Introduction
The Falkland Islands (Malvinas) are some of a number of remote islands and archipelagos at high to polar latitudes in the Atlantic, Indian and Southern oceans that once supported rainforest and rainforest scrub (woody spp. <5 m tall) in the pre-Quaternary period (Cantrill & Poole Reference Cantrill and Poole2012). Evidence for the former presence of tree-dominated vegetation includes in situ tree stumps, wood, leaves and fossil pollen and spores (Edwards 1921, Cookson Reference Cookson1947, Birnie & Roberts Reference Birnie and Roberts1986, Poole & Cantrill Reference Poole and Cantrill2007, Seward & Conway Reference Seward and Conway1934, Stone et al. Reference Stone, Aldiss and Edwards2005, Truswell et al. Reference Truswell, Quilty, McMinn, Macphail and Wheller2005). In almost all cases, the palaeofloras are assumed to have colonized the islands via transoceanic dispersal of seeds and spores by wind, ocean currents and birds from adjacent larger landmasses during globally warm periods (e.g. Late Cretaceous or Eocene; Zachos et al. Reference Zachos, Pagani, Sloan, Thomas and Billups2001, Macphail & Cantrill Reference Macphail and Cantrill2006, Ortiz-Jaureguizar & Cladera Reference Ortiz-Jaureguizar and Cladera2006).
Here, we report the discovery of a buried lignitic deposit during excavations for the foundations of the Tussac House aged care centre on the foreshore at Stanley, the capital city on the eastern island of the Falkland Islands (Fig. 1). This is the second of two lignitic deposits (forest beds) found in the Falkland Islands that preserve evidence of forest or woody plant communities growing on this now-treeless archipelago (Macphail et al. Reference Macphail, Alley, Truswell and Hill1994). The first discovery in 1899 comprised a lignitic deposit with tree trunks > 1.5 m in diameter found outcropping on the foreshore of West Point Island, a small island on the western side of the archipelago (Halle Reference Halle1911). While it was initially proposed that this deposit may have been an accumulation of driftwood (Baker Reference Baker1924), analysis of its stratigraphic relationships confirmed that the lignite was in situ, while the associated fossil pollen and spores suggested that the deposit was Late Neogene in age, representative of a time period when climate conditions allowed the growth of forests (Birnie & Roberts Reference Birnie and Roberts1986). More recent palynological investigation by Macphail & Cantrill (Reference Macphail and Cantrill2006) and an investigation of the wood assemblage (Poole & Cantrill Reference Poole and Cantrill2007) of the West Point Island deposit narrowed its age limits to Middle Miocene to Early Pliocene. Here, we document the microfloras and wood fragments preserved in the Tussac House deposit. We infer the age limits by a combination of stratigraphic distribution of fossil pollen species transported by wind/birds from southern Patagonia and by comparing coeval floras present in Patagonia-Tierra del Fuego, the South Shetland Islands and West Antarctica.

Figure 1. Location of Tussac House (green dot) and West Point Island (purple dot) on the Falkland Islands (inset, map of the South Atlantic region). Map produced using Generic Mapping Tools (GMT).
Setting
The Falkland Islands lie at 52°S, 540 km east of the coast of South America. Unlike many volcanic islands off the Chilean coast, the Falkland Plateau is considered to be a rifted part of the African Plate associated with the break-up of Gondwana and the opening of the Atlantic Ocean during the Mesozoic (Stanca et al. Reference Stanca, McCarthy, Paton, Hodgson and Mortimer2022). The modern climate is made of up two main zones: subpolar oceanic climate (Köppen classification: Cfc) in lowland areas and polar tundra climate (Köppen classification: ET) in upland areas (Beck et al. Reference Beck, Zimmermann, McVicar, Vergopolan, Berg and Wood2018), being highly influenced by the surrounding cool South Atlantic waters. There are some isolated areas of cold desert (BWk) and cold steppe (BSk) in lowland areas influenced by rain shadows. The mean annual temperature is 5.5°C, with high mean monthly and annual wind speeds of ~8.5 m s-1 (with prevailing westerly winds) and relatively low annual precipitation of ~600 mm, distributed uniformly throughout the year (Lister & Jones Reference Lister and Jones2014). Although the Falkland archipelago is ~8° latitude north of the southern limits of tree growth in southern South America, trees do not now grow naturally on the islands, and vegetation is dominated by acid grasslands, including whitegrass (Cortaderia pilosa) and diddle-dee (Empetrum rubrum), with tussac grass (Poa flabellata) found in coastal areas. Woody shrubs, such as native boxwood (Veronica elliptica), are relatively uncommon and confined to topographically sheltered areas. Reasons for the lack of timber-sized trees (and tree species) include the subdued topography, very extensive periglacial activity but limited glacier formation during Pleistocene glacial stages and prevalence of gale-force winds (Clark & Wilson Reference Clark and Wilson1992, Rosenbaum Reference Rosenbaum1996, Hamson et al. Reference Hamson, Evans, Sanderson, Bingham and Bentley2008).
The Tussac House site is located on the foreshore of Stanley Harbour (51.6949°S, 57.8315°W). Geotechnical boreholes drilled on the site indicate that the subsurface geology consists of ~2 m of Holocene peats sequentially underlain by periglacial sediments comprising a 1 m-thick blue-grey clay and ~3 m thick clay-rich diamicton, which unconformably overlie the lignitic organic deposit. The total thickness of this organic deposit is unknown due to it meeting water table depth, but it is likely to be > 2 m thick.
Methods
A geotechnical excavation in March 2020 uncovered a lignitic wood-rich matrix at ~6 m depth. During a subsequent geotechnical dig in May 2020, the section was sampled, with the recovery comprising a 60 cm lignitic section (Fig. 2) and several large wood fragments (Supplemental Table 1a), probably representing tree-sized parent plants.

Figure 2. Lithostratigraphy of the Tussac House site.
