1. Introduction
A reliable understanding of the processes involved in climatic change at regional or global scale is closely related to the possibility of obtaining long-time, high-resolution records of climatic and environmental direct indicators or proxy-markers. In this way, the forcing factors and the negative or positive feedback mechanisms of the climatic system can be studied and effectively interpreted. to do this, it is essential to have available natural archives able to provide palaeoclimatic and palaeoenvironmental data over the longest period and with the highest time resolution possible. Among the natural palaeo-data archives, ice cores appear to constitute the best compromise between the maximum temporal range and the minimum sampling interval, at least for detailed studies of environmental and climatic changes in the last-glacial/interglacial cycles (Reference Bradley, Eddy and BradleyBradley and Eddy, 1991).
To obtain continuous, time-resolved palaeo-datasets, it is necessary to analyze ice-core sections with a depth resolution of a few centimetres. This means that a very large number of subsamples has to be produced, making the analytical work tedious, time-consuming and expensive. In the 1980s, some relevant parameters, such as liquid or solid (electrical conductivity measurements (ECM) and dielectric profiling (DEP)) conductivity, were continuously measured in the field (Reference HammerHammer, 1980; Reference Moore, Mulvaney and ParenMoore and others, 1989), but chemical analysis was carried out on single subsamples, leading to many contamination problems, mainly caused by sample manipulation. the fast, high-resolution determination of chemical compounds, carried out in series with drilling activity and subsampling, allows an immediate interpretation of temporal profiles of climatic and environmental markers, provides a useful indication for a reliable subsampling (driving a higher-resolution sampling where the profile trends look particularly interesting), identifies temporal horizons (e.g. volcanic signatures) and reveals post-depositional processes affecting the stratigraphic distribution of some species. Recently, chemical flow analysis (CFA) methods, based on continuous ice-core melting followed by spectrofluorimetric and spectrophotometric flow analysis, have been developed and used for measurements in the field (Reference Fuhrer, Neftel, Anklin and MaggiFuhrer and others, 1993; Reference Sigg, Fuhrer, Anklin, Staffelbach and ZurmühleSigg and others, 1994; Reference RöthlisbergerRöthlisberger and others, 2000b). Nevertheless, some ionic components, especially chloride and sulphate, were not measured (chloride) or were partially determined (sulphate), because the continuous methods at present do not have sufficient sensitivity to determine low levels. to overcome this information gap, we set up a semi-continuous ion chromatographic method (Reference UdistiUdisti and others, 2000), named fast ion chromatography (FIC), able to carry out one determination of chloride, nitrate and sulphate every minute. In this way, 788m of European Project for Ice Coring in Antarctica (EPICA) DomeC (EDC 1996) ice core were continuously analyzed with a mean resolution of 4 cm. Some preliminary results on the comparison between chemical (chloride and sulphate) and electrical (ECM, DEP) profiles have already been reported (Reference UdistiUdisti and others, 2000).
In this paper we report the first complete record of chloride, nitrate and sulphate concentrations by FIC for the 6 –788m EDC 1996 depth range, covering Holocene, transition, Last Glacial Maximum (LGM) and the glacial age up to about 45 000 years BP. the data discussion is divided into the climatic periods mentioned above, and comparisons with mean values from other Antarctic ice cores (Vostok, Old Dome C, Byrd, Dome Fuji) are performed. Site variability was also investigated comparing EDC1996 depth/ concentration profiles with those obtained on a parallel 100m deep firn core (Firetracc, drilled in 1998/99). Finally, some evidence of post-depositional effects, able to change the initial concentration of snow depositions in superficial firn layers, is discussed in order to emphasize their role in the palaeoatmospheric reconstruction from ice-core analysis.
2. Sampling and Methods
2.1. Sampling station
Dome C, East Antarctica (Pacific–Indian Ocean sector; 3233 ma.s.l.;75˚06’06’’ S, 123˚23’42’’ E), is one of the two sites (the other being located in Dronning Maud Land, East Antarctica (Atlantic sector)) chosen by the EPICA Steering Committee to carry out deep ice coring down to the bedrock. the main scientific goal of the EPICA Dome C project is to obtain continuous, high-resolution profiles (>3000m depth) of chemical and physical parameters which can be used to reconstruct the environmental and climatic variations over the last 500 000 years (probably 4–5 glacial/ interglacial cycles). In the 1997/98 campaign, about 300 m of the ice core (100–400m depth range) were analyzed by FIC. In the 1998/99 field season, FIC analysis was carried out on the 6–100 and 400–585m depth ranges. Finally, the remaining 200 m ice-core sections (585–788m), whose analysis was not possible at the time of coring because of ice fragility (brittle zone), were processed at the Alfred Wegener Institute, Bremerhaven, Germany, in October 2000. Other parameters measured contemporaneously or after the FIC measurements include CFA, DEP, ECM, classical ion chromatography (IC), stable isotopes, dust, physical properties, heavy metals, etc. (see, e.g.,Reference Wolff, Basile, Petit and SchwanderWolff and others, 1999; Reference MulvaneyMulvaney and others, 2000; Reference JouzelJouzel and others, 2001; Reference MonninMonnin and others, 2001; Reference Delmonte, Petit and MaggiDelmonte and others, 2002).
