Introduction
Most visitors to Antarctica are tourists (74,400 visitors in the 2019–2020 summer; International Association of Antarctica Tour Operators 2020), yet some of the more obvious and persistent direct human impacts are related to established research stations (see Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey and Moreno2009, Aronson et al. Reference Aronson, Thatje, McClintock and Hughes2011) that are inhabited by far fewer people (peak station population of ~5000 in 2019/2020; Council of Managers of National Antarctic Programs 2020). Approximately 60 of the 76 currently operational Antarctic research stations (40 open year-round, 36 open seasonally) are situated on, or accessed by, the coast (Council of Managers of National Antarctic Programs 2020). Each of these stations inevitably is a source of contamination to the marine environment to varying degrees. These anthropogenic contaminants include petrochemicals from fuel spills, leachate from historic disposal sites, organic enrichment and chemical disposal via sewage systems and the release of persistent contaminants from processes such as fuel combustion and building decay (Bargagli Reference Bargagli2005, Tin et al. Reference Tin, Fleming, Hughes, Ainley, Convey and Moreno2009). However, the environmental significance of any marine contamination is ultimately related to the extent of associated biological effects in the surrounding natural environment (Chapman Reference Chapman2007). Therefore, it is important to consider the effects of contamination on marine biota to determine the extent and significance of contamination at coastal Antarctic research stations.
Two commonly measured biological indicators of marine pollution in Antarctica include macrobenthic faunal community composition and bioaccumulation of contaminants in benthic faunal tissues (Bargagli Reference Bargagli2005). Macrobenthic faunal communities are ideal indicators of pollution because they are sedentary, long-lived (relative to plankton), widespread and sensitive to changes in water and sediment qualities. Macrobenthic communities have been used to determine pollution effects adjacent to several Antarctic research stations including extensively at McMurdo Station (e.g. Dayton & Robilliard Reference Dayton and Robilliard1971, Conlan et al. Reference Conlan, Kim, Lenihan and Oliver2004, Palmer et al. Reference Palmer, Klein, Sweet, Montagna, Serciano and Hyde2021), Casey Station (e.g. Thompson et al. Reference Thompson, Riddle and Stark2003, Stark et al. Reference Stark, Kim and Oliver2014), Davis Station (Stark et al. Reference Stark, Corbett, Dunshea, Johnstone, King and Mondon2016) and Palmer Station (Hyland et al. Reference Hyland, Laur, Jones, Shrake, Cadian and Harris1994). Like macrofauna communities, tissue bioaccumulation has been used as a bioindicator at many research stations in Antarctica, including McMurdo Station (Kennicutt et al. Reference Kennicutt, McDonald, Sericano, Boothe, Oliver and Safe1995, Negri et al. Reference Negri, Burns, Boyle, Brinkman and Webster2006), Palmer Station (Kennicutt et al. Reference Kennicutt, McDonald, Sericano, Boothe, Oliver and Safe1995), Scott Base (Negri et al. Reference Negri, Burns, Boyle, Brinkman and Webster2006), Rothera Station (Krasnobaev et al. Reference Krasnobaev, ten Dam, Boerrigter-Eenling, Peng, van Leeuwen and Morley2020, Webb et al. Reference Webb, Hughes, Grand, Lohan and Peck2020), in the vicinity of Henryk Arctowski, Comandante Ferraz and Machu Picchu stations in King George Island (Ahn et al. Reference Ahn, Kim and Choi2002, Trevizani et al. Reference Trevizani, Figueira, Ribeiro, Theophilo, Majer and Petti2016) and Terra Nova Bay stations (Bargagli Reference Bargagli2001). Contaminants accumulate in benthic faunal tissues directly from benthic sediments and hard surfaces as well as through the ingestion of contaminated food and suspended particulate material (Kennish Reference Kennish1997). The accumulation of contaminants into an organism's tissues indicates the presence of biologically available contaminants (Kennicutt et al. Reference Kennicutt, McDonald, Sericano, Boothe, Oliver and Safe1995, Cabrita et al. Reference Cabrita, Padeiro, Amaro, Dos Santos, Leppe and Verkulich2017). Molluscs (e.g. the bivalve Laternula elliptica and the limpet Nacella concinna) have been advocated as useful bioindicators of anthropogenic change throughout Antarctica because they are relatively sedentary, easy to collect, large enough to analyse, have a broad distribution and accumulate contaminants in their tissues in concentrations high enough for detection when contaminants are present (see Bargagli Reference Bargagli2005, Webb et al. Reference Webb, Hughes, Grand, Lohan and Peck2020).
Contamination varies among Antarctic research stations because of multiple factors, including different management practices, station sizes and extents of activities. Although all nations managing Antarctic research stations have accepted the Antarctic Treaty's Protocol on Environmental Protection (ATPEP; www.ats.aq), each station has different standards and environmental management practices. For example, approximately half of Antarctic research stations have sewage treatment capability, but this treatment ranges widely from simple maceration to tertiary treatment (Smith & Riddle Reference Smith, Riddle, Kerry and Riddle2008, Gröndahl et al. Reference Gröndahl, Sidenmark and Thomsen2009). Although the size of a station's footprint is difficult to define and measure (Brooks et al. Reference Brooks, Jabour and Bergstrom2018), a station's size and level of activity can be approximated by its population. Ignoring McMurdo Station, which has a peak seasonal population of 1200, peak populations of the coastal Antarctic stations range from 6 to 170 people, with a median and mean of 45 and 52 people, respectively (Council of Managers of National Antarctic Programs 2020). This research documents the extent and effects of station-derived contamination on the marine environment at the United States Antarctic Program (USAP) Palmer Station, a medium-sized Antarctic research station (peak population of 46). Contamination extent is assessed by measuring sediment contaminant concentrations and contamination effects are determined using macrobenthic faunal communities and limpet (N. concinna) tissues as biological indicators.
This research is part of a larger project that was designed to establish the framework for a long-term monitoring programme that could be used to partially fulfil the USA's obligations as a signatory to the Antarctic Treaty to monitor the local effects of humans on the environment (see ATPEP; www.ats.aq). Similar monitoring techniques that have successfully been used at USAP's McMurdo Station (see Kennicutt et al. Reference Kennicutt, Klein, Montagna, Sweet, Wade, Palmer and Denoux2010, Klein et al. Reference Klein, Sweet, Wade, Sericano and Kennicutt2012, Palmer et al. Reference Palmer, Klein, Sweet, Montagna, Serciano and Hyde2021) were applied to the smaller and environmentally different Palmer Station to test the transferability of techniques, methodologies and designs to other areas of Antarctica with human occupation. This marine environmental study at Palmer Station builds on previous work that documented sediment and limpet tissue contamination in the vicinity from 1989 to 1992 (see Kennicutt et al. Reference Kennicutt, McDonald, Denoux and McDonald1992a, Reference Kennicutt, McDonald, Denoux and McDonald1992b).
Methods
Study area
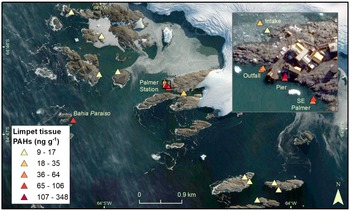
Palmer Station (64°46'S, 64°03'W) is a USAP research station established adjacent to Arthur Harbour, on Anvers Island, on the western Antarctic Peninsula. The station in its present location near Gammage Point was established in 1968 and has recently been occupied year-round by ~20 (winter) to 46 (summer) people. The current Palmer Station is ~1.5 km from the sites of two former stations on Amsler Island: the original Palmer Station (Old Palmer Station, 1965–1968) and the British Antarctic Survey's Base N (1955–1958; Fig. 1) (Khan et al. Reference Khan, Klein, Katich and Xian2019). Also in Arthur Harbour is the wreck of the Bahía Paraíso, a ship that grounded ~2 km from Palmer Station in 1989, spilling 600,000 l of diesel fuel arctic (Kennicutt Reference Kennicutt1990), the largest spill in Antarctic history (Filler et al. Reference Filler, Kennicutt, Snape, Sweet, Klein and Fingas2014).

