorded in total phosphorus. Except for iron, potassium and phosphorus, the other chemical constituents had their highest concentrations in Rockpool, where highest pH and colour values were also measured. In contrast, in Suvivesi, the concentrations were generally lowest among the sites. The EC correlated strongly with the concentrations of sodium, calcium and magnesium (for each r > 0.9, P < 0.0001). In addition, potassium, manganese and iron concentrations were correlated with the EC (r > 0.58, P < 0.05). Furthermore, the different cation (K, Na, Ca, Mg, Mn and Fe) concentrations were inter-correlated when the results of the study sites were considered (all combinations had r > 0.7 and P < 0.01). Among the epiglacial and supraglacial lakes and ponds, the highest EC values and cation concentrations were measured in Ringpond and Velodrome (Table III). Sodium, magnesium and calcium were the three cations with the greatest range of variability in concentration among the waterbodies.
In line with cations, nitrogen and phosphorus concentrations also varied substantially between the waterbodies in summer 2014–15. The highest concentrations of phosphorus and nitrogen were found in Rockpool. The lowest nitrogen concentrations appeared in Suvivesi, Ringpond and Plogen, while the lowest phosphorus concentrations were found in Toppond1, Toppond2, Fossilryggen and Ringpond (Table III). Because of the low nitrogen and phosphorus concentrations, their inorganic fractions could be reliably measured in only a few waterbodies. In Rockpool, ammonium (NH4-N) and nitrite-nitrate (NO2 + NO3-N) comprised together 10% of the total nitrogen, and phosphate (PO4-P) comprised 40% of the total phosphorus. In summer 2010–11, total nitrogen and phosphorus concentrations were predominantly low, but occasionally higher concentrations were measured, in of up to 30 μg l−1 in Ringpond and Toppond2 and of up to 700 μg l−1 in a small brook flowing into Suvivesi.
Hierarchical clustering based on geochemistry classified the study lakes and ponds into four to five groups depending on the variables included in the analysis. When all of the data were included, Suvivesi, Plogen and Penaali formed one group and Fossilryggen, Toppond1, Velodrome and Ringpond formed the second group, while Toppond2 and Rockpool formed their own individual groups (Fig. 6). However, if all of the measured variables were included, Suvivesi and Penaali formed one group, Plogen, Ringpond and Fossilryggen formed the second one, Toppond1, Toppond2 and Velodrome formed the third one and Rockpool formed its own fourth group. Very similar groupings were given for pH, EC, colour and total phosphorus and total nitrogen concentrations.

Fig. 6. Hierarchical cluster analysis of three of the study lakes. The cluster is based on the data of pH, electrical conductivity, colour, total phosphorus, total nitrogen, potassium, sodium, calcium, magnesium, manganese and iron concentrations.
In summer 2014–15, with a few exceptions, DO concentrations and saturation levels varied within a narrow range in the waterbodies (Table II). The saturation level was mostly below the equilibrium with the atmosphere. However, in Rockpool and Fossilryggen, the DO concentrations were close to 20 mg l−1, with saturation close to 150%. The highest DO concentration was measured in Fossilryggen on 21 January, at ~30 mg l−1, corresponding to 250% saturation. In Rockpool, the maximum DO concentration was 21.4 mg l−1 or 174% saturation, measured on 27 December and 3 January. The highest DO value recorded by the DO/temperature data logger in Rockpool was 17.4 mg l−1, 144% saturation, at noon on 28 December. In contrast to 2014–15, in summer 2010–11, DO supersaturation was measured only twice, with both cases in Velodrome pond.
Comparing the geochemistry between the summers of 2003–04 and 2014–15, it is seen that the element concentrations were similar. The differences can be largely explained by the differences in the weather conditions and in the sampling techniques, and by random variations. For example, the stratification of temperature and chemistry was strong in Fossilryggen pond, and it is therefore understandable that the exact sampling depth plays a major role. In the ponds on the top of Basen, biological activity was very high, which makes the timing of the sampling important. Our supraglacial lakes were disturbed by inflow from nunataqs close to their edges in localized streams, as is suggested by observations. In pure supraglacial conditions, the lake water quality would be influenced only by the local ice sheet and atmospheric deposition.
Discussion
Relative to the nunataqs and ice sheets, the study lakes and ponds can be divided into three major groups. This classification follows the approaches of Vincent et al. (Reference Vincent, Hobbie, Laybourn-Parry, Vincent and Laybourn-Parry2008) and Hodgson (Reference Hodgson, Bengtsson, Herschy and Fairbridge2012) but includes only the types of lakes present in the Vestfjella region: supraglacial and epiglacial waterbodies, which are situated on the ice sheet, and nunataq ponds on top of nunataqs (Table I). The location of a waterbody relative to the neighbouring nunataq and ice sheet affects its hydrological and chemical characteristics. The formation of supraglacial and epiglacial waterbodies is dependent on the existence of blue ice, which allows light penetration through the topmost layer of the ice sheet. Plogen and Penaali also received meltwater inflow from the ice sheet, as was clearly indicated by the chemistry results. The closure of the seasonal lakes is expected to progress by 1.0–1.5 cm per day for up to 3–4 months for the deepest lakes observed (Hawes et al. Reference Hawes, Safi, Sorrel, Webster-Brown and Arscott2011a). There are very small water pockets (below 1 m scale) in the ice sheet surface layer, called cryoconite holes, initiated by impurities in the ice sheet surface (Hawes et al. Reference Hawes, Howard-Williams, Fountain, Vincent and Laybourn-Parry2008), but they have not been considered here.