In the 1998/99 campaign, a 120m firn core, Firetracc, was drilled 300m south of the main drilling site, and the top 100m were analyzed in the field for chloride, nitrate and sulphate by FIC in the same field season.
2.2. FIC measurements
The FIC method used here is based on a coupling of IC and flow analysis techniques (Reference UdistiUdisti and others, 2000). Ice-core sections were continuously melted by a melter device (Reference RöthlisbergerRöthlisberger and others, 2000b). the liquid sample taken from the inner part of the melter, to avoid superficial contamination due to drilling and cutting procedures, is sent to a modified ion chromatograph. A distribution valve allows the sample to be sent alternatively in a pre-concentrator or in the waste. Considering the IC cycle (1 measurement min–1) and the mean melting rate (4 cm min–1), the sampling resolution is 4 cm, corresponding to about 1.5 and 3.5 deposition years in the Holocene and LGM, respectively.
In the first campaign (1997/98) the method was not optimized for low chloride and nitrate values, so their measurements in the 100–360m depth range have to be considered just an indication. Later method improvement allowed reliable determination of nitrate and chloride in the rest of the ice core (6–100 and 360–788m), as confirmed by comparison with classic IC measurements (Reference LittotLittot and others, 2002).
3. Results and Discussion
3.1. Data discussion
Figure 1shows high-resolution Cl–, NO3 – and SO4 2– profiles for the 6–788m depth range of EDC 1996 ice core. the depth scale is divided into four sections, corresponding to the Holocene (6–360m), the transition (360–480m), the LGM (480–600m) and the last third of the glacial period (600–788m). the different periods were approximately established by deuterium (δD) profile by Reference JouzelJouzel and others (2001) and on the basis of the proposed dating by Reference Schwander, Jouzel, Hammer, Petit, Udisti and WolffSchwander and others (2001). Figure 2 shows the median concentration distributions (as mg L–1) of Cl–, NO3 – and SO4 2– in the four periods. the statistical parameters are reported in Table 1. the mean values are heavily influenced by the concentration spikes, especially frequent for sulphate and chloride, while median values are not significantly affected by sudden, low-frequency peaks. Therefore, median values are considered representative of the background levels.
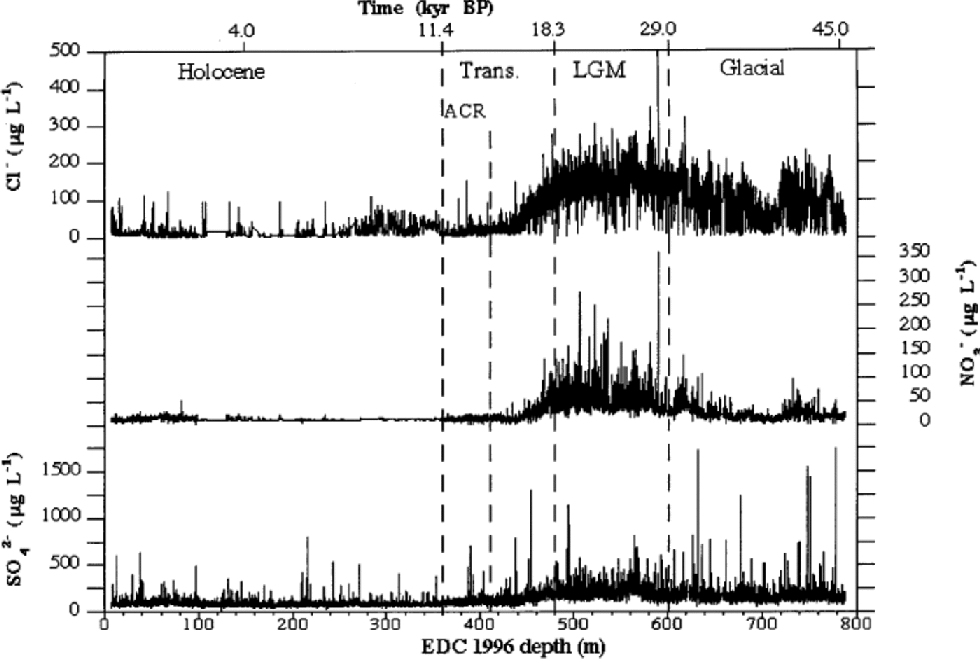
Fig. 1 High-resolution concentration/depth profiles of chloride, nitrate and sulphate along EDC 1996 ice core (6–788 m depth range).