Ten samples of 1 cc of sediment spaced along the 60 cm section were prepared for palynostratigraphic investigation (Macphail Reference Macphail2021). Concentrating the oxidation-resistant fossil pollen and spores (miospores) and fungal spores preserved in cultural deposits and soils requires the removal of the inorganic matrix and organic matrix (see Wood et al. Reference Wood, Jasonius and McGregor1996). Sample process included 1) treatment with 10% potassium hydroxide, 2) sieving residue through a 100 μm mesh sieve, 3) addition of a lycopodium tablet and 10% hydrochloric acid, 4) sodium polytungstate heavy liquid separation at a specific density of 1.8 g/cm2 to remove mineral matrix and finally 5) treatment with acetolysis solution (sulphuric acid and acetic anhydride). Aliquots of the organic extracts were set on permanent strew mounts using Petropoxy 154.
The plant microfossil component in each sample was counted using a Zeiss™ Photomicroscope II fitted with Plan-Neofluar™ and Planapo™ objectives, which provide magnifications up to 2000×. Specimens of fossil pollen and spores were counted until statistically robust numbers of fossil pollen and spores were recorded (> 250 specimens), and then the remainder of the strew mounts and additional strew mounts for each sample were scanned for additional rare morphospecies; in the case of low-yield samples, the pollen count comprises the total numbers of fossil pollen and spores present on the microscope slide.
Samples of wood from the section were taken for radiocarbon measurements at the University of New South Wales Chronos 14Carbon-Cycle Facility to determine whether the material returned infinite radiocarbon ages (i.e. > 50 000 years). Each sample type was pre-treated following the protocols outlined in Turney et al. (Reference Turney, Becerra-Valdivia, Sookdeo, Thomas, Palmer and Haines2021). The wood specimens were also examined by light microscopy to determine their taxonomic class. To investigate the anatomical characteristics, the material was fractured to expose the three planes of section (transverse and radial and tangential longitudinal) mounted on aluminium stubs. Specimens were not treated/coated prior to imaging. Backscattered electron imaging was conducted using a Hitachi TM4000Plus scanning electron microscope at 15 kV in standard vacuum mode (~30 Pa). Wood specimens are described using the terminology of the IAWA Committee (1989, 2004) wherever possible. We compared samples with the published literature on wood samples from West Point Island (Poole & Cantrill Reference Poole and Cantrill2007) to facilitate identification.
To provide further context for our plant proxy evidence, we performed a brief comparison of the Falklands climate with palaeoclimate modelling scenarios of the Miocene and Oligocene. Here, we used the simulations of Farnsworth et al. (Reference Farnsworth, Lunt, Robinson, Valdes, Roberts and Clift2019), who ran palaeoclimate simulations for each of the periods of interest: namely, the Langhian (13.8–16.0 Ma), Burdigalian (16.0–20.4 Ma), Aquitanian (20.4–23.0 Ma), Chattian (23.0–27.8 Ma) and Rupelian (27.8–33.9 Ma), as well as a pre-industrial control simulation. These simulations were performed using the HadCM3BL model, using palaeogeography reconstructions generated by Getech. The model simulations were coarse resolution (2.50° latitude × 3.75° longitude) to cover many geological periods within the computational resources available. This means that each simulation typically had no land grid cell assigned to the Falkland Islands, or in some cases only a single grid cell, depending on the configuration. Thus, the model can produce a good representation of South American topography and climate through time, whereas the Falkland Islands generally fall below the model resolution.
To overcome the resolution limits, we used the palaeoclimate simulations to reconstruct anomalies of temperature and precipitation at the Falkland Islands geographical coordinates rather than absolute values. These anomalies were then used to adjust a modern high-resolution (30 arc-seconds) climatology of temperature and precipitation (WorldClim version 2; Fick & Hijmans Reference Fick and Hijmans2017) to create palaeoclimatologies. These palaeoclimatologies were reconstructed using an additive anomaly for temperature and a multiplicative anomaly for precipitation (following Beck et al. Reference Beck, Zimmermann, McVicar, Vergopolan, Berg and Wood2018).
Results
Palynostratigraphic analysis
All samples yielded large to very large numbers of mostly well-preserved terrestrial palynomorphs in organic extracts dominated by cuticle fragments and organic fines. Cysts of marine algae (dinoflagellates) and miospores of freshwater aquatic or semi-aquatic plants are absent except for occasional Botryococcus and a single specimen of Sparganiaceaepollenites (Sparganium, Typha) at 20–21 cm in TH20-3-1. Whether or not frequent to common ‘amorphous spheres’ (cf. Leiosphaeridia) in the samples represent freshwater algae is unknown.
Taxonomy
The majority of commonly occurring and some rare miospores in the Tussac House samples (Figs 3 & 4) can be assigned to fossil morphospecies formally described from Palaeogene-Neogene deposits in southern Australia, New Zealand and South America (references in Macphail et al. Reference Macphail, Alley, Truswell and Hill1994, Barreda & Palazzesi Reference Barreda and Palazzesi2007, Palazzesi & Barreda Reference Palazzesi and Barreda2007, Raine et al. Reference Raine, Mildenhall and Kennedy2011). However, the morphological diversity exhibited by some morphospecies and genera is unusually high, particularly within morphotypes assigned to Podocarpidites (nearest living relative (NLR) Podocarpus-Prumnopitys) and Nothofagidites (Nothofagus). In both cases, individual pollen grains can vary markedly in size and shape. Reasons for this include deposition of both immature and mature pollen grains representing plants whose remains form the lignitic deposit or were growing close to the site (M.K. Macphail, personal observation 2023). In addition, curtailed pollen development may have occurred due to the short growing season at high latitudes and the associated climate stress on flower development.

Figure 3. Selected photomicrographs of fossil pollen produced by gymnosperm and angiosperm living relatives in the rainforest of southern South America (nearest living relatives in parentheses): a. Araucariacites australis (Araucaria), b. Podocarpidites sp. (cf. P. microreticuloidata) (Podocarpus-Prumnopitys), c. Podocarpidites type 2 (P. marwickii complex) (Podocarpus-Prumnopitys), d. Nothofagidites americanus type (Nothofagus subgenus Lophozonia), e. Nothofagidites acromegacanthus complex (Nothofagus subgenus Brassospora?), f. Nothofagidites brachyspinulosus (Nothofagus subgenus Fuscospora), g.-i. Nothofagidites flemingii-rocaensis-saraensis complex (Nothofagus subgenus Nothofagus), j. Dicolpites sp. (Tepualia stipularis type), k. & l. Myrtaceidites verrucosus complex (includes Myrceugenia, Temu, Ugni), m. Polycolporopollenites esobalteus (Polygonaceae), n. Pseudowinterapollis cranwellae (P. couperi) (Drimys) and o. Tricolpites reticularis (Gunnera).