Fig. 1. Sampling locations adjacent to Palmer Station.
The shallow (< 15 m) marine environment adjacent to Palmer Station is dominated by hard substrates with pockets of soft sediment, whereas deeper depths are dominated by soft sediment (Moe & DeLaca Reference Moe and DeLaca1976, Richardson & Hedgepeth Reference Richardson, Hedgepeth and Llano1977). Macroalgae (especially Desmarestia spp. and Himantothallus grandifolius) are common on the shallow rocky areas (Richardson & Hedgepeth Reference Richardson, Hedgepeth and Llano1977, Amsler et al. Reference Amsler, Rowley, Laur, Quetin and Ross1995). Sunlight persists year-round at Palmer Station (5 h in winter, 19 h in summer), and fast ice only occurs for several weeks each year (Shabica Reference Shabica1976). Anchor ice is limited to < 3 m deep, but brash ice and small icebergs are common (Shabica Reference Shabica1972). Arthur Harbour has water temperatures ranging from 0.6°C at the surface and -0.1°C at the bottom in January to -1.9°C (surface and bottom) in August (Lowry Reference Lowry1975). Salinities range seasonally from 33.7 in April to 34.6 in July and August (mean = 34.1).
Study design
Sampling occurred at different depths along transects radiating from Palmer Station, following Kennicutt et al. (Reference Kennicutt, McDonald, Denoux and McDonald1992a, Reference Kennicutt, McDonald, Denoux and McDonald1992b). Transects were sampled in probably contaminated and reference areas, as conducted at McMurdo Station (Kennicutt et al. Reference Kennicutt, Klein, Montagna, Sweet, Wade, Palmer and Denoux2010, Palmer et al. Reference Palmer, Klein, Sweet, Montagna, Serciano and Hyde2021). Documenting the potential effects of humans on the marine environment required depth-dependent methods to compensate for vertical zonation in bottom substrate that occurs adjacent to Palmer Station. Sampling for sediment chemistry and macroinfaunal community composition occurred at depths of 18 and 24 m as soft sediment was rare at depths shallower than 15 m. Marine sediments were sampled along four transects adjacent to Palmer Station (Fig. 1). Two transects were sampled near suspected anthropogenically impacted areas: adjacent to the sewage outfall (Outfall) and adjacent to the ship loading dock (Pier). Two transects north of Palmer Station were sampled as potential reference transects: adjacent to the water intake (Intake) and north-east of Palmer Station (North Palmer; NP).
Sampling of the limpet N/ concinna occurred at depths of 1.5 and 4.5 m because the species was most abundant in shallow depths (< ~5 m) and because previous studies indicated that total polycyclic aromatic hydrocarbon (PAH) concentrations in limpet tissues were greater at shallow depths (Kennicutt et al. Reference Kennicutt, McDonald, Sericano, Boothe, Oliver and Safe1995). As with the sediment samples, limpets were sampled adjacent to the sewage outfall, pier and water intake jetty, but also further south-east of Palmer Station (South Palmer), from the hull of the Bahía Paraíso wreck (at 4.5 m deep), and at ten previously sampled potential reference sites (at 1.5 m deep) further from Palmer Station: one further up Hero Inlet and nine on offshore islands > 1 km away from Palmer Station Inlet (Kennicutt et al. Reference Kennicutt, Sweet, Fraser, Stockton and Culver1991). Limpet tissues were previously studied to determine the biological effects of the Bahía Paraíso spill in Arthur Harbour, including adjacent to Palmer Station (Kennicutt et al. Reference Kennicutt, McDonald, Denoux and McDonald1992b).
Sampling and laboratory analysis
Marine sediments at each sampling location were sampled by SCUBA divers in April 2015 using hand-held 6.3 cm-diameter cores (35.3 cm2) to a sediment depth of 10 cm. Triplicate sediment samples were taken for chemical, grain size and macrobenthic community assessment at each sampling station. Approximately 10 limpets were collected by SCUBA divers from every limpet site. Limpet tissues collected at each station were combined into one composite sample.
Sediment and tissue chemistry samples were frozen at -20°C and shipped to the Geochemical Environmental Research Group (GERG) at Texas A&M University, College Station for chemical analyses. Sediments were analysed for hydrocarbons, organochlorines (e.g. dichloro-diphenyl-trichloroethane (DDT), polychlorinated biphenyls (PCBs)), mercury and total inorganic and organic carbon concentrations (TIC and TOC, respectively). Sediment metal concentrations (aside from mercury) were not analysed in this study because there was minimal variability in metal concentrations measured at similar locations in 2014 (Figs S1 & S2). Tissues were analysed for hydrocarbons, organochlorines and metals. The laboratory analysis of organochlorines (PCBs and other pesticides) in limpet tissues was not completed for all samples after none were detected in the analysis of an initial subset of samples.
In brief, PAH, PCB and pesticide concentrations were determined using gas chromatography/mass spectrometry (GC-MS), metal concentrations (except for mercury) were determined using inductively coupled plasma mass spectrometry (ICP-MS), mercury concentrations were determined using by cold vapour atomic absorption spectroscopy (CVAAS) and carbon concentrations (TIC and TOC) were determined using an induction furnace and infrared detector (see Sweet & Wade Reference Sweet, Wade, Lauenstein and Cantillo1998, Aly et al. Reference Aly, Casillas, Luo, McDonald, Wade and Zhu2021 and Protocol S1) using methods from the National Oceanic and Atmospheric Administration ‘Status and Trends Program’ (National Oceanic and Atmospheric Administration 1993) and the United States Environmental Protection Agency (Wade et al. Reference Wade, Atlas, Brooks, Kennicutt II, Fox and Sericano1988, Telliard Reference Telliard1989, Creek et al. Reference Creek, Brockhoff and Martin1994; see also Morehead et al. Reference Morehead, Montagna and Kennicutt2008, Klein et al. Reference Klein, Sweet, Wade, Sericano and Kennicutt2012). Quality assurance/quality control (QA/QC) was performed on each set of up to 20 samples. The QA/QC procedures evaluated a procedural blank, a blank spike, a matrix spike, a duplicate of a sample (to test for sample homogeneity and analytical variability) and a standard reference material (SRM) when available. Details of the acceptance criteria that were met for all reported data are listed within Protocol S1.
Sediment grain size samples were stored at 4°C until analysis at GERG. Grain size was determined using the methods of Folk (Reference Folk1980) using a combination of sieve and pipette analysis.
Macrobenthic samples were split into 0–3 and 3–10 cm vertical sections, fixed with 5% buffered formalin and sent to Texas A&M University-Corpus Christi. The macrobenthos were then washed and extracted on a 0.5 mm sieve, identified to the lowest practical taxonomic level (usually species or genus) and enumerated (for abundance). Organisms were then pooled into higher taxonomic groups, dried at 50°C for 24–48 h and weighed (for biomass). The abundance and biomass of large nematodes (> 0.5 mm) are included in the results to provide consistency with two previous studies in the vicinity of Palmer Station (Lowry Reference Lowry1975, Richardson & Hedgepeth Reference Richardson, Hedgepeth and Llano1977). However, nematodes are not included in the statistical analyses because they are generally considered to be meiofauna.