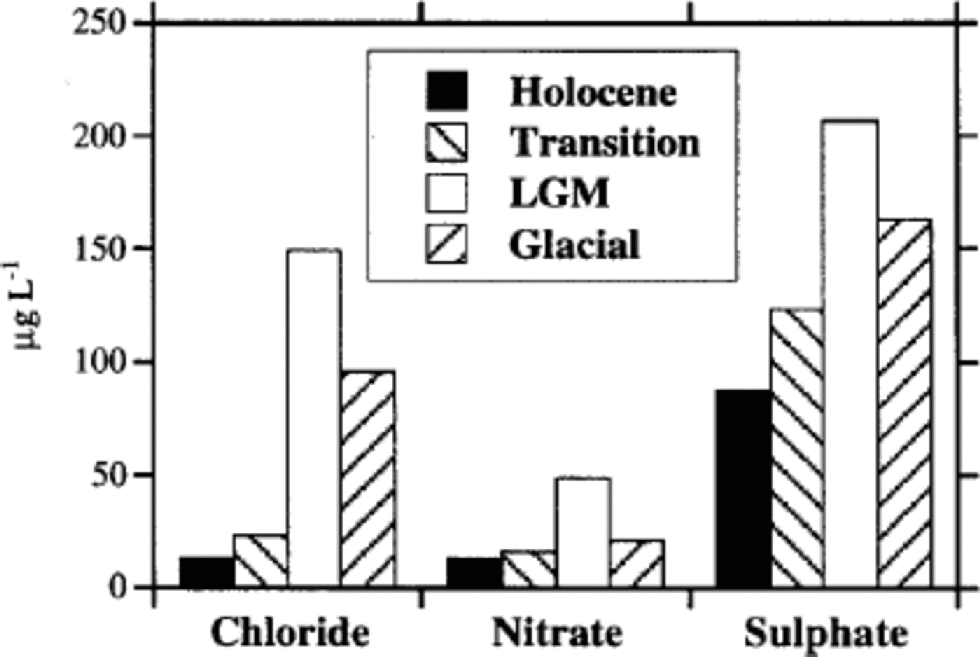
Fig. 2 Histograms reporting mean anionic (Cl–, NO3 –, SO4 2–) content (as mg L–1) in the considered climatic stages (Holocene, transition, LGM, glacial period).
From Figure 1, a common pattern can be observed for all three components. In the glacial age, relatively high concentrations are measured, with a progressive and sharp increase (especially for nitrate) toward the LGM period, when all species reach the highest concentrations. the sulphate, nitrate and, especially, chloride background (median) values are highly modulated by volcanic spikes (sulphate) and by the effects of the fast climatic changes (chloride and nitrate) that characterized this climatic period (Reference MayewskiMayewski and others,1994; Reference PetitPetit and others, 1999). In the glacial/interglacial transition, a sharp concentration decrease, dephased with respect to δD variations, is evident. Indeed, the ``ionic transition’’ from glacial to interglacial conditions appears to end about 80m before the isotopic temperature reaches the typical Holocene high values, with a temporal shift of about 4000 years.
Holocene values are generally constant along the whole climatic period, with low and stable background values, especially for nitrate. the median concentrations range from 2.5 (sulphate) to 11 (chloride) times lower than those measured in the LGM.
Figure 2 shows that sulphate is the dominant anion in all climatic conditions, but its percentage contribution is correlated to temperature, because of the higher anticorrelation with temperature of chloride and, to a lesser extent, nitrate. In fact, the very large concentration decrease of chloride from glacial to interglacial conditions drives the percentage changes of anion composition. In the cold period, the sulphate contribution is about 50% of the main anions total budget, but this percentage significantly increases through the transition, reaching a value of >75% in the Holocene. This pattern is due to the sharp concentration decrease of chloride and nitrate in the warm period.
Chloride concentrations are higher than nitrate concentrations in the cold period, but such differences decrease in the transition and become negligible in the Holocene.
3.2. Chloride
Chloride depositions involve salt species (mainly coming from sea spray: NaCl, MgCl2) and HCl, directly emitted into the atmosphere by natural (e.g. volcanic emissions) or anthropogenic sources, or originated from acid/base exchange between NaCl and atmospheric acidity (mainly H2SO4 and HNO3, but also methanesulphonic acid (MSA) and organic acids) (Reference Shaw, Oeschger and LangwayShaw,1989). In order to evaluate different source contributions and to explain the effects of changes in source intensity, transport efficiency and persistence in snow layers, chloride profiles should be compared with the Na profile, but such a comparison will be made in another paper (personal communication from R. Röthlisberger, 2001). Meanwhile, some interesting features in chloride pattern, in comparison with the other determined anions, can be pointed out.