Figure 4. Selected photomicrographs of pollen of age-diagnostic and phytosociologically significant taxa (nearest living relatives in parentheses; all morphogenera and species are now extinct in southernmost South America and on the Falkland Islands: a. Canthiumidites cf. bellus (Randia type), b. Clavatipollenites glarius (Hedyosum), c. Thymelaepollis sp. of Macphail & Cantrill (Ovidia type), d.-f. Cupanieidites reticularis (Sapindaceae tribe Cupanieae), g. Dacrycarpidites australiensis (Dacrycarpus), h. Dacrydiumites florinii (Dacrydium), i. Microalatidites palaeogenicus (Phyllocladus), j. Phyllocladidites mawsonii (Lagarostrobos franklinii), k. Phyllocladidites elongatus (extinct Lagarostrobos sp.), l. Trichotomosulcites subgranulosus complex (extinct Podocarpaceae) and m.-o. Gen. et sp. nov. of Macphail & Cantrill (Reference Macphail and Cantrill2006).
In this study, the Podocarpidites component has been assigned to two broad groups based on the size and mode of attachment of the sacci to the corpus of the pollen grain, not to species identified in the Neogene flora of Tierra del Fuego by Zamaloa & Romero (Reference Zamaloa and Romero2005). The Nothofagidites component is assigned to three broad morphospecies groups comprising Nothofagidites acromegacanthus (NLR Nothofagus subgenus Brassospora), Nothofagidites americanus/tehuelchesii (Nothofagidites subgenus Lophozonia) and Nothofagidites flemingii/rocaensis/saraensis (Nothofagidites subgenus Nothofagus). Unidentified triporate, tricolpate and tricolporate pollen grains have been grouped into Proteacidites spp. (Proteaceae), Tricolpites spp. and Tricolporites spp., respectively.
More generally, the usage of Australian and New Zealand names for morphologically similar pollen and spore morphotypes in South America has led to a de facto broadening of the original fossil species diagnosis as used in the South American literature, and it is probable that the fossil taxon represents more than one ecotype, species or genus, some of which are extinct and potentially had different ecological tolerances to their NLRs (Macphail et al. Reference Macphail, Alley, Truswell and Hill1994, Macphail & Cantrill Reference Macphail and Cantrill2006).
Many of these and less commonly occurring taxa have NLRs in Argentina and/or Chile (Table I), although the taxonomic resolution is mostly low (to genus and family levels), or they are ancestral members of the living clades in South America at least to the family level (cf. Heusser Reference Heusser1971, Markgraf & D'Antoni Reference Markgraf and D'Antoni1978, Palazzesi & Barreda Reference Palazzesi and Barreda2007). At least one novel morphotype (Gen. et sp. nov., this study) appears to be unique to the Falkland archipelago (Macphail & Cantrill Reference Macphail and Cantrill2006).
Table I. Modern affinity of selected fossil morphospecies and genera with Eocene to Miocene time distributions in Patagonia.

Dominance and diversity
All fossil assemblages are dominated by fossil species of Podocarpaceae, Nothofagaceae and Myrtaceae (NLRs in parentheses) in which Phyllocladidites mawsonii (Lagarostrobos franklinii) is the dominant morphospecies and Podocarpidites (Podocarpus/Prumnopitys/Retrophyllum), Nothofagidites acromegacanthus, Nothofagidites flemingii/rocaensis/saraensis (Nothofagidites subgenus Nothofagus), Myrtaceidites verrucosus (numerous South American Myrtaceae genera) and Proteacidites (Proteaceae) occur in frequent to common numbers (Table II). Estimates of relative abundance are based on statistically robust counts of > 450 fossil pollen and spores (excluding fungal spores) for each sample. Percentages < 1% and morphospecies recorded outside the pollen sum are indicated by ‘+’ and ‘×’ in Table II, respectively.
Table II. Relative abundances of commonly occurring and selected uncommon to rare morphospecies recovered from the Tussac House samples. Rare taxa (< 1%) within the spore-pollen count are indicated by ‘+'; very rare taxa recorded outside the spore-pollen count are indicated by ‘×'.

Wood analysis
Samples of wood from the section were taken for radiocarbon dating at the University of New South Wales Chronos 14Carbon-Cycle Facility. All gave infinite radiocarbon ages of > 50 ka (Table III & Supplemental Table 1b), confirming the geological antiquity of the deposit.
Table III. Tussac House wood anatomical characteristics.