Nunataq ponds are more strongly influenced by the nunataq water balance, as there is significant meltwater inflow from the surrounding drainage area. Toppond1 was clearly influenced by local melting, while Toppond2 was seemingly influenced by the snow and ice melting around the pond itself. Toppond1 had a nearly 1 m thick ice cap, but Toppond2 was very shallow, and the lake was mostly ice free in daytime if the weather was sunny and warm, but it was frozen if the day was cloudy and cold. Rockpool was very shallow and far from glaciers, and it gained water from seasonal snow melting. All of the snow in its drainage basin had already melted by early summer. The water level started to decline and the pool, as well as several other similar waterbodies, started to dry up. However, in Rockpool, some water remained for as long as we continued our measurements (until the end of January). In nunataq snow patches, meltwater runoff was ~1 mm snow water equivalent per day (Leppäranta et al. Reference Leppäranta, Järvinen and Lindgren2013a).
Our study lakes in Vestfjella, Dronning Maud Land, are oligotrophic or ultra-oligotrophic, characterized by low EC and nutrient concentrations, similarly to most Antarctic surface lakes (Lyons & Finlay Reference Lyons, Finlay, Vincent and Laybourn-Barry2008). Their life history depends on the local climate. In much colder environments the summer warming would not reach the melting stage, while in much warmer environments the lakes would become perennial. Our study lakes appear to be quite different climatologically from these extreme cold and warm situations.
According to their locations relative to the nunataqs and physical and chemical characteristics, the studied waterbodies can be grouped into several categories. Lake Suvivesi forms its own category because of its large surface area and its consequently weak dependence on the meltwater from the nunataq. However, in this case the nunataq has an indirect impact on the lake evolution, because Basen gives shelter against the wind, which is a prerequisite for blue ice formation. In all of the lakes, liquid water exists only in the uppermost layer of the ice. However, lakes that are in close contact with the nunataqs are influenced by the erosion material transported by meltwater to the lakes and ponds.
In Lake Suvivesi, the element concentrations showed large spatial variability. Compared with the concentrations in the snow cover in the neighbourhood (Kärkäs et al. Reference Kärkäs, Teinilä, Virkkula and Aurela2005, Lehtinen & Luttinen Reference Lehtinen, Luttinen and Ojala2005), the medians were approximately the same for sodium, potassium, sulphate and nitrate, while calcium and magnesium were enriched and chloride was lower in the lake. These differences indicate a significant impact resulting from the nunataq via inflow.
When the transport of inorganic material is strong enough, meltwater discharge affects the pH, EC and other geochemical variables. Previous studies have shown that the nunataqs and the surface waters of the present study may have abnormally high cation concentrations, particularly for magnesium and calcium, but low levels of nitrogen and phosphorus (Lehtinen & Luttinen Reference Lehtinen, Luttinen and Ojala2005, Keskitalo et al. Reference Keskitalo, Leppäranta and Arvola2013).
In those lakes and ponds that are situated on top of Basen nunataq or in close contact with it on the glacier, nitrogen and phosphorus may partially originate from the sea, where snow petrels (Pagodroma nivea) feed and carry food to their nesting areas in Basen and Plogen. In summer 2014–15, the populations consisted of 30–50 pairs in these nunataqs. In addition, in Basen, one pair of south polar skuas (Stercorarius antarcticus) was nesting and feeding on the snow petrels and thus releasing nutrients into the environment. Occasionally, two other petrel species (Oceanites oceanicus, Thalassoica antarctica) also visited the area, mostly seen above Basen.
In small ponds on top of Basen nunataq, cyanobacteria may have supported nitrogen reserves. In small rock pools in particular, plankton mat communities have large numbers of cyanobacteria that can fix atmospheric nitrogen (Bergman et al. Reference Bergman, Gallon, Rai and Stal1997, Charpy et al. Reference Charpy, Palinska, Casareto, Langlade, Suzuki, Abed and Golubic2010). The importance of in situ assimilated nitrogen or phosphorus released through erosion relative to that transported from the sea by birds is unknown, however.