Table 1. Statistical parameters for EDC 1996 ice core in the climatic period examined

Chloride profile is modulated by a number of spikes superimposed on the background, especially in the first 100 m. Some of these peaks are in phase with ice electric conductivity signatures (ECM and DEP) (Reference UdistiUdisti and others, 2000), revealing acidic species contribution (HCl). Other spikes instead show low ice conductivity and could be related to sea-spray inputs. the environmental and/or climatic significance of these spikes needs to be considered carefully because HCl is heavily affected by post-depositional processes (re-emission into the atmosphere) closely related to the snow acidity and the accumulation rate. Besides, opposite to post-depositional losses, a contamination by HCl uptake from atmosphere in the storehouse at Dome C cannot be excluded in the porous firn (first 100 m). Figure 3 shows a comparison between the first 25m of EDC 1996 and Firetracc ice core. the EDC 1996 ice core shows a larger number of chloride spikes and a higher background value than Firetracc. This difference cannot be due to changes in snow composition (the two ice cores are situated very close together), and a contamination effect appears probable. Indeed, this firn-core section was stored 2 years before analysis, while the Firetracc firn core was analyzed just after the coring. This hypothesis is confirmed by observation of the trend of background chloride differences between EDC 1996 and Firetracc with depth. In the most superficial part (0–25m), where the firn porosity is high, the median Cl– value in the EDC 1996 ice core is about 2.5 times higher than that measured in Firetracc (13.4 and 5.0 μg L–1, respectively). In the deeper firn-core sections (75–100 m), the two values are practically coincident (8.2 and 8.0 μg L–1).

Fig. 3 Chloride and nitrate profiles for the top 25 m depth of Firetracc and EDC 1996 ice cores.
Chloride shows the highest sensitivity to climate changes, with a sharp anticorrelation with temperature (Fig. 3; Table 1). In cold periods Cl– concentration is relatively high, reaching a maximum mean value in the LGM(149.6+40.3 μg L–1). In the Holocene, a very low mean value (19.1+15.3 μg L–1) is measured. Since the mean snow accumulation rate in the Holocene (around 2.8 cm ice equivalent) is higher than in the LGM (around 1.1 cm) (Reference Schwander, Jouzel, Hammer, Petit, Udisti and WolffSchwander and others, 2001), the concentration values have to be corrected by the dilution effect. In fact, assuming similar atmospheric load, the aerosol components are scavenged by a lower wet-deposition quantity (and also dry deposition becomes more important), leading to higher snow concentrations. Assuming a ratio of 2.5 between Holocene and LGM mean deposition rates, the chloride concentration appears to be at least 4 times higher in the LGM than in the Holocene. In the glacial period (Fig. 4) the chloride profile also shows the highest variability, at both high and low frequencies. In particular, in the 680– 720 m depth range, the chloride profile shows a large dip, followedby two other minor dips around 740 and 760 mdepths. Other alternating sequences of peaks and dips are visible in the 530–680m depth range. Reference JouzelJouzel and others (2001) report the δD isotopic profile related to the first 585 m. When the δD isotopic data become available for the 585–788m depth range, the correspondence between chloride and δD trends (anticorrelated) will be checked.

Fig. 4 Detail of chloride profile in 480–788 m depth range corresponding to LGM (a) and the rest of the examined glacial period (b).
Chloride appears to be sensitive to Antarctic Cold Reversal (ACR) variation, quickly increasing background concentrations (twice as high as surroundings) corresponding to the isotopic temperature minimum (about 385 m).
Sources:1This paper. 2 Reference Legrand and DelmasLegrand and Delmas (1988). 3 Reference Legrand, Lorius, Barkov and PetrovLegrand and others (1988). 4 Reference Langway, Osada, Clausen, Hammer, Shoji and MitaniLangway and others (1994). 5 Reference Watanabe, Kamiyama, Motoyama, Fujii, Shoji and SatowWatanabeand others (1999) (estimated from picture).
The sharp changes of chloride in the different climatic conditions are the result of several concomitant and opposite effects. A higher sea-spray atmospheric load in the glacial period was probably related to higher source intensity and more efficient transport processes caused by more intense winds and atmospheric turbulence (Reference PetitPetit and others, 1999). Besides, the lower atmospheric humidity leads to a lower precipitation frequency, increasing the residence time of sea-spray atmospheric aerosol. Finally, changes in chemical composition of precipitation and in snow-accumulation rate also need to be considered. Reference Wagnon, Delmas and LegrandWagnon and others (1999) and Reference Traversi, Becagli, Castellano, Largiuni, Udisti, Colacino and GiovannelliTraversi and others (2000) found that HCl is not stable when the accumulation rate is <50–80kgm–2 a–1 and it is re-emitted into the atmosphere because of its volatility and its mobility in the firn lattice. This appears to be the case with Dome C (Holocene ice accumulation rate ~2.8cma–1, Reference Schwander, Jouzel, Hammer, Petit, Udisti and WolffSchwander and others, 2001). In fact, Figure 3 shows a dramatic decrease of chloride with depth in the uppermost snow layers of the Firetracc ice core. Low Holocene values represent the residual of total chloride deposition after the volatile species (HCl) has been re-emitted into the atmosphere. When snow acidity decreases drastically, as in the glacial period due to the higher dust load (Reference De Angelis, Barkov and PetrovDe Angelis and others, 1987; Reference PetitPetit and others, 1999), more HCl can be fixed as chloride salt in the snow by atmospheric or post-depositional neutralizing processes (acid/base reactions with carbonate and bicarbonate particles) (Reference Röthlisberger, Hutterli, Sommer, Wolff and MulvaneyRöthlisberger and others, 2000a). the lower accumulation rates occurring in glacial periods worked as negative factors towards chloride conservation, but the higher neutralizing effect of the alkaline dust was a prevailing positive factor, resulting in larger chloride concentrations in glacial ice.