Statistical analysis
Sediment chemical concentrations and macrobenthic communities were compared among transects over time using univariate and multivariate statistics. The sediment chemistry and grain size characteristics of each station were compared using principal component analysis (PCA) on arcsine-root-transformed grain size and carbon content data and loge(x + 1)-transformed metal and contaminant data.
Univariate variables used to compare macrofaunal differences at sites and transects include total abundance, total biomass, species richness, Hill's N1 diversity and Pielou's J' evenness. Non-metric multi-dimensional scaling (nMDS) was used to characterize macrobenthic community composition among sites over time. Groupings of samples were highlighted using the similarity profile routine (SIMPROF; Clarke et al. Reference Clarke, Somerfield and Gorley2008). The nMDS and SIMPROF analyses used family-level taxonomic resolution to reduce noise and better discriminate between disturbed and reference communities (Warwick Reference Warwick1988a, Reference Warwick1988b). Determining the effects of large disturbances on macrobenthic communities using family-level taxonomic resolution has been used successfully in other studies in Antarctica (Hyland et al. Reference Hyland, Laur, Jones, Shrake, Cadian and Harris1994, Thompson et al. Reference Thompson, Riddle and Stark2003, Conlan et al. Reference Conlan, Kim, Lenihan and Oliver2004, Palmer et al. Reference Palmer, Klein, Sweet, Montagna, Serciano and Hyde2021).
The relationships among contaminants and macrobenthic communities were compared by correlating the first two principal components (PCs) from the PCA and individual sediment variables with macrobenthic abundance, biomass, Pielou's J' evenness and N1 diversity. Bio-Env analysis, a routine for linking biota to the environment, was used to match the best combination of sediment chemical components with spatiotemporal community assemblage data (Clarke & Ainsworth Reference Clarke and Ainsworth1993). Bio-Env and nMDS analyses were conducted using PRIMER 7 software (Clarke et al. Reference Clarke, Gorley, Somerfield and Warwick2014). Community composition data were root-transformed prior to multivariate analyses. Sediment chemistry and grain size data were log-transformed prior to Bio-Env analysis. All univariate analyses, PCA and data management were completed using SAS 9.4 software (SAS Institute Inc. 2019).
Results
Sediment chemistry and grain size
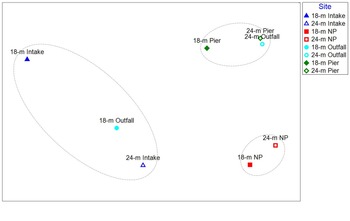
PC1 and PC2 represent 47.8% and 23.2% of the total variation within the sediment chemistry and grain size datasets, respectively (total = 71.0%; Fig. 2). PC1 represents differences in sediment grain size among stations. Sites with higher PC1 scores (Pier and NP sites, 24 m Outfall site) generally had higher silt and clay contents and, to a lesser extent, higher TOC and total petroleum hydrocarbon (TPH) concentrations. Sites with lower PC1 scores (Intake sites, 18 m Outfall site) had higher gravel and sand contents. PC2 represents differences in contamination among stations. Sites with higher PC2 scores (Pier and Outfall sites) generally had higher DDT and PCB concentrations and, to a lesser extent, higher PAH, TPH and TIC concentrations. Mercury concentrations were low in all samples (0.001–0.012 μg g-1; Table I & Fig. S3), so they were excluded from the PCA.

Fig. 2. a. Chemical loads and b. station scores from the principal component analysis of sediment chemistry and grain size for each station. DDT = dichloro-diphenyl-trichloroethane; NP = North Palmer; PAH = polycyclic aromatic hydrocarbon; PC = principal component; PCB = polychlorinated biphenyl; TIC = total inorganic carbon; TOC = total organic carbon; TPH = total petroleum hydrocarbon.
Table I. Sediment chemistry concentrations of sampling sites adjacent to Palmer Station and other coastal research stations in Antarctica. All units are in ng g-1 except for mercury (μg g-1).

a From Council of Managers of National Antarctic Programs (2020).
b Sample concentrations.
c Site means over multiple years.
DDT = dichloro-diphenyl-trichloroethane; PAH = polycyclic aromatic hydrocarbon; PCB = polychlorinated biphenyl; TPH = total petroleum hydrocarbon; WQB = Winter Quarters Bay.
Both Intake sites and the 18 m Outfall site had coarser sediment (and lower PC1 scores) than the other five sites. The two Intake sites and the 18 m Outfall site had low concentrations of clay and silt (1.0–3.2% clay, 3.7–12.8% silt) and higher concentrations of sand and gravel (59.6–83.7% sand, 7.8–31.9% gravel) than the other sites (5.0–15.1% clay, 46.9–73.1% silt, 11.2–42.2% sand, 0–7.6% gravel; Figs S4 & S5 & Table S1). Gravel and sand concentrations were both inversely correlated with silt and clay concentrations (-0.93 ≤ R ≤ -0.66, P ≤ 0.0005; Table II)
Table II. Spearman rank correlations among sediment chemistry and grain size variables. Spearman correlation coefficients (ρ; top), P values (bottom). N = 24. Correlations where R ≥ 0.5 are highlighted in bold.

DDT = dichloro-diphenyl-trichloroethane; PAH = polycyclic aromatic hydrocarbon; PCB = polychlorinated biphenyl; TIC = total inorganic carbon; TOC = total organic carbon; TPH = total petroleum hydrocarbon.
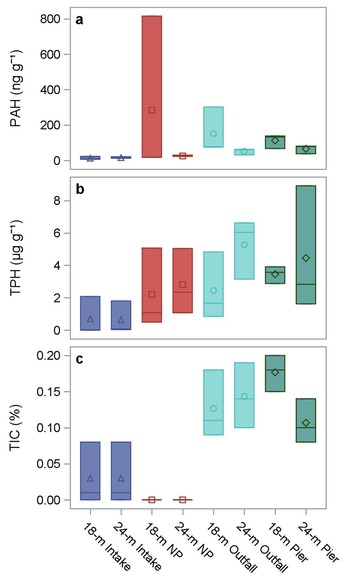
Sediment total PAH concentrations were lowest at the Intake transect (6.2–22.7 ng g-1) and at the NP transect except for one replicate at the 18 m NP site (17.3–30.0 and 815.6 ng g-1; Fig. 3a & Table I). Sediment total PAH concentrations were highest at the 18 m Outfall (74.5–301.8 ng g-1) and Pier sites (67.4–138.0 ng g-1). Total PAH concentrations were positively correlated with both TPH (R = 0.65, P ≤ 0.0005) and TIC (R = 0.56, P ≤ 0.005; Table II).

Fig. 3. Polycyclic aromatic hydrocarbon (PAH), total petroleum hydrocarbon (TPH) and total inorganic carbon (TIC) concentrations in sediments adjacent to Palmer Station. Symbols represent mean concentrations. Horizontal lines represent concentrations in each replicate sample. NP = North Palmer.
Sediment TPH concentrations were lower at the Intake transect (0–2.1 μg g-1) than all other transects (NP: 0.5–5.1 μg g-1, Outfall: 0.8–6.6 μg g-1, Pier: 1.6–8.9 μg g-1; Fig. 3b & Table I). Sediment TPH was positively correlated with fine sediment concentrations (silt: R = 0.57, P ≤ 0.003; clay: R = 0.51, P ≤ 0.01) and negatively correlated with coarse sediment concentrations (sand: R = -0.57, P ≤ 0.004; gravel: R = -0.61, P ≤ 0.002; Table II).