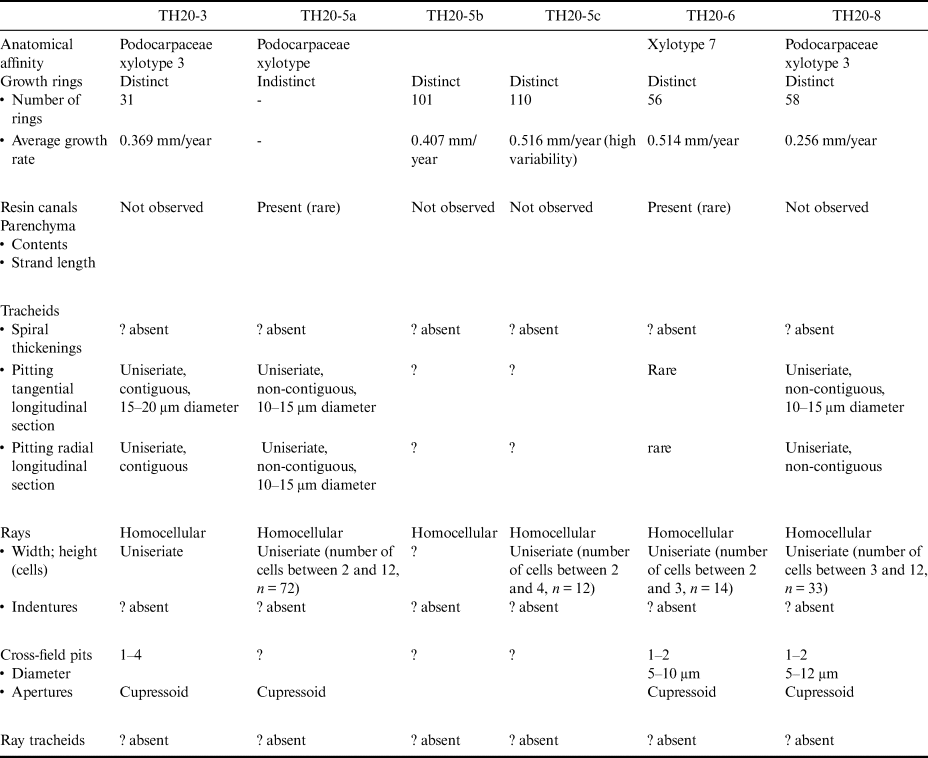
Widespread compression made it difficult to diagnose anatomical characteristics of many of the wood fragments (Table III & Supplemental Tables 1a & 1b). Where possible, xylotype was inferred from available diagnostic characteristics and compared directly to the xylotype classification of Poole & Cantrill (Reference Poole and Cantrill2007).
The material observed is assigned to xylotype 3 (Podocarpaceae) documented from the West Point Island deposit (Poole & Cantrill Reference Poole and Cantrill2007). Although widespread compression makes identification of certain features difficult, some anatomical characteristics are observable (Fig. 5). Samples TH20-3, TH20-5 and TH20-8 appear to have consistent anatomical characteristics where these are observable. Rays are exclusively uniseriate and range between 2 and 12 cells. Cell end walls, when observed, tend to be smooth. In the radial section view, cross-field pits are generally cupressoid. This xylotype appears to have anatomical similarities to podocarpaceous wood. Xylotype 7 appears to be represented by only one sample: TH20-6. This xylotype is characterized by rare or absent pitting and the presence of resin canals. More material with better preservation is needed for further taxonomic assessment.

Figure 5. Scanning electron microscopy images of wood fragments. a. TH20-3 radial section - homocellular rays, b. TH20-3 radial section - smooth cell end wall, c. TH20-3 tangential longitudinal section - low-height uniseriate rays, d. TH20-5a tangential longitudinal section - low- to medium-height uniseriate rays, e. TH20-5a tangential longitudinal section - smooth end walls, f. TH20-5a tangential longitudinal section, g. TH20-6 tangential longitudinal section - low-height uniseriate rays and h. TH20-6 radial section - cross-field pits.
Discussion
Most of the fossil pollen and spores preserved in the Tussac House forest bed (Macphail Reference Macphail2021) closely resemble pollen (gymnosperms, angiosperms) and spores (ferns, fern allies) produced by plants in the modern floras of South America and other landmasses in the Southern Hemisphere, although time distributions in southern South America (cf. Tables I & II; Macphail Reference Macphail1999, Partridge Reference Partridge1999, Raine et al. Reference Raine, Mildenhall and Kennedy2011) may vary from, for example, New Zealand and Australia. Some herbs and shrubs remain extant on the Falkland Islands (e.g. Huperzia fuegianum, Amaranthaceae, Asteraceae, Gunnera, Ericaceae (Empetrum, Gaultheria) and Myrtaceae (Myrteola); Broughton & McAdam Reference Broughton and McAdam2005). Other taxa almost certainly are ancestral members of the living clades at least to the family level. In many instances, it is probable that the fossil taxon represents more than ecotype, species or genus, some of which are extinct and potentially had different ecological tolerances to their NLRs (Macphail et al. Reference Macphail, Alley, Truswell and Hill1994).
Absolute and probable age limits of the Tussac House forest bed
Unlike Argentina (see Barreda Reference Barreda1996a,Reference Barredab, Barreda & Palamarczuk Reference Barreda and Palamarczuk2000a,Reference Barreda and Palamarczukb,Reference Barreda and Palamarczukc, Reference Barredab, Barreda & Palamarczuk Reference Barreda and Palamarczuk2000a,Reference Barreda and Palamarczukb,Barreda et al. Reference Barreda, Palazzesi and Marenessi2009, Amenabar et al. Reference Amenabar, Guerstein, Alperin, Daners, Casadio and Raising2023), no formal pollen and spore-based palynostratigraphy has been developed to date and correlate Palaeogene and Neogene sediments on the Falkland Islands or independently dated marine sediments on the submerged Falkland Plateau or Malvinas Basin (Amenabar et al. Reference Amenabar, Guerstein, Alperin, Daners, Casadio and Raising2023 and references therein). Accordingly, the age limits of the West Point Island forest bed and the Tussac House forest bed (Macphail & Cantrill Reference Macphail and Cantrill2006, Macphail Reference Macphail2021) have been inferred using the time distribution of fossil pollen species (morphospecies) whose NLRs occur in mega- and meso-thermal rainforest in Patagonia, commonly defined as encompassing the Argentinean provinces of Neuquén, Río Negro, Chubut, Santa Cruz and Tierra del Fuego (Quattrocchio et al. Reference Quattrocchio, Volkheimer, Borromei and Martinez2011, Palazzesi et al. Reference Palazzesi, Vizcaíno, Barreda, Cuitiño, del Río and Goin2021 and references therein).