In the Holocene, when acidity content does not change significantly, accumulation rate could be the dominant factor in the chloride snow preservation. In fact, a significant increase of background Cl– values is visible in the 270– 360m depth range (roughly corresponding to time period 8420–11400BP; Reference Schwander, Jouzel, Hammer, Petit, Udisti and WolffSchwander and others, 2001), where chloride concentrations 4–5 times higher than typical Holocene levels are measured. Sodium does not show an analogous pattern (unpublished data from R. Röthlisberger), and ECM and DEP measurements, which are more sensitive to acidic contributions, show profiles similar to those of chloride (Reference UdistiUdisti and others, 2000). Therefore, an increase of HCl persistence, probably related to changes in snow-accumulation rates, appears probable. In fact, in the same depth range, the δD profile (Reference JouzelJouzel and others, 2001) shows the highest isotopic temperatures.
Table 2 shows the chloride mean values measured in Old Dome C,Vostok and Byrd ice cores. the three East Antarctic ice cores show similar mean values in both the glacial and Holocene periods. Vostok ice core shows the highest climate sensitivity, with LGM mean concentration 410 times higher than the Holocene value.
Table 2. Mean concentration of chloride, nitrate and sulfate (as μg L–1) from various Antarctic deep ice cores (EDC 1996, Old Dome C, Vostok, Byrd and Dome Fuji) for the four climatic stages

In the Holocene, Byrd ice core shows mean concentrations 2–3 times higher than those measured at Dome C and Vostok. This increase is coherent with the relatively high snow-accumulation rate (about three times higher than at Dome C: R. Udisti, unpublished information) measured at Byrd Station, able to decrease or to prevent HCl re-emission.
3.3. Nitrate
The main contributions of nitrate in the Antarctic ice cap could come from lightning, stratospheric N2O oxidation, cosmic-ray production and continental sources (Reference Legrand and KirchnerLegrand and Kirchner, 1990). Reference Wagenbach, Legrand, Fischer, Pichlermayer and WolffWagenbach and others (1998) concluded that stratospheric inputs (polar stratospheric cloud sedimentation processes and stratospheric to tropospheric transport) control the seasonal pattern of nitrate. Reference JonesJones and others (1999) demonstrated that marine biogenic alkyl-nitrate makes a significant contribution to the summer nitrate budget in coastal areas. In Antarctica, nitrate is supposed to be deposited mainly as gaseous HNO3, dry deposition being considered negligible (Reference Legrand and KirchnerLegrand and Kirchner, 1990).
Nitrate potentially constitutes a proxy-marker to reconstruct the NO x content in the palaeo-atmosphere and the past solar activity (Reference Legrand and KirchnerLegrand and Kirchner, 1990). However, nitrate records in Greenland and Antarctica will yield full information on values and trends of natural and anthropogenic contribution to the global nitrogen budget only when the atmosphere–snow transfer functions are understood, since many aspects of its atmospheric scavenging, deposition processes and post-depositional effects are not yet clear. In addition, there are many uncertainties in the identification and attribution of main and secondary sources of the ice-sheet nitrate budget.
Table 1 and Figure 2 report nitrate mean concentrations in the different climatic periods, and Table 2 shows a comparison with other ice cores.
In the glacial period, nitrate does not show the relatively high values shown by chloride, and also the profile variability is lower, except for large waves in the 600–640 and 720– 760m depth ranges. A very large increase is visible only in the LGM, where a mean value of 53.9+23.8 μg L–1 is measured. In the transition, concentrations rapidly drop to the low Holocene values, with a trend in-phase with the chloride profile. Nevertheless, nitrate does not show the same climatic sensitivity as chloride to the ACR. In fact, no significant increase is observed around 380 m, where the ACR shows the minimum temperature.
Unlike chloride and sulphate, nitrate shows very constant Holocene values without spikes. Although the 1997/98 nitrate measurements (100–360m depth range) were not optimized, the FIC method was able to determine values higher than 5–10 μg L–1, so that nitrate spikes were revealed if they occurred.
The Holocene mean value (12.9+3.0 μg L–1) is similar to that atVostok (15.5 μg L–1) and 50% lower than those at Old Dome C (19.2 μg L–1) and Dome F (20 μg L–1). the low EDC 1996 mean value could be due to bias on nitrate determined by FIC in this depth range, but CFA nitrate data from Reference Röthlisberger, Hutterli, Sommer, Wolff and MulvaneyRöthlisberger and others (2000a) show a mean Holocene concentration around 15.0 μg L–1, confirming our data. the highest Holocene values are measured at Byrd (47.1 μg L–1), where the accumulation rate is three times higher than at Dome C, demonstrating the effect of this parameter on nitrate conservation in snow layers, like chloride.