PCBs and DDT were undetectable in any sediment sample except for two replicates at the 18 m Outfall site (total PCB: 353 and 0 ng g-1, total DDT: 25.3 and 3.15 ng g-1 for replicates 1 and 2; Table I & Fig. S3). Total DDT in the two DDT-contaminated samples was comprised of 67% p,p'-DDT, 16–18% o,p'-DDT and 13–15% p,p'-dichloro-diphenyl-dichloroethane (DDD). Sediment mercury concentrations were lower at both Intake sites and the 18 m Outfall site (0.001–0.003 μg g-1) than at all other sites (0.003–0.012 μg g-1) except for the 18 m NP site (0.001–0.004 μg g-1).
Sediment TIC occurred in lower concentrations at the Intake and NP sites (0–0.08%) than the Outfall and Pier sites (0.08–0.20%; Fig. 3c). Mean sediment TOC concentrations were lower at the shallow Intake (0.07%) and Outfall (0.16%) sites than the other sites (0.23–0.38%). However, the within-site variation in TOC at most sites was too great to be able to infer any differences among transects (total range 0.02–0.75%). TOC concentrations were positively correlated with fine sediment concentrations (silt: R = 0.65, P ≤ 0.002; clay: R = 0.76, P < 0.0001) and negatively correlated with sand concentrations (R = -0.76, P < 0.0001; Table II).
Macrofauna community
Total macrofaunal abundance at each station ranged from 24,109 n m-2 at the 18 m Intake site (26,757 n m-2 with nematodes) to 102,110 and 102,300 n m-2 at the 24 m Intake and Pier sites (117,711 and 107,689 n m-2 including nematodes; Table III & Fig. S6). The infauna community immediately surrounding Palmer Station was numerically dominated by nematodes (unidentified, 17,361 m-2, 24.8% of total abundance), the amphipod Podoceropsis sp. (Photidae, 10,270 n m-2, 14.6%), oligochaetes (unidentified, 8805 n m-2, 12.6%) and the cumacean Eudorella splendida (Leuconidae, 5531 n m-2, 7.9%; Table S2). The most abundant families were Photidae (Amphipoda, 10,270 n m-2), Leuconidae (Cumacea, 5531 n m-2), Maldanidae (Polychaeta, 3226 n m-2) and Rissoidae (Gastropoda, 2505 n m-2; Table S3). On a coarser taxonomic level, the mean macrobenthic community was dominated by nematodes, amphipods (16,829 n m-2, 24.0%) and polychaetes (14,194 n m-2, 20.2%; Table S4).
Table III. Most abundant taxa (family resolution).

Macrofaunal community composition based on family-level resolution (excluding nematodes) is clustered into three groups (SIMPER test; Fig. 4). Group A contains the two Intake sites and the 18 m Outfall site. Group B contains both NP sites. Group C contains both Pier sites and the 24 m Outfall site. Group A is characterized by having high relative abundances of Oligochaeta and the Lasaeidae and Apistobranchidae families and low abundances of the Leuconidae, Photidae and Phoxocephalidae families. Group B is characterized by having high relative abundances of the Maldanidae, Capitellidae and Spionidae families and low relative abundances of Oligochaeta and the Ophelidae and Rissoidae families. Group C has high relative abundances of the Photidae, Oedicerotidae and Aoridae families and low relative abundances of the Cirratulidae and Spionidae families.

Fig. 4. Non-metric multi-dimensional scaling plot of macrofauna communities at each station overlaid with cluster groupings from SIMPROF analysis (P ≤ 0.004). NP = North Palmer.
Excluding megafauna, the biomass of the macrofauna community was dominated by polychaetes (13.5 g m-2, 46.5%), molluscs (8.9 g m-2, 30.5%) and crustaceans (5.5 g m-2, 18.7%; Table S5 & Fig. S6). The megafauna sampled were large individuals of the bivalve Laternula elliptica (mean = 2070 mg), which occurred at the 18 m Outfall (n = 5, 1010 g m-2) and 18 m Intake (n = 1, 164 g m-2) stations and dominated total biomass at these stations. Macrofauna biomass including L. elliptica was greatest at the shallow station (18 m) within each transect. Crustacean biomass was higher in the Pier (8.6 and 12.7 g m-2) and Outfall (6.7 and 7.9 g m-2) transects than the NP (3.0 and 3.3 g m-2) and Intake transects (0.1 and 1.7 g m-2). Crustacean abundance was higher at the Pier (35,645 and 66,467 n m-2) and 24 m Outfall sites (52,190 n m-2) than the 18 m Outfall site (3214 n m-2), Intake sites (946 and 8887 n m-2) and NP sites (13,048 and 9360 n m-2).
Mean N1 diversity was higher at the 24 m Intake site (11.2 ind. 35-cm-2), the 18 m Pier site (9.3 ind. 35-cm-2) and the 18 and 24 m NP sites (8.6 and 10.3 ind. 35-cm-2) than at all other sites (5.0–7.3 ind. 35-cm-2; Fig. S7). Mean J' evenness was higher at the Intake sites (0.73 and 0.69 35-cm-2), NP sites (0.77 and 0.84 35-cm-2) and the 18 m Pier site (0.72 35-cm-2) than the Outfall sites (0.59 and 0.61 35-cm-2) and 24 m Pier site (0.53 35-cm-2). There was enough intra-site variability that no differences among transects could be determined, except that J' evenness was lower at the Outfall than at the NP sites.
Linking macrofauna communities with sediment qualities
Macrofauna community composition was most highly correlated with the combination of TOC, TIC, gravel, silt and mud (R = 0.854, P ≤ 0.003, Tables IV & S6). The highest ten correlations between community composition and environmental variables (P ≥ 0.833) all include TOC. The single variables that correlate highest with community composition are mud (R = 0.704, P ≤ 0.014), silt (R = 0.693, P ≤ 0.017) and TOC (R = 0.674, P ≤ 0.017).
Table IV. Highest correlations of sediment variables (vars) with macrobenthic community composition for combinations of one to five trial variables. Further results are listed in Table S6.

TIC = total inorganic carbon; TOC = total organic carbon.
Hill's N1 diversity was weakly correlated (Pearson correlation) with PC2 (which represents contamination, R = -0.52, P ≤ 0.19), TIC (R = -0.51, P ≤ 0.20) and TPH (R = -0.62, P ≤ 0.10; Table S7). Pielou's J' evenness was weakly correlated with PC2 (R = -0.62, P ≤ 0.10) and TIC (R = -0.64, P ≤ 0.09). Species richness was weakly correlated with gravel content (R = 0.59, P ≤ 0.12). Total biomass (excluding L. elliptica) was positively correlated with PAH concentrations (R = 0.75, P ≤ 0.03). There were no other relationships among univariate macrofauna variables and sediment characteristics (R < 0.50).
Limpet tissues
PAH concentrations in limpet tissues were higher close to Palmer Station (17–348 ng g-1) and on the Bahía Paraíso (106 ng g-1) than at the outlying islands (9–13 ng g-1; Figs 5 & S8 & Table V). The highest concentration occurred at the Pier (1.5 m deep: 348 ng g-1; 4.5 m deep: 86 ng g-1), followed by the 1.5 m deep South Palmer site (79 ng g-1). The lowest PAH concentrations in limpet tissues adjacent to Palmer Station occurred at the Intake transect (17 and 35 ng g-1). Tissue PAH concentrations were higher at 1.5 than 4.5 m depths at the Pier transect and south-east of Palmer Station, but lower at 1.5 m depths for the Outfall and Intake transects.