The present study focuses on the time distribution of fossil pollen of three rainforest thermophiles preserved in marine sedimentary formations in Patagonia, viz. Canthiumidites cf. bellus Clavatipollenites and Cupanieidites, whose NLRs, respectively, are Randia (now found in dry forest communities in northern South America), Hedyosmum (Chloranthaceae; now found in lowland to montane rainforest, including disturbed areas at low elevations, from 18°N to 25°S) and Cupania (Sapindaceae; now found north of 30°S in southern South America; see Marchant et al. Reference Marchant, Almeida, Behling, Berrio, Bush and Cleef2002). Isotopic (86Sr/87Sr, Ar/Ar, U-Pb) foraminifer and marine dinoflagellate dinocysts of these formations provide independent age control for the time distributions of the morphospecies in Patagonia (Guler et al. Reference Guler, Estebenet, Navarro, Fuentes, Cuitino and Palazzesi2021 and references therein, Parras & Cuitino Reference Parras and Cuitino2021).
Supporting evidence is provided by 1) objective (Jaccard coefficient) comparisons of Oligocene-Miocene palynofloras in Patagonia and Tierra del Fuego, 2) the presence or absence of pollen from wind-pollinated plants dominating Patagonian steppe vegetation (e.g. Nanez et al. Reference Nanez, Quattrochio and Ruiz2009, Cornu et al. Reference Cornou, Martinez, Quattrocchio and Asensio2012, Reference Cornou, Quattrocchio and Martinez2014) and 3) stratigraphic distributions of taxa that are now prominent in Patagonian steppe (Amaranthaceae, Asteraceae, Poaceae) and Randia type (Canthiumidites cf. bellus), which may be evidence for sourcing of pollen from northern Argentina in the Oligocene-Miocene.
Complicating the palynostratigraphic record of the establishments, extirpations and extinctions of these and other mesotherm taxa are (Barreda & Palazzesi Reference Barreda and Palazzesi2007, Barreda et al. Reference Barreda, Guler, Palazzesi and Rabassa2008, Quattrocchio et al. Reference Quattrocchio, Volkheimer, Borromei and Martinez2011, Vizcaíno et al. Reference Vizcaíno, Bargo and Fernícola2013, Estebenet et al. Reference Estebenet, Guerstein and Raising2014, Sachse et al. Reference Sachse, Strozyk, Anka, Rodriguez and di Primo2015, Warny et al. Reference Warny, Kymes, Askin, Krajewski and Tatui2019, Parras et al. Reference Parras, Guerstein, Perez Panera, Griffin, Nanez, Cusminsky and Quiroga2020, Palazzesi et al. Reference Palazzesi, Vizcaíno, Barreda, Cuitiño, del Río and Goin2021, Amenabar et al. Reference Amenabar, Guerstein, Alperin, Daners, Casadio and Raising2023): 1) geographical, geomorphic and climatic factors such as the progressive tectonic uplift of the Andes during the Palaeogene-Neogene,) opening of Drake Passage and development of the Antarctic Circumpolar Current in the Late Eocene and earliest Oligocene c. 34–23 Ma, 3) two marine inundation events (c. 22–15 Ma) during the latest Oligocene and Early to Middle Miocene represented by marginal and shallow- to deep-water marine deposits, 4) extinction in Patagonia by the mid-Eocene of previously established mega- and meso-therm taxa followed by the associated emergence of Nothofagus-Podocarpaceae communities in the Middle Eocene to Early Oligocene and by the expansion of arid-adapted Patagonian steppe communities in the Late Oligocene and Miocene and 5) marked differences between the palaeofloras occupying coastal and hinterland regions.
Much of the published age-range data from Argentina come from outcrops deposited during the Late Oligocene to Early Miocene Patagoniense transgression. Representative sections occur from the Andean Foothills in the north-west to Tierra del Fuego in the extreme south of Argentina, although these represent significantly different periods within the Oligocene to Middle Miocene time (Guerstein et al. Reference Guerstein, Guler and Casadio2004, Blisniuk et al. Reference Blisniuk, Stern, Chamberlain, Idleman and Zeitler2005, Parras et al. 2012, Cornou et al. Reference Cornou, Quattrocchio and Martinez2014, Estebenet et al. Reference Estebenet, Guerstein and Raising2014, Kohn et al. Reference Kohn, Strömberg, Madden, Dunn, Evans, Palacios and Carlini2015, Guler et al. Reference Guler, Estebenet, Navarro, Fuentes, Cuitino and Palazzesi2021, Parras & Cuitino Reference Parras and Cuitino2021). For example, the palynostratigraphically well-studied Santa Cruz Formation (Parras et al. Reference Parras, Guerstein, Perez Panera, Griffin, Nanez, Cusminsky and Quiroga2020) began accumulating at c. 17.5 Ma in the south-west, c. 19.8 Ma in the north-west and c. 19. 3 Ma in the south-east in Santa Cruz Province and at c. 16 Ma in the Golfo San George Basin in the adjacent Chubut Province but is absent further to the north and west in the Valdes and Neuguén basins. Underlying Eocene to Early Miocene formations are mostly confined to specific basins in Patagonia (e.g. the Monte Leon, Río Leona, Río Turbio and San Julien formations (Austral-Magallanes Basin), the Chenque and Centinela formations (Golfo San Jorge Basin) and Slogget Formation (Tierra de Fuego); cf. Guerstein et al. Reference Guerstein, Guler and Casadio2004, Parras et al. Reference Parras, Guerstein, Perez Panera, Griffin, Nanez, Cusminsky and Quiroga2020, Parras & Cuitino Reference Parras and Cuitino2021, Amenabar et al. Reference Amenabar, Guerstein, Alperin, Daners, Casadio and Raising2023). Palynostratigraphic data from marine cores on the Argentine continental shelf are limited to two wells, as are data from confirmed Middle and Late Miocene and Pliocene continental outcrops in Patagonia.