The LGM/Holocene concentration ratio is about 4.2, similar to that measured at Dome F, whileVostok shows a higher nitrate sensitivity to climate changes (LGM/Holocene ratio = 5.6). Assuming a dilution factor of 2.5 between the snow-accumulation rates in warm and cold conditions, we conclude that a net nitrate increase can be observed only in the LGM, when nitrate content twice that in the Holocene was memorized in the snow layers at Dome C.
Nitrate concentration is shown to be heavily influenced by post-depositional effects in stations with low accumulation rate. Recent studies on superficial snow at Vostok showed a decrease from 100 μg L–1 in the uppermost layers to 20 μg L–1 at 2m depth (Mayewsky and Legrand, 1990; Reference Wagnon, Delmas and LegrandWagnon and others, 1999). This pattern suggests that a large fraction of nitrate is deposited at Vostok as the volatile species HNO3 , and later re-emitted into the atmosphere by processes not yet well understood (Reference Mulvaney, Wagenbach and WolffMulvaney and others, 1998). Photolytic decomposition may be another cause of nitrate depletion in the uppermost snow layers (Reference Honrath, Peterson, Guo, Dibb, Shepson and CampbellHonrath and others, 1999). Figure 3 shows the concentration drop of nitrate in the most superficial layers of Firetracc ice core. Such a nitrate decrease is faster than that of chloride. Indeed, the superficial nitrate concentration (up to 100 μg L–1) drops to Holocene background values (<20 μg L–1) in the first 0.5 m.The analogous chloride concentration decrease occurs in about 3 m. for deeper layers, the very similar median values in the range 0–100m (16.2 μg L–1 for EDC 1996 and 16.5 mg L–1 for Firetracc) confirm the absence of contamination during the ice-core storage.
Like chloride, the higher alkaline dust load in glacial ice neutralized a higher nitrate fraction as non-volatile salt, increasing its persistence in the snow layers (Reference Wolff and DelmasWolff, 1995; Reference Legrand, Léopold, Dominé, Wolff and BalesLegrand and others, 1996). A clear demonstration of this effect is the close correlation between non-sea-spray Ca and nitrate in glacial sections at Dome C (Reference Röthlisberger, Hutterli, Sommer, Wolff and MulvaneyRöthlisberger and others, 2000a).
In the cold period, two opposite effects may have influenced the nitrate preservation in the snow layer: a higher fixation by larger quantities of alkaline aerosol particles and an easier re-emission into the atmosphere due to the reduced snow-accumulation rates. In the LGM, the very high dust content (Reference De Angelis, Barkov and PetrovDe Angelis and others, 1987; Reference PetitPetit and others, 1999) may have been decisive, fixing more nitrate as salt, assuming no variations in source intensity and transport processes. In the remaining glacial age, the greatly reduced dust content may have prevented nitrate preservation, with the result that nitrate concentration drops to quasi-Holocene levels, except for particular ice layers characterized by high Ca content (Reference Röthlisberger, Hutterli, Sommer, Wolff and MulvaneyRöthlisberger and others, 2000a). Probably for the same reason, nitrate appears insensitive to the ACR, when the snow accumulation rate and dust content were relatively low.
The nitrate profile was used to determine whether the chloride increase in the depth range 270–360m could be attributed to an increase in snow-accumulation rate. However, in this depth range, no significant variation in nitrate profile is detectable. Anyway, since chloride is more conservative than nitrate, a slight accumulation-rate increase, able to fix higher chloride content but insufficient to fix higher nitrate concentration, could have occurred.
We conclude that the relationships between nitrate variation in Antarctic ice cores and changes of source intensity or transport processes in the different climatic period need to be considered carefully before mechanisms able to modulate nitrate fixing in snow layers are well understood.
3.4. Sulphate
In Antarctica, the sulphate sources are mainly constituted by primary (sea-spray) and secondary (biogenic emission) marine inputs, and to a lesser extent by crustal and anthropogenic contributions (e.g. Reference Shaw, Oeschger and LangwayShaw, 1989; Reference Legrand and MayewskiLegrand and Mayewski, 1997; Reference MinikinMinikin and others, 1998). Sulphate spikes, attributed to volcanic emissions, are frequently recorded in snow layers. Such sulphate peaks heavily perturb the concentration/depth profile, but only in particular snow layers, and do not affect long-time sulphate background. In the inner ice-sheet regions, the sulphate primary contribution (sea salt and dust) progressively decreases with altitude and distance from the sea (Reference Udisti, Becagli, Castellano, Traversi, Vermigli and PiccardiUdisti and others, 1999), then the main continuous sulphate source is constituted by SO2 produced by oxidation of dimethylsulphide (DMS), emitted into the atmosphere by oceanic phytoplanktonic activity (for a review, see Reference Legrand and MayewskiLegrand and Mayewski,1997).
Biogenic emissions of H2SO4 and MSA can play a relevant role as forcing factors and negative feedback mechanisms of climate, constituting a large percentage of cloud condensation nuclei in remote oceanic regions (Reference Charlson, Lovelock, Andreae and Warren.Charlson and others, 1987). These compounds, once the conservative character of their snow deposition is assessed, could permit the reconstruction of the marine biogenic activity in the different climatic cycles (Reference Legrand and MayewskiLegrand and Mayewski, 1997; Reference MinikinMinikin and others, 1998).