Fig. 5. Total polycyclic aromatic hydrocarbon (PAH) concentrations in limpet tissues.
Table V. Limpet tissue chemistry concentrations of sampling sites adjacent to Palmer Station and other research stations in Antarctica. All units are in μg g-1 except for polycyclic aromatic hydrocarbons (PAHs; ng g-1). Concentrations are listed as ranges or means ± standard deviations.

a Council of Managers of National Antarctic Programs (2020).
Copper and lead concentrations were much higher at the Bahía Paraíso (Cu: 6.25 μg g-1; Pb: 1.92 μg g-1) than near Palmer Station (Cu: 1.14–2.12 μg g-1; Pb: 0.11–0.45 μg g-1), which were generally higher than at the sites far from Palmer Station (Cu: 0.84–1.18 μg g-1; Pb: 0.04–0.25 μg g-1; Table V & Figs S12 & S15). Zinc concentrations were highest at the Outfall and Pier sites (12.1–18.4 μg g-1) than at all other sites (7.8–10.5 μg g-1, Fig. S16). Barium, beryllium, cobalt, chromium, iron, mercury, manganese and vanadium were higher at most sites close to Palmer Station than at most sites far away, including at the Bahía Paraíso (Figs S8–S16). Conversely, cadmium and magnesium concentrations were generally lower at sites near Palmer Station than at the more distant sites.
Discussion
Sediment chemistry
Aside from one or two samples containing high DDT and PCB concentrations, the marine sediment contaminants adjacent to Palmer Station generally had lower or similar concentrations to those reported at other Antarctic research stations and were lower than any Effects Range Low limits (ERL, 10th percentile of effects; Long et al. Reference Long, MacDonald, Smith and Calder1995; Table I). This suggests that there are minimal effects of individual contaminants on the benthos (not accounting for multiple stressor effects). These low levels of anthropogenic contamination relative to other stations could be attributed to several possible factors. First, physical transport processes (e.g. currents, waves, iceberg scouring) are greater at Palmer Station than at some other stations (Kennicutt et al. Reference Kennicutt, McDonald, Sericano, Boothe, Oliver and Safe1995), meaning that the dispersal of any contamination is also relatively greater in sediments adjacent to Palmer Station. Second, the peak population at Palmer Station (46 people) is smaller than those of most of the stations compared in this study (peak populations of 8–66 and 1200 people), and the relatively lower activity level may result in lower levels of anthropogenic contamination. Third, waste management may be more effective at Palmer Station; contaminant concentrations were generally lower at the Intake and north side of the Station than adjacent to the Pier and Outfall, where most of the anthropogenic activity occurs.
The patchy distribution of concentrated DDT and PCB compounds adjacent to the sewage outfall is surprising given that there are relatively low concentrations of most other contaminants in the local marine sediments. Total DDT was between the ERL (1.58 ng g-1) and the Effects Range Median (ERM, 50th percentile of effects; total DDT = 46.1 ng g-1) in two out of three samples taken at the 18 m Outfall site (3.2 and 25.3 ng g-1) but not detected elsewhere (Long et al. Reference Long, MacDonald, Smith and Calder1995). In comparison, the highest DDT concentration at Palmer Station (25.3 ng g-1) is higher than any sediment sample taken at two contaminated locations adjacent to McMurdo Station from 2000 to 2013 (Winter Quarters Bay: 24.1 ng g-1 and McMurdo sewage outfall: 22.6 ng g-1; Palmer et al. Reference Palmer, Klein, Sweet, Montagna, Serciano and Hyde2021). The second highest DDT concentration at Palmer Station is similar to mean DDT concentrations at contaminated sampling sites (2.8–3.5 ng g-1) and higher than mean concentrations at reference sampling sites (0.2–0.5 ng g-1) adjacent to McMurdo Station. DDT and its derivatives dichloro-diphenyl-dichloroethylene (DDE) and DDD also occurred in Arthur Harbour sediments between 1989 and 1993; however, their locations and concentrations are not reported (Kennicutt et al. Reference Kennicutt, McDonald, Sericano, Boothe, Oliver and Safe1995).
The total PCB concentration was well above the ERM (180 ng g-1) in one sample at the 18 m Outfall at Palmer Station (353 ng g-1) but also not detected elsewhere. This PCB concentration is higher than total PCB concentrations in three soft sediment samples taken in Arthur Harbour between 1989 and 1993 (2.8–4.2 ng g-1, Kennicutt et al. Reference Kennicutt, McDonald, Sericano, Boothe, Oliver and Safe1995) and mean total PCB concentrations at reference sampling sites adjacent to McMurdo Station (9–35 ng g-1), but within the range of mean concentrations at contaminated sites adjacent to McMurdo Station (263–1561 ng g-1; Palmer et al. Reference Palmer, Klein, Sweet, Montagna, Serciano and Hyde2021).
Most persistent organic pollutants (POPs), including PCB and DDT compounds, reach Antarctica through atmospheric transport (Risebrough et al. Reference Risebrough, Walker, Schmidt, de Lappe and Connors1976), and despite a global reduction in their use since the 1970s, snow and glacial ice that have entrapped these compounds eventually release them into the aquatic environment (Lukowski & Ligowski Reference Łukowski and Ligowski1987, Corsolini Reference Corsolini2009). DDT has been detected in glacial meltwater in the vicinity of Palmer Station (0.0187 ng l-1), and this meltwater has been speculated to cause local elevated DDT concentrations in sea-ice algae, plankton and Adélie penguins (Chiuchiolo et al. Reference Chiuchiolo, Dickhut, Cochran and Ducklow2004, Geisz et al. Reference Geisz, Dickhut, Cochran, Fraser and Ducklow2008). However, the presence of concentrated patches of PCBs and DDT at the Palmer Station outfall relative to immediately surrounding sediments and known contaminated sediments at McMurdo Station indicate a local rather than a global source of contamination of these POPs. This patchy distribution adjacent to the outfall suggests small, isolated contamination events, possibly via the wastewater outfall.
Risebrough et al. (Reference Risebrough, Walker, Schmidt, de Lappe and Connors1976) reported high concentrations of PCBs in snow at Palmer Station in 1975 (0.004–0.010 ng g-1) as a consequence of burning ‘considerable quantities of waste materials’. PCBs in marine sediments adjacent to McMurdo Station were mostly station-derived and mostly resembled a mixture used in fluid-filled electrical conductors (Aroclor 1260) until 1971 (United States Environmental Protection Agency 1976, Kennicutt et al. Reference Kennicutt, Klein, Montagna, Sweet, Wade, Palmer and Denoux2010). PCBs from electrical components used at historical military sites, including US sites, are also thought to be the largest source of localized PCB contamination in the Canadian Arctic (Kuzyk et al. Reference Kuzyk, Stow, Burgess, Solomon and Reimer2005, Stow et al. Reference Stow, Sova and Reimer2005). It is probable that the PCB contamination at Palmer Station is derived from similar electrical components because Palmer Station, like McMurdo Station, was initially run by the US military. DDT also has a military connection despite its more widely known use as an insecticide in agricultural practices prior to the 1970s. The military of the USA and other nations heavily used DDT to combat insect-borne diseases such as malaria and typhus during World War II (1939–1945) and continued to use it on military ships and bases as a means of controlling mosquitos, lice, scabies, fleas, flies, cockroaches and bedbugs for decades after (United States Department of Agriculture 1946, Russell Reference Russell1999). Even up until 1972, when DDT was banned in the USA for civilian use, it was still being used by the US military to exterminate mice and bats (United States Environmental Protection Agency 1975). Although there are no native nuisance insects near Palmer Station, DDT could have been brought on the ships that serviced Palmer Station in early years of its existence (1968 onwards) to exterminate insects that were present in warmer climates en route to Antarctica and could have entered the marine environment from those ships. The dominance of p,p'-DDT (67%) rather than the main degradation product p,p'-DDE occurring in Palmer Station sediments in 2015 indicates an undegraded state (Corsolini & Sarà Reference Corsolini and Sarà2017), which is most probably the result of the slow degradation rates in cold environments with long winter darkness (i.e. polar regions; Mangano et al. Reference Mangano, Sarà and Corsolini2017) rather than a new source. The limited historical sampling for PCBs and DDT at Palmer Station makes it difficult to conclusively determine when PCB and DDT contamination of marine sediments occurred and to distinguish temporal changes from spatial heterogeneity. While it is possible that PCBs and DDT are still entering the marine environment via the outfall or runoff near the outfall, this seems extremely improbable given the current careful environmental practices at Palmer Station. Regardless of the source, it is important to acknowledge the sewage outfall area as a source of POPs that may be entering the food web adjacent to Palmer Station.