For this reason, and due to the possibility that other relevant records are unpublished, the age limits of samples from the Tussac House forest bed are assigned to broad periods of geological time only. The inferred age limits assume that pollen of mesotherm taxa have been transported long distances by wind, birds or insects prior to their extinction or extirpation in continental Patagonia (see Macphail & Cantrill Reference Macphail and Cantrill2006, Macphail Reference Macphail2021). This approach includes caveats that are difficult to test given that the morphospecies are mostly preserved in lignitic sediments where much of the associated palynoflora represents plants growing in or around the site. The reasons for this are as follows:
1) The Falklands flora show a strong affinity with Patagonian South America and Tierra de Fuego and more limited links with the sub-Atlantic Islands, New Zealand and, especially in the past, Australia (see McDowell Reference McDowell2005). However, any colonization of the Falkland archipelago by mega- and meso-thermal plants in the early Palaeogene is most likely to have occurred during periods of global warmth, particularly the Early Eocene hyperthermals. Nevertheless, we recognize that rainforest thermophiles with broad ecological tolerances might have survived after they became extinct in Patagonia (due to increasing aridity) or West Antarctica (due to cold conditions; cf. Zachos et al. Reference Zachos, Dickens and Zeebe2008, Hyland & Seldon Reference Hyland and Sheldon2018, Fernandez et al. Reference Fernadez, Santamarina, Palazzesi, Telleria and Barreda2021, Srivastava et al. Reference Srivastava, Bhatia, Verma, Singh, Utescher and Mehrotra2023). Nevertheless, given the biologically profound impact of the major global cooling event at the Eocene-Oligocene boundary (Mi-1) elsewhere at mid- to high latitudes in the Southern Hemisphere (cf. Macphail et al. Reference Macphail, Alley, Truswell and Hill1994, Reference Macphail, Hill, Carpenter and McKellar2014), we consider it improbable that any thermophiles survived this event given the exposed habitats on the topographically subdued Falkland archipelago. Whether any recolonization of the Falklands by thermophiles growing in northern Argentina occurred during the Middle Miocene Climatic Optimum (MMCO) at c. 15–17 Ma is unknown.
2) Transoceanic dispersal of seeds by wind or water from South America into the Atlantic Ocean is well documented (cf. Renner Reference Renner2004, Turney et al. Reference Turney, Jones, Fogwill, Hatton, Williams and Hogg2016, Thomas et al. Reference Thomas, Jones, Fogwill, Hatton, Williams and Hogg2018, Zwier et al. Reference Zwier, van der Bilt, de Stigter and Bjune2022), but successful establishment on remote oceanic islands such as the Falklands is markedly reduced if the ‘target’ environment(s) differ significantly from those occupied by the parent plants (see McDowell Reference McDowell2005, Ali Reference Ali2017). Most of these constraints do not apply to pollen or spores of temperate and subtropical plants in South America that are dispersed into the south-west Atlantic or Pacific oceans, although the marine depositional conditions tend to favour long-term preservation more than many terrestrial environments (Kappen & Straka Reference Kappen and Straka1988, Montade et al. Reference Montade, Nebout, Kissel and Mulsow2011).
3) The rarity of Canthiumidites cf. bellus, Clavatipollenites and Cupanieidites and the absence of other South American thermophile pollen types in the Tussac House forest bed is likely to reflect deposition in an environment where much of each microflora is derived from locally growing plants (see Barreda et al. Reference Barreda, Palazzesi and Marenessi2009).
4) There is an unknown likelihood that some earlier or later occurrences of Canthiumidites cf. bellus, Clavatipollenites and Cupanieidites in Patagonia are unpublished.
Records of Cupania type (Cupanieidites), Hedyosmum type (Clavatipollenites) and Randia type (Canthiumidites cf. bellus) obtained from review surveys of the published palynostratigraphic literature for Patagonia and adjacent regions in southern South America (see Barreda et al. Reference Barreda, Guler, Palazzesi and Rabassa2008) are given in Table IV. The fossil record of Randia type (Canthiumidites cf. bellus) in Patagonia is notable as this thermophile is an important element in dry forests in northern South America (Marchant et al. Reference Marchant, Almeida, Behling, Berrio, Bush and Cleef2002).
Table IV. Chronostratigraphic distribution of trace records of fossil Cupania-type, Hedyosmum-type and Randia-type pollen in Patagonia, Argentina. (+) indicates species is present; (-) indicates species was not recorded, (cf.) indicates comparable pollen.

a After Parras & Cuintino (Reference Parras and Cuitino2021) and Amenábar et al. (Reference Amenabar, Guerstein, Alperin, Daners, Casadio and Raising2023).
NA = not applicable.
Maximum age limits of the Tussac House forest bed
On the data available, the parent source(s) of Clavatipollenites but apparently not Cupanieidites or Canthiumidites cf. bellus were present in Patagonia in or by the Middle Eocene. Assuming the former became extinct during the Mi-1 global cooling event, the maximum age limit of the Tussac House forest bed is inferred to be Late Oligocene based on records of Clavatipollenites, Cupanieidites and Canthiumidites cf. bellus in the Austral-Magallanes, Chubut and Golfo San Jorge basins in (or by) the Late Oligocene sediments (Río Leona, Monte Léon and San Julian formations; Barreda Reference Barreda1997, Barreda et al. Reference Barreda, Palazzesi and Marenessi2009, Heredia et al. Reference Heredia, Paez, Guerstein and Parras2012). Whether any of the parent sources had reached as far south as Tierra del Fuego by this time is uncertain.
Minimum age limit of the Tussac House forest bed
The minimum age limit of the Tussac House forest bed is no older than Middle Oligocene based on multiple records of Cupanieidites and Clavatipollenites in the Austral-Magallanes Basin (Monte Leon and San Julian formations) and, less certainly, Golfo San Jorge Basin (Perfiles Mazarredo Formation), but it might be as young as Middle Miocene (Cupanieidites, Canthiumidites cf. bellus) based on 86Sr/87SR dates for the Chenque Formation in the Golfo San Jorge (cf. Barreda Reference Barreda1996a, Parras et al. Reference Parras, Guerstein, Perez Panera, Griffin, Nanez, Cusminsky and Quiroga2020). Whether or not the latter age limit is supported by the trace records of pollen representing the wind-pollinated/dispersed families Amaranthaceae (Chenopodipollis chenopodiaceoides) and Poaceae (Graminidites media) in the Tussac House microfloras is uncertain as the parent plants are recorded in Nothofagaceae-Podocarpaceae communities prior to the Late Miocene expansion of steppe vegetation in Patagonia. The absence of Asteraceae (Tubulifloridites spp.) pollen is less easily explained unless this is due to its lower pollen production and more limited dispersal by wind and animals.