The sulphate profile (Fig. 1) shows many volcanic signatures distributed along the ice core with variable frequencies. These peaks are caused by depositions of sulphuric acid (or, more rarely, neutralized sulphate salt) emitted by volcanicactivity.The reliability of volcanic signatures in the first 100 mis confirmed by the comparison with the sulphate Firetracc profile (Fig.5). This comparison also confirms the background (median) sulphate value, around 100 μgL–1 in the range 0–100m, with a difference <5% between the two ice cores.
The volcanic signatures were used to compare EDC 1996 and Firetracc depth scales: the very good regression line (R = 0.9999) gives slope = 1.0 and intercept = 0.89 m. This depth difference between the two ice cores is probably due to uncertainties in surface estimation at the two sites. the sulphate profile for the most superficial layers shows no post-depositional effects and no increase in background values, confirming that the increasing contribution of anthropogenic sulphur emissions is not significant in Antarctic inner regions (Reference MinikinMinikin and others, 1998).
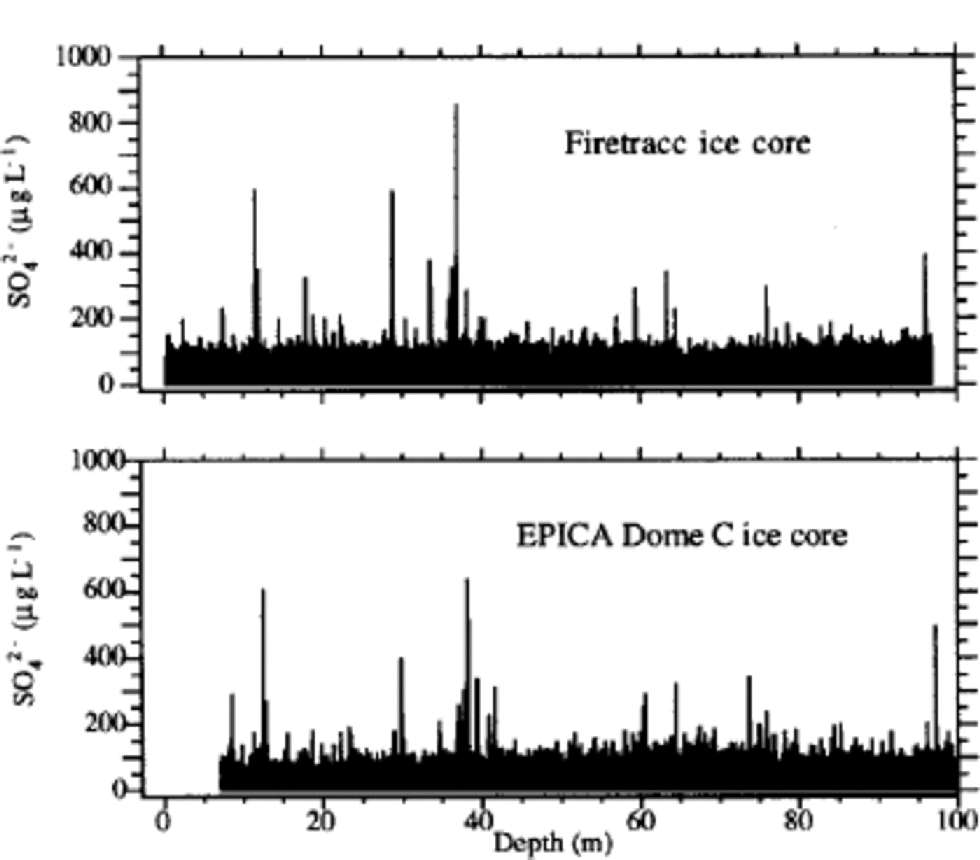
Fig. 5 Sulphate profile for the top 100 m depth of Firetracc and EDC 1996 ice cores.
Figure 3 shows the mean concentration of sulphate in the four climatic periods, and Table 1 reports the basic statistical parameters.
Sulphate median concentrations decrease by about 2–2.5 times from the LGM to the Holocene, showing a lower slope with respect to the chloride and nitrate pattern. This decrease is of the same order of magnitude as the snow-accumulation rate change between the cold and warm periods, so the sulphate increase in glacial ice could be driven almost completely by the reduced frequency of atmospheric scavenging processes, with a relatively low increase of sulphate load in the atmospheric aerosol because of increases in source intensity or transport efficiency.
Table 2 shows the sulphate concentration measured in different Antarctic ice cores for the four climatic periods. By comparing Holocene values, an anticorrelation between concentration and accumulation rate can be observed; the highest values were measured at Vostok, where the snow accumulation rate is about 30% lower than at Dome C (R. Udisti, unpublished information), and the lowest mean concentrations are related to Byrd Station, characterized by a relatively high accumulation rate. Where available, sulphate data show a similar trend among the different stations in the four climatic periods, with concentration about 2–2.5 times higher in the LGM than in the Holocene. the comparison between EDC 1996 and Old Dome C gives very similar values, in spite of the very different resolution (Reference Legrand, Lorius, Barkov and PetrovLegrand and others, 1988).