Total PAH concentrations in sediments adjacent to the Pier and Outfall in the present study (32–302 ng g-1) were generally lower than those measured adjacent to the Station in 1991, 2 years after the Bahía Paraíso grounding and spill (< 10–14,491 ng g-1) and those occurring adjacent to the defunct Old Palmer Station (USAP) and Base N (British Antarctic Survey), ~2 km north-west of Palmer Station in 1991 (5643–59,478 ng g-1; Kennicutt et al. Reference Kennicutt, McDonald, Denoux and McDonald1992a). The Bahía Paraíso spill in 1989 especially contaminated intertidal sediments and organisms but had lesser effects on subtidal sediments (Kennicutt & Sweet Reference Kennicutt and Sweet1992, Kennicutt et al. Reference Kennicutt, McDonald, Denoux and McDonald1992a, Hyland et al. Reference Hyland, Laur, Jones, Shrake, Cadian and Harris1994). In as early as 2 years after the spill, it was concluded that local inputs from ship, boating and on-land station activities had contaminated subtidal sediments to a greater extent than the Bahía Paraíso spill except immediately adjacent to the sunken ship. High soil PAH concentrations occurred at Old Palmer Station and Base N in the 1990s despite the removal of buildings and some remediation.
Total PAH concentrations in sediments adjacent to the Palmer Station Pier and Outfall were lower than two contaminated locations at McMurdo Station (2000–2013 mean at Winter Quarters Bay: 1091 ng g-1; and McMurdo Station outfall: 755 ng g-1; Kennicutt et al. Reference Kennicutt, McDonald, Denoux and McDonald1992a, Palmer et al. Reference Palmer, Klein, Sweet, Montagna, Serciano and Hyde2021). Aside from one sample, total PAH concentrations at the Intake and North Palmer transects in this study (815.6 and 6.2–30.0 ng g-1) were less than the mean values at two reference locations adjacent to McMurdo Station (Cape Armitage: 40.8 and 27.5 ng g-1). Total PAH concentrations at Palmer Station in 2015 were similar to those occurring adjacent to Signy and Ferraz stations, but lower than those adjacent to Carlini Station (Cripps Reference Cripps1992, Martins et al. Reference Martins, Bícego, Taniguchi and Montone2004, Curtosi et al. Reference Curtosi, Pelletier, Vodopivez and Mac Cormack2007).
The high concentration of total PAHs detected in one sample collected from the 18 m North Palmer transect was dominated by pyrogenic PAHs (335 ng g-1, 41%), including a relatively high concentration of chrysenes (142 ng g-1; Table S8). A high total PAH concentration (4858 ng g-1) occurred in water 9 m deep along the North Palmer transect in 1991, although not at 5 and 16 m depths along the same transect. The majority of the Palmer Station marine sediments that were highly contaminated with PAHs in 1991, including at 9 m deep along the North Palmer transect, were mostly made up of non-degraded fuel residues (high Group-A PAH concentrations and a high (> 1) n-C17/pristane ratio). The difference in location and composition of PAHs at the North Palmer transect in 2015 compared to 1991 and the occurrence of the high concentration at only one out of three replicates indicate patchy PAH contamination of incinerated or other non-fuel waste dumped north of Palmer Station. It is important to recognize that total sediment PAH concentrations occurring at Palmer Station in 2015 (6–302 and 816 ng g-1) are lower than the ERL of 4022 ng g-1 and considerably lower than the ERM of 44,792 ng g-1 of sediment quality guidelines (Long et al. Reference Long, MacDonald, Smith and Calder1995). Similarly, the likelihood of contaminant effects on macrofauna and meiofauna was considered to be low (< 20%), where total PAH concentrations in deep Gulf of Mexico sediments following the Deepwater Horizon spill were 4000 ng g-1 (Balthis et al. Reference Balthis, Hyland, Cooksey, Montagna, Baguley, Ricker and Lewis2017). Therefore, these recent concentrations at Palmer Station are unlikely to have any biological effects despite being above background concentrations.
Sediment TPH concentrations were low adjacent to Palmer Station (0–9 μg g-1), especially adjacent to the water intake (0–2 μg g-1). The TPH concentrations adjacent to Palmer Station are similar to or lower than those occurring adjacent to Davis Station (1–62 μg g-1; Green & Nichols Reference Green and Nichols1995) and reference locations at McMurdo Station (mean concentrations from 2000 to 2013 of 4 and 15 μg g-1; Palmer et al. Reference Palmer, Klein, Sweet, Montagna, Serciano and Hyde2021) and Casey Station (0–73 μg g-1, Stark et al. Reference Stark, Kim and Oliver2014) and much lower than contaminated locations at McMurdo Station (180 and 238 μg g-1) and Casey Station (145–698 μg g-1). The likelihood of adverse biological effects on deep-sea macrofauna and meiofauna in the Gulf of Mexico is low (< 20%) at much higher concentrations (606 and 700 μg g-1) than that occurring at Palmer Station (Balthis et al. Reference Balthis, Hyland, Cooksey, Montagna, Baguley, Ricker and Lewis2017). This indicates that TPH contamination at Palmer Station is also unlikely to have biological effects.
Sediment concentrations of mercury (0.001–0.012 μg g-1) adjacent to Palmer Station are lower than the ERL (Hg = 0.15 μg g-1, total PAH = 4022 ng g-1; Long et al. Reference Long, MacDonald, Smith and Calder1995), indicating a low likelihood of biological effects. Mercury concentrations at Palmer Station (0.001–0.012 μg g-1) were similar to or lower than those occurring adjacent to other Antarctic research stations, including Zucchelli Station (Italian National Antarctic Research Program), Terra Nova Bay (0.006–0.027 μg g-1; Bargagli et al. Reference Bargagli, Monaci, Sanchez-Hernandez and Cateni1998), Scott Base (Antarctica New Zealand, < 0.001–0.012 μg g-1; Negri et al. Reference Negri, Burns, Boyle, Brinkman and Webster2006) and Ross Island and McMurdo Station (USAP) reference locations (Cape Armitage), Ross Island (0.01–0.03 μg g-1), and much lower than those occurring in Winter Quarters Bay (0.02–0.07 μg g-1) and the sewage outfall (0.09–0.19 μg g-1), McMurdo Station (Palmer et al. Reference Palmer, Klein, Sweet, Montagna, Serciano and Hyde2021; Table I). These subtidal sediment mercury concentrations at Palmer Station are similar to or lower than those of Deception Island supratidal and intertidal beach sediments (0.001–0.118 μg g-1), despite natural, volcanic sources of mercury (1.3–10 μg g-1) also being in the vicinity (Mão de Ferro et al. Reference Mão de Ferro, Mota and Canário2014). The uniformly low concentrations of mercury at Palmer Station suggests a global atmospheric and/or natural geological source.