Depositional environment
Relative level data indicate that the top depth of the Tussac House lignitic deposit is ~1.5 m above mean high water mark and that the base of the deposit extends below present-day mean sea level and therefore may have accumulated during a period of low relative sea level. The local depositional environment is more likely to have been a damp depression given the virtual absence of obligate or semi-obligate algal cysts or pollen of herbs such as Sparganiaceae (Sparganiaceaepollis) and Menyanthaceae (Striasyncolpites laxus), which occur in ‘marsh’ deposits in Patagonia during the Oligocene-Miocene. One possibility, suggested by the strong east to west alignment of the basement rocks and ongoing tectonic uplift of the archipelago (Stanca et al. Reference Stanca, McCarthy, Paton, Hodgson and Mortimer2022), is a depression on the back slope on a tilted fault block.
Palaeoflora and vegetation
As the microfloras represent essentially the same Podocarpaceae-Nothofagus rainforest or rainforest shrub community (Table II), the data more compelling suggests that the lignites were deposited over a short period of geological time under perhumid cool or, less likely, cold conditions. The closest modern analogues are Magellanic evergreen Nothofagus rainforest (see table 10.1 in Veblen et al. Reference Veblen, Donoso, Kitzberger, Rebertus, Veblen, Hill and Read1996). Shared characteristics include the rarity of Araucaria and the prominence of Nothofagus spp., whose NLRs may be ancestral to extant species in the subgenus Fuscospora (e.g. Nothofagus antarctica, Nothofagus betuloides and Nothofagus pumilo) given the high-latitude position of the Falkland Islands throughout the Late Palaeogene and Neogene. The parent plants of Nothofagidites americanus and Nothofagus teheulchesii may be extinct, as subgenus Lophozonia spp. in South America (Nothofagus alpina, Nothofagus obliqua) are restricted to latitudes above ~40°S in southern Chile and Argentina.
This palaeo-community, however, has no clear modern analogue, as the NLRs of most Podocarpaceae morphospecies are endemic to Tasmania (Lagarostrobos franklinii), New Zealand and/or Southeast Asia (e.g. Dacrycarpus (Dacrycarpites australiensis), Dacrydium (Dacrydiumites florinii) and Phyllocladus (Microalatidites palaeogenicus)). Two podocarp clades are extinct (Podosporites parvus, Trichotomosulcites subgranulosus). The NLRs of most rare ferns and angiosperm shrubs represent humid-demanding species with broad geographical distributions in South America. Examples are the probable tree fern Cyathidites australis (Cyatheaceae), the club-moss Foveotriletes palaequetrus (Huperzia fuegianum) and Embothrium (Diporites granulosus), all of which occur as far south as Tierra del Fuego, and tree ferns in Andean ‘cloud forests’ at lower latitudes (Broughton & McAdam Reference Broughton and McAdam2005). The same is likely to be true of the herb flora whether the unassigned Tricolpites/Tricolporites counts represent herbaceous or woody plants. The absence of Lophosoria (Cyatheacidites annulatus) is surprising given that this ground fern became established elsewhere on sub-Antarctic islands (e.g. the Kerguelen Islands; Cookson Reference Cookson1947) during the Miocene.
Although the scanning electron microscopy analysis of the wood assemblage was able to identify two distinct xylotypes, poor sample preservation precluded a species-level identification and as such is unable to inform on the probable age limits of or genera presented in the Tussac House forest bed. An interesting characteristic observed in the West Point Island deposit was the preponderance of Podocarpaceae pollen (60%) compared to the wood, which is largely cf. Austrocedrus. Although the wood assemblages analysed from the Tussac House deposit were significantly lower in number, a similar trend is apparent. This is probably a reflection of the pollination strategy in Podocarpaceae (wind pollinated) as well as of taphonomic effects. The pollen grains are saccate and tend to float on surface water, so different concentrations of grains from the same vegetation are found depending on whether the grains were deposited from water or onto some sort of peat surface (Traverse Reference Traverse and Traverse2007). Even within standing water the deposition can fluctuate due to differential transport.
Comparison of the Tussac House and West Point Island forest beds
Comparisons between the West Point Island forest bed (Macphail & Cantrill Reference Macphail and Cantrill2006) and Tussac House forest bed microfloras suggest that the latter are slightly older than the former. With few exceptions, all common and most rare morphospecies recorded in the Tussac House forest bed also occur in the West Point Island forest bed. The only significant differences are that the relative abundance of Phyllocladidites mawsonii is lower in the West Point Island microfloras (15–25%) compared to the Tussac House microfloras (34–48%) and that Chenopodipollis chenopodiaceoides, Graminidites and Myrtaceidites eucalyptoides were not recorded in the Tussac House microfloras. Cupanieidites reticularis was not recorded in the West Point Island samples. Although trace occurrences of Myrtaceidites eucalyptoides in the West Point Island microfloras may represent contamination from drilling equipment, the significance of the other differences provides insights into probable age limits given the wide dispersal of Amaranthaceae and Poaceae pollen by wind around the Southern Hemisphere (Macphail Reference Macphail1979) vs the probable dispersal of Cupanieae pollen by insects.
On these data, the probable age limits of the West Point Island forest bed are more likely to be Late Oligocene to Early (Middle?) Miocene than the Middle(?) Miocene to Early Pliocene age limits (Table IV) based in part on Sparganiaceaepollenites and Thymelaepollis sp. (Macphail & Cantrill Reference Macphail and Cantrill2006). If correct, the revised age limits for the West Point Island forest bed indicate that Lagarostrobos populations on the Falkland Islands had undergone a significant contraction during the Early to Middle Miocene (i.e. during the warming period leading up to the MMCO at c. 15 Ma). This seems ecologically improbable given the broad distribution of extant Lagarostrobos franklinii from the lowlands to the subalpine/alpine zone in perhumid western Tasmania. Possible explanations are that the sources of Phyllocladidites mawsonii pollen on the Falkland Islands were now extinct, cold-demanding ecotypes or, less likely, that warming leading up to the MMCO was accompanied by a significant reduction in precipitation across the archipelago. In either case, the most probable period when Lagarostrobos and any other potentially tree-sized podocarps became extirpated from the Falkland Islands is the Late Miocene, a conclusion that is ecologically consistent with the earlier development of tundra on the South Shetland Islands during the Mi-1 glaciation (Warny et al. Reference Warny, Kymes, Askin, Krajewski and Bart2016).