The increase of sulphate concentration in the glacial age is more evident in Greenland, where it is correlated with Ca content (Reference MayewskiMayewski and others, 1994). That suggests that the larger increase arises from a large increase of crustal sulphate contribution. Acontemporaneous decrease in MSA (Reference Hansson and Saltzman.Hansson and Saltzman, 1993), used as biogenic indicator, seems to exclude an increased biogenic contribution. Therefore, the different sulphate pattern in Arctic and Antarctic ice sheets can be explained by the different crustal inputs in areas with different geographical distribution of continental masses.
From sulphate measurements carried out by Reference Legrand, Lorius, Barkov and PetrovLegrand and others (1988,Reference Legrand, Feniet-Saigne, Saltzman, Germain, Barkov and Petrov1991) onVostok ice core, the low interglacial and higher glacial sulphate values are confirmed for the last interglacial period (Eemian, 98+17 μg L–1) and during the Penultimate Glacial Maximum (208+50 μg L–1).
Assuming that volcanic spikes do not affect median values in warm and cold periods, the residual slight sulphate increase in glacial periods, once concentrations are corrected by accumulation rate, may have been caused by an increased oceanic biogenic activity (Reference Legrand, Feniet-Saigne, Saltzman, Germain, Barkov and PetrovLegrand and others, 1991). This hypothesis is consistent with the supposed tripling of marine productivity during the LGM, with respect to mean Holocene values, in upwelling areas (Reference Sarnthein, Winn, Zahn, Berger and LabeyrieSarnthein and others, 1987).
4. Conclusions
FIC proved to be a reliable and accurate method for analyzing, directly in the field, continuous melted firn- and ice-core sections. Measurements performed on the first 788m of the EDC 1996 ice core yielded high-resolution (about 19 000 determinations for each component, with a resolution of about 4.0 cm) depth/concentration profiles of chloride, nitrate and sulphate for the last 45 000 years of snow deposition in central Antarctica.
From the dataset obtained, the following conclusions can be drawn.
As expected, the Holocene is shown to be a climatic period characterized by high environmental stability, demonstrated by low and stable background concentration values. Whereas the background levels of sulphate depend on source inputs, transport processes and scavenging efficiency, chloride and nitrate concentration values are the result of depositional and post-depositional processes, the latter consisting mainly in the re-emission into the atmosphere of acidic species (HCl and HNO3). Spikes in sulphate concentration are related to volcanic activity, while chloride peaks need to be considered carefully because they can be due to effective source changes (volcanic inputs, sea-spray depositions) but also to changes in snow acidity and/or in accumulation rate, which may change the chloride persistence in the snow layers. ContaminationbyHCluptake from storehouse is highly probable in the firn sections (down to 100m depth).
The glacial/interglacial transition shows the highest variability for all the three compounds. the concentrations decrease rapidly from high glacial values to lower Holocene levels in only 40 m of ice core, covering about 3000 years (18 300–15 300 years BP). This depth interval is just one-third of the entire isotopic temperature transition. Since the temperature increase and chloride, nitrate and sulphate decrease occur almost contemporaneously at the start of deglaciation, the climatic forcing factors must have been the same for climatic and environmental variations, but the atmosphere aerosol composition reached the typical Holocene composition in advance of about 4000 years with respect to the temperature, showing little climatic sensitivity to the ACR (with the exception of chloride).
High background values and spike frequency are visible in the LGM, when chloride and nitrate show net concentration increases, considering the reduced snow-accumulation rate. Despite the lower accumulation rate which may enhance post-depositional HCl and HNO3 re-emissions into the atmosphere, the decreased snow acidity probably caused a higher content of chloride and nitrate in snow layers, by fixing them as non-volatile salts. While source-intensity and transport-efficiency variations appear to explain glacial chloride increase, nitrate increase needs to be considered carefully, because of the higher nitrate sensitivity to change in neutralizing effects and accumulation rate. As a consequence, while chloride also shows high background levels in the rest of the glacial period (45 000–29000 years BP), nitrate deposition is analogous to that in the Holocene, with higher concentration values only in snow layers characterized by high dust content.
A better understanding of atmosphere/snow transfer functions and mechanisms is fundamental for reliable use of chloride and, especially, nitrate, as a proxy-marker of climatic and environmental changes.
Acknowledgements
This work is a contribution to the ``European Project for Ice Coring in Antarctica’’ (EPICA), a joint European Science Foundation (ESF)/European Commission (EC) scientific programme, funded by the EC and by national contributions from Belgium, Denmark, France, Germany, Italy, the Netherlands, Norway, Sweden, Switzerland and the United Kingdom. This is EPICA publication No. 34.