Macrofauna community
Aside from total macrofaunal biomass being higher at the 18 m than the 24 m sites at the Pier, Outfall and North Palmer transects, there were no obvious spatial trends for any univariate macrofauna community variable analysed in this study (total abundance, diversity, species richness, evenness). However, crustacean biomass was greater at the Outfall and Pier sites (6.7–12.7 g m-2) than at the Intake and North Palmer transects (0.1–3.3 g m-2; Table S5), and crustacean abundance was high at the Pier sites and 24 m Outfall site (35,645–66,467 n m-2) than at all other sites (946–9360 n m-2; Table S4). Crustaceans are recognized as being disturbance-sensitive at McMurdo Station and disturbance indicators at Casey Station (Stark et al. Reference Stark, Kim and Oliver2014). It is suspected that increased anthropogenic organic enrichment at Casey Station provides food for crustaceans, whereas the higher metal and possibly persistent organic compounds at McMurdo Station restrict crustaceans from accessing any similarly enriched areas. Crustacean biomass at the 18 m Outfall site at Palmer Station was dominated by the larger lysianassid amphipod Hippomedon kergueleni (maximum length of 22 mm), while the 24 m Outfall and Pier sites were dominated by the smaller but more abundant photid amphipod Podoceropsis spp. (maximum length of ~6–8 mm; De Broyer Reference De Broyer and Llano1977). H. kergueleni and other Lysianassid amphipods are known deposit feeders and scavengers (Slattery & Oliver Reference Slattery and Oliver1986, Marine Ecosystems Research Programme 2020), and Lysianassidae have been associated with increased organic carbon loads at Casey Station (Stark et al. Reference Stark, Kim and Oliver2014). The dominant feeding mode of Podoceropsis spp. is thought to be suspension feeding, but they are probably opportunistic like other amphipods (Marine Ecosystems Research Programme 2020). Podoceropsis spp. may prefer the finer sediments that occur at the Pier sites and the 24 m Outfall site rather than the 18 m Pier site. However, Podoceropsis spp. abundances are much lower at the North Palmer sites where similarly fine sediments exist. Differences in amphipod assemblages could be attributed to the different exposure levels on the north and south coasts of Palmer Station and potential associated differences in benthic micro- and macro-algal composition (De Laca & Lipps Reference De Laca and Lipps1976).
Macrofauna community composition adjacent to Palmer Station is divided into three clusters that do not separate entirely by geography (Fig. 4). While the macrofauna community at the North Palmer sites is different from those occurring at the other locations, the two Outfall communities separate: the 18 m Outfall community is more similar to the Intake communities and the 24 m Outfall community is more similar to the Pier communities. Sediment grain size and carbon content (both TOC and TIC) explained the distribution of communities better than spatial zones and contaminant concentrations. Macrofauna community composition was most highly correlated with the combination of TOC, TIC, gravel, silt and mud (R = 0.854, P ≤ 0.003; Table IV & Table S6), with the most highly correlated single chemical and grain size variables being mud (R = 0.704, P ≤ 0.014), silt (R = 0.693, P ≤ 0.017) and TOC (R = 0.674, P ≤ 0.017). N1 diversity was lowest when TIC concentrations, TPH concentrations and the summary variable PC2 (= high DDT, PCB, PAH, TPH and TIC concentrations; Fig. 2) were high, although the correlations between these variables were weak (-0.62 ≤ R ≤ -0.51; Table S7). There were very few other relationships among univariate macrofaunal variables and chemistry and grain size variables.
Macrofauna communities were correlated with different sediment variables at Palmer and McMurdo stations. Macrofauna community composition was most highly correlated with TPH, barium and TIC in heavily polluted marine sediments adjacent to McMurdo Station (Palmer et al. Reference Palmer, Klein, Sweet, Montagna, Serciano and Hyde2021). Barium and TPH were indicators of an intense spatial gradient in contamination at McMurdo Station, whereas TIC varied temporally and is possibly related to temporal changes in benthic production and/or decomposition of deposited organic material (Norkko et al. Reference Norkko, Thrush, Cummings, Gibbs, Andrew, Norkko and Schwarz2007). Although TIC is highest at the Outfall and Pier transects at Palmer Station, the cause of the relatively high TIC concentrations may be unrelated to anthropogenic activity. It is possible that the greater abundance and biomass of crustaceans that occur adjacent to the Outfall and Pier sites allow for the greater decomposition of organic carbon or decrease benthic primary production, which would result in additional TIC. Alternatively, the concentration of dead crustacean exoskeletons could provide the additional TIC. The presence of an amphipod species (Corophium volutator) and two other macrofauna species (polychaete Hediste diversicolor and gastropod Hydrobia acuta neglecta) facilitated an increase in organic decomposition and therefore higher TIC in sediments from a Danish estuary (Andersen & Kristensen Reference Andersen and Kristensen1992). The much lower concentrations of contaminants over a smaller spatial extent of contamination at Palmer Station than at McMurdo Station means that natural variations in grain size and carbon content directly or indirectly play a greater role in macrofauna community composition than does contamination at Palmer Station.
Sediment grain size is known to directly and indirectly influence macrofauna community composition throughout the world, in part because it is associated with other factors, such as organic content, porosity and current flow (Gray Reference Gray and Barnes1974, Snelgrove & Butman Reference Snelgrove and Butman1994, Grebmeier et al. Reference Grebmeier, Frey, Cooper and Kędra2018). After the Bahía Paraíso spill, sediment grain size and depth were thought to play more important roles in structuring macrobenthic community compositions than small concentrations of hydrocarbon contamination in depths of 30–115 m at Arthur Harbour (Hyland et al. Reference Hyland, Laur, Jones, Shrake, Cadian and Harris1994). Long-term (1971–1989) changes in macrofauna communities at Palmer Station were speculated by Hyland et al. (Reference Hyland, Laur, Jones, Shrake, Cadian and Harris1994) to be related to natural factors, such as ice scouring, glacial calving and glacial retreat. Diversity and benthic community composition were more highly correlated with grain size and organic content than iceberg scour in a study near Rothera Point, Antarctic Peninsula (Vause et al. Reference Vause, Morley, Fonseca, Jażdżewska, Ashton and Barnes2019). The positive correlation of TOC content with mud, silt and clay contents and its negative correlation with sand content (Table II) mean that the relative importance of grain size and organic content for facilitating community composition cannot be separated in this current study.
The macrofauna communities at the 18 m Pier and Outfall sites were compared with similar sites sampled in 1971 (stations 6 and 10 in Richardson & Hedgepeth Reference Richardson, Hedgepeth and Llano1977) in an attempt to determine any temporal differences that might be attributable to changes in anthropogenic activity. The macrofauna communities in 1971 shared some similar numerically dominant taxa to those occurring in this current study. Both the 1971 and 2015 communities had high relative abundances of the cumacean Eudorella spp. (E. gracilor in 1971, E. splendida in 2015), the polychaetes Ophelina syringopyge (Opheliidae) and Rhodine antarctica (Maldanidae) and the bivalve Mysella sp. The 2015 communities had greater relative abundances of the gastropod Onoba subantarctica than the 1971 communities. The 18 m Pier site had higher relative abundances of amphipods (e.g. Podoceropsis sp., Haplocheira plumosa, Monoculodes scabriculosus), nematodes and oligochaetes in 2015 than those reported for 1971 and the 2015 18 m Outfall site. The differences in total abundances of each dominant species between 1971 and 2015 can be attributed to many possible factors. The most obvious factors are that the sampling locations were not identical in 1971 and 2015 or that there has been a long-term change over time, as documented in Arthur Harbour from 1971 to 1989 by Hyland et al. (Reference Hyland, Laur, Jones, Shrake, Cadian and Harris1994). Other potential natural factors include seasonal variation, which occurs in the benthos at Arthur Harbour and extends to benthic microflora composition and benthic megafauna behaviour (1971 samples were taken in January–February, 2015 samples were taken in April), or iceberg and ice disturbances (Kauffmann Reference Kauffman1974). It is not apparent that any temporal changes can be attributed to changes in human activities.