Climate modelling and interpretation
Anomaly-based reconstructions of annual mean temperature and annual mean precipitation over the Falkland Islands were developed from palaeoclimate modelling scenarios of the Miocene and Oligocene generated by Farnsworth et al. (Reference Farnsworth, Lunt, Robinson, Valdes, Roberts and Clift2019) using the HadCM3BL coupled climate model to provide further context for the palaeoflora evidence. Although the modern annual mean temperature of the Falklands is ~6°C, the reconstructed values are significantly warmer, ranging from ~12°C to 13°C in the Early to Middle Miocene (13.8–23.0 Ma), up to 14–15°C in the Late Oligocene (23.0–27.8 Ma) and then cooler again (11–12°C) in the Early Oligocene (27.8–33.9 Ma; Supplemental Fig. 3). Precipitation varies significantly, from ~500 mm/year in the modern down to 300–400 mm/year for the Middle Miocene, becoming significantly wetter (~700–900 mm/year) in the Early Miocene (20.4–23.0 Ma) and Late Oligocene (23.0–27.8 Ma) and then trending lower again in the Early Oligocene (27.8–33.9 Ma; Supplemental Fig. 4). These patterns are consistent with a hypothesized decrease in precipitation from the Early to Middle Miocene, a possible cause of the changes in Lagorostrobos pollen discussed above.
We also use the temperature and precipitation climatologies to generate Köppen climate classifications for each of the Miocene and Oligocene time slices (Supplemental Fig. 5). Based on WorldClim version 2 data (Fick & Hijmans Reference Fick and Hijmans2017), the modern Falklands climate is predominantly subpolar oceanic (Cfc), with areas of polar tundra (ET) at higher elevations. Going back in time, the Falklands are classified as predominantly cold steppe (BSk) for both the Langhian (13.8–16.0 Ma) and Burdigalian (16.0–20.4 Ma) due to relatively low precipitation and slightly warmer average temperatures. For the Aquitanian (20.4–23.0 Ma) and Chattian (23.0–27.8 Ma) the Falklands become predominantly temperate oceanic (Cfb; i.e. a similar zone to modern-day western Europe), in accordance with higher precipitation during those periods. Further back to the Rupelian (27.8–33.9 Ma) the Falklands are again dominated by cold steppe (BSk) climate.
Palaeoclimate time series for the closest grid point to Tussac House show summer (December–February), winter (June–August) and annual mean reconstructed temperatures and precipitation rates over the mid- to late Cenozoic (Fig. 6). These time series indicate the substantial variations in precipitation for the palaeo-reconstructions (with periods both wetter and drier than modern conditions), and the temperature reconstructions show that Tussac House was consistently 6–7°C warmer than modern in terms of the annual mean, and with greater seasonal variability, ranging from 6–8°C in winter to 16–18°C in summer. These climatic conditions would have been amenable for the development of the rainforest/scrub communities identified in the pollen spectra within the Tussac House lignite deposit.

Figure 6. Reconstructed a. temperature and b. precipitation time series at Tussac House based on WorldClim v2 and anomaly-based temperature/precipitation reconstructions for the five palaeo-simulations of the Langhian, Burdigalian, Aquitanian, Chattian and Rupelian. Ann = annual; DJF = December, January, February; JJA = June, July, August.
Conclusion
The Tussac House deposit has a number of similarities to and differences from the West Point Island deposit that was first discovered in 1906. This study affirms that the South Atlantic region hosted diverse rainforests during the mid- to late Cenozoic period, perhaps in several episodic phases. All fossil pollen and spore assemblages (microfloras) recovered from Tussac House samples represent the same palaeo-community - Nothofagus-Podocarpaceae cool temperate rainforest or rainforest scrub - growing on the eastern Falkland Islands sometime between the Late Oligocene (maximum age limit) and the Middle Miocene (minimum age limit). In the absence of local independent age controls, these age limits rely on the time distribution of pollen from mesothermal rainforest plants found in southern Argentina, particularly Cupanieidites reticularis (NLR Sapindaceae tribe Cupanieae) and Clavatipollenites glarius (NLR Hedyosum). The minimum age limit is supported by palaeoclimate modelling and the absence of wind-transported pollen types from the arid-adapted steppe vegetation that developed in Argentina during the late Palaeogene-Neogene.
Acknowledgements
Special thanks to the South Atlantic Environmental Research Institute (SAERI) for facilitating this work. Thanks also to Sammy Hirtle, Daniel Ford and the construction team on the Tussac House site, who kindly paused their excavation to allow sampling. The authors acknowledge the facilities and the scientific and technical assistance of Microscopy Australia at the Electron Microscope Unit (EMU) within the Mark Wainwright Analytical Centre (MWAC) at UNSW Sydney. We would like to express our sincere gratitude to the two reviewers for their valuable comments and suggestions, which greatly improved the quality of this manuscript.
Financial support
This study was funded by the Australian Research Council Fellowship (DE200100907) awarded to ZAT. DKH is supported by DE220100279.
Competing interests
The authors declare none.
Author contributions
ZAT led the field expedition. ZAT, HC, CT and SC collected the samples. MM and HC undertook the Palaeogene palynostratigraphic and Quaternary pollen analyses and interpretation, respectively. DJC, HAH and KP undertook the wood analysis. DKH undertook the climate modelling. ZAT and MM drafted the manuscript, and all authors contributed to the preparation of the manuscript.
Climate data availability
The HadCM3BL climate data presented in this study were downloaded from an open access repository: https://www.paleo.bristol.ac.uk/ummodel/scripts/papers/Farnsworth_et_al_2019b.html. WorldClim version 2 climatologies were downloaded from https://www.worldclim.org/data/worldclim21.html.
Supplemental material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0954102024000129.