Antarctic lakes are mostly considered to be phosphorus-limited, although nitrogen and phosphorus concentrations can vary considerably among them (Laybourn-Parry Reference Laybourn-Parry, Huiskes, Gieskes, Rozema, Schorno, van der Vies and Wolff2003), and in some areas nitrate-rich waters can be found (Vincent & Howard-Williams Reference Vincent and Howard-Williams1994). In our study, nutrient limitation of lakes also seems to be a major feature (Keskitalo et al. Reference Keskitalo, Leppäranta and Arvola2013), although each waterbody behaves differently and thus the impact on water chemistry varies from lake to lake. Our results indicate that, among the group of nunataq ponds, two to three different subgroups can be identified based on their hydrological and water chemistry properties. The meltwater inflow from the nunataq strongly influences the water chemistry in Penaali and Plogen, and this explains why their water chemistry characteristics are close to those of Suvivesi. Shallow rock pools with their rich cyanobacterial mat communities are unique ecosystems due to their food webs and very high photosynthetic potential. In Antarctica, cyanobacterial mat communities can be abundant in many lake and pond ecosystems (e.g. Hawes et al. Reference Hawes, Howard-Williams and Vincent1992, Hodgson et al. Reference Hodgson, Vyverman, Verleyen, Sabbe, Laevitt and Taton2004, Singh & Elster Reference Singh, Elster and Seckbach2007). According to Hawes et al. (Reference Hawes, Safi, Sorrel, Webster-Brown and Arscott2011a), benthic communities usually predominate over planktonic ones in small ponds in ice-free areas of Antarctica.
Regarding the water chemistry and heat balance, inorganic bottom sediment is an important characteristic in small ponds (Toppond1, Toppond2, Rockpool and Fossilryggen) on top of the nunataqs. The presence of sediment differentiates them from the rest of our waterbodies, where the liquid water layer was above ice. As a result of the inorganic sediment, small ponds and rock pools on top of the nunataqs had higher water pH values and nitrogen and phosphorus concentrations than the other waterbodies. Very high pH values (> 10) and DO concentrations (up to 100 mg l−1) have been found previously by Hawes et al. (Reference Hawes, Safi, Webster-Brown, Sorrel and Arscott2011b) in small ponds in McMurdo Ice Shelf close to Bratina Island.
Among our waterbodies, Rockpool was a unique environment with an overwhelmingly rich cyanobacterial community. Due to it receiving meltwater from the surrounding seasonal snow patches, despite having bottom sediment equal to the two nunataq ponds (Toppond1 and Toppond2), we suggest that the rock pool should be considered as a specific water formation. The reason for this is that its lifespan is shorter than that in other lakes and ponds, and thermal conditions are more variable. In addition, organisms in such rock pools are exposed to greater ultraviolet radiation than in deeper lakes and ponds with much thicker ice cover.
Conclusions
In Vestfjella, western Dronning Maud Land, three main ‘lake categories’ were found: 1) supraglacial lakes, 2) nunataq ponds and 3) epiglacial ponds. Category 2 can be divided into two subgroups regarding whether the meltwater source is glacial or just from seasonal snow patches. Supraglacial waters are ultra-oligotrophic, while in nunataq ponds the concentrations of dissolved and suspended solids vary over a wide range. Each waterbody behaves uniquely and thus the impact on the water chemistry varies from lake to lake. Our results indicate that, among the group of nunataq ponds, two to three different subgroups can be identified based on their hydrological and water chemistry properties.
The heat budget of the Vestfjella lakes is dominated by solar radiation and longwave radiation losses, with turbulent heat exchange acting strongly only episodically. Sublimation can become a significant part of the mass balance. The limnological quality of the waterbodies ranges from ultra-oligotrophic supraglacial lakes to small hypereutrophic nunataq ponds.
Water temperature may also vary widely among the lakes and ponds. Shallow ponds usually freeze over at night and during snowstorms and cold weather, while during sunny days they are mostly ice-free. We propose that the three lake types are common in the vicinity of nunataqs in the western and northern Dronning Maud Land.
Acknowledgements
We thank the Finnish Antarctic Research Program (FINNARP) for logistical and field support during the expedition, and the Department of Physics and the Lammi Biological Station (LBS), University of Helsinki, for the equipment and laboratory analyses. The FINNARP expedition chiefs, Mr Petri Heinonen and Mr Mika Kalakoski, are thanked for their guidance and help, and Dr Onni Järvinen, Dr Eija Kanto, Ms Anne Lehtinen and Mr Olli-Pekka Mattila and thanked for their contributions to the fieldwork. Special thanks are given to Riitta Ilola and Jaakko Vainionpää at the LBS laboratory for their chemistry analyses. Dr Warwick F. Vincent is thanked for his careful and constructive review of the manuscript.
Author contributions
ML took part in the three fieldtrips in 2004–15 reported here. AL conducted the lake research in the first fieldtrip (2003–04). LA joined the last fieldtrip (2014–15). All authors contributed equally to the analysis and writing the manuscript. ML was mainly responsible for the physics. AL and LA were mainly responsible for the geochemistry and biology.