Limpet tissues
Limpet (N. concinna) tissues served as successful bioindicators of contamination from Palmer Station as they indicated the presence of bioavailable contaminants in the local marine environment. There was no evidence of PCBs in limpet tissues in 2015, despite low concentrations (29–76 ng g-1) occurring from 1989 to 1993 (Kennicutt et al. 2015). In 2015, limpet tissues adjacent to Palmer Station, especially at the pier, outfall and south-east of the station, had high concentrations of PAHs, copper, lead, zinc and several other metals (Ba, Be, Co, Cr, Fe, Hg, Mn, V; Table 5 & Figs S8–S16). Historically (1989–1991), PAHs in limpet tissues at Palmer Station have been primarily low-molecular-weight, lipid-soluble compounds that enter the marine environment as slicks and/or runoff from the Station (Kennicutt et al. Reference Kennicutt, McDonald, Sericano, Boothe, Oliver and Safe1995). Although tissue PAH concentrations are higher adjacent to Palmer Station in 2015 than at the surrounding islands, those at the water Intake and adjacent to the Pier are an order of magnitude lower than concentrations that occurred after the Bahía Paraíso spill (1989–1991; Table V; Kennicutt et al. Reference Kennicutt, McDonald, Denoux and McDonald1992b). The 2015 tissue PAH concentrations at Palmer Station are lower than the 1989–1991 PAH concentrations at Old Palmer Station/Base N by one to two orders of magnitude. Tissue PAH concentrations have been reduced by up to five orders of magnitude in Arthur Harbour from 1989–1991 to 2015. While low concentrations of anthropogenic hydrocarbons can be difficult to detect because of the presence of naturally occurring biotic hydrocarbons (Cripps Reference Cripps1994), sampling at reference stations allows for the detection of concentrations above a natural range.
Trace metals concentrations in limpet tissues at Palmer Station were generally comparable to most tissues at other stations, although some stations had concentrations that were much higher (e.g. higher iron concentrations at Rothera Station, Adelaide Island and several stations on King George Island, higher manganese concentrations at Rothera Station; see Table V). Caution must be taken when comparing metal concentrations at different stations because of varying natural background concentrations attributed to sediment and rock minerology. For example, Admiralty Bay, King George Island, has high natural zinc and copper concentrations (Trevizani et al. Reference Trevizani, Figueira, Ribeiro, Theophilo, Majer and Petti2016). Although more toxic than some metals, cadmium also naturally occurs in high concentrations in waters and organisms of the Southern Ocean, especially where upwelling occurs (see Kennish Reference Kennish1997, Bargagli Reference Bargagli2005). It is possible that the higher concentrations of cadmium in tissues in Arthur Harbour compared with those adjacent to Palmer Station are because higher concentrations might occur in deeper, less stratified water further away from the larger landmass. Cadmium concentrations were higher in sediments in deeper (24 m) than shallow (12–18 m) environments adjacent to Palmer Station (Fig. S2). Spatial differences in sediment minerology and volcanic activity may also explain differences in iron and mercury concentrations in different locations, although limpets do not exist at some of the high-latitude stations that have some of the highest levels of contamination (e.g. McMurdo Station).
Elevated metal and PAH concentrations in limpet tissues at Palmer Station relative to the surrounding islands indicate that the station's activities are introducing bioavailable contaminants to the marine environment. While these contaminant concentrations generally appear to be low, contaminants such as mercury and cadmium can biomagnify up the food web into species that feed on limpets, such as birds (e.g. kelp gulls (Larus dominicanus) and sheathbills (Chionis albus)), asteroids (e.g. Diplasterias brucei, Odontaster validus and Perknaster spp.), fish (Notothenia coriiceps) and echinoids (Sterechinus neumayeri; Favero et al. Reference Favero, Silva and Ferreyra1997, Bargagli et al. Reference Bargagli, Monaci, Sanchez-Hernandez and Cateni1998, Bargagli Reference Bargagli2005, Suda et al. Reference Suda, Vani, de Oliveira, Rodrigues, Rodrigues and Lavrado2015, Cipro et al. Reference Cipro, Montone and Bustamente2017). Therefore, there may be effects further up the food chain at Palmer Station even though contamination concentrations in limpets are low.
Summary
Palmer Station is a medium-sized coastal Antarctic research station with generally similar or lower levels of marine sediment contamination compared to other Antarctic research stations. This is despite patches of elevated PCB and DDT concentrations at the sewage outfall and an elevated PAH concentration at an otherwise reference area, all of which are suspected to be of local and historical origin. Contaminants were generally higher at Palmer Station adjacent to the sewage outfall and pier than adjacent to the water intake and north side of the station. Quantifying the effects of this contamination on infauna communities is challenging because of the high variability in grain size and unmeasured natural variables (e.g. waves, currents, ice scour) in the relatively high-energy environment at Palmer Station, although anthropogenic effects are probably low. The limpet N. concinna is a successful bioindicator that was used to indicate that bioavailable hydrocarbons and metals continue to be introduced to or recycled within the marine waters adjacent to Palmer Station. However, sediment and limpet hydrocarbon concentrations are generally much lower than those occurring within a few years following the Bahía Paraíso spill in 1989. Sampling marine sediment and limpet tissues for contaminants should be incorporated into future monitoring plans because they are easily sampled compared to many other contamination and pollution indicators, they can be directly linked to human activities and they represent effects of humans on the marine food web. Environmental assessments such as this study are important for characterizing the localized effects of humans at Antarctic research stations, but they need to be continued in order to determine the consequences of changes in environmental management over time. This study will be particularly useful for determining anthropogenic effects prior to proposed development at Palmer Station, including the major construction of a new shipping pier planned for the 2021–2022 summer (National Science Foundation, personal communication 2021).
Acknowledgements
We especially thank USAP divers Shawn Harper, Rob Robbins and Steve Rupp for assisting with sample collection and diving logistics. We also thank several people for assisting with laboratory analyses for macrobenthic fauna and sediment chemistry, especially Elani Morgan and Rick Kalke. The USAP staff both at Palmer Station and in the USA were instrumental in providing support for this project, as was the parent body of USAP, the National Science Foundation's Division of Polar Programs (especially Polly Penhale). We would like to thank Jeffrey Hyland and an anonymous reviewer for their constructive criticisms of the original submitted version of this manuscript.
Financial support
This study was funded by the US Army Corps of Engineers Cold Regions Research and Engineering Laboratory (grant number W913E5-16-C-0006). The funding sources had no involvement in study design, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the article for publication.
Author contributions
AGK, STS and TAP conceived, designed and implemented the fieldwork. LJH, STS and TAP conducted laboratory analyses. TAP performed data management, statistical analyses and interpretation of findings. TAP prepared the manuscript with input from all authors.
Supplemental material
Eight supplemental tables and 16 supplemental figures will be found at https://doi.org/10.1017/S0954102021000535.