Introduction
One of the main ice properties that have a major influence on drilling technology is the ice creep that causes borehole deformation under conditions of negative pressure difference on the hole walls. To compensate ice-overburden pressure, drilling in glacial ice requires the use of a borehole fluid that prevents borehole closure.
The ideal borehole fluid will meet the following criteria: (1) fluid density is approximately equal to or a little higher than the ice density; (2) fluid viscosity does not adversely affect the drill’s optimal lowering/hoisting rate; (3) fluid freezing point is lower than not only the minimal temperature in the borehole but also the minimum temperature of the air outside the drilling shelter (where the fluid is usually stored); (4) fluid is not aggressive to the ice core, borehole walls, drill and cable components; (5) fluid does not change its chemical or physical properties during storage, transportation and use in borehole; (6) fluid is non-flammable and non-explosive; (7) fluid is non-toxic to humans, biodegradable and has no other environmental effects; (8) fluid is inexpensive and readily available from markets.
In the practice of deep ice drilling, only three types of borehole fluid have been used: (1) petroleum-based products (e.g. kerosene) with added densifier; (2) aqueous glycol or ethanol solutions; and (3) n-butyl acetate. All of these are far from ideal for use as a borehole fluid because of safety, environmental and other issues (Reference Talalay and GundestrupTalalay and Gundestrup, 2002a).
The borehole fluids of the first category – two-component systems based on petroleum products – impose a high environmental risk since, in the event of a spill, they have a long resistance time, particularly in a cold environment. Even at very low concentrations in the water environment (<1 mgm–3), petroleum fluids can poison microorganisms. The materials used as densifiers in these two-component systems (CFC-11, CFC-113, HCFC-141b) cause depletion of the ozone layer when released into the atmosphere and their use has been limited or banned by the Montreal Protocol.
The borehole fluids of the second category – aqueous glycol or ethanol solutions – have generally low environmental hazard potential. From the technological standpoint, these hydrophilic liquids have two major disadvantages that limit their use as borehole fluids. First, they continuously dissolve the borehole walls, the core and chips until slush plugs are formed in the borehole, resulting in a high risk of equipment loss down the hole. Second, they are extremely viscous at temperatures below 0˚C.
The use of the third borehole fluid – n-butyl acetate – was proposed and introduced by the Polar Ice Coring Office (PICO), University of Alaska Fairbanks, USA. They conducted a chemical literature survey in an effort to identify a borehole fluid (1) with the necessary viscosity and density characteristics to minimize health and safety risks for drillers, (2) which would cause minimal environmental impact, and (3) which would not compromise the scientific integrity of the ice core. Of nearly 250 000 compounds electronically surveyed, 11 potential drilling fluids were identified: anisole, hexanol, heptanol, octanol, p-cymene, s-butyl benzene, pseudocumene, propyl, n-butyl acetate, amyl acetate and propyl proprionate. PICO concluded that of these 11, only n-butyl acetate met their requirements (Reference GosinkGosink, 1989; Reference Gosink, Tumeo, Koci and BurtonGosink and others, 1989).
n-butyl acetate was subsequently used as the borehole fluid on the GISP2 project in Greenland (Reference GosinkGosink and others, 1994), and at Dome Fuji, Antarctica (Reference FujiiFujii and others, 2002). The main problem with its use is the hazard posed to the health of the people working at the coring site. The recommended exposure limit to n-butyl acetate adopted in various countries varies from 40 to 200 ppm (Reference Talalay and GundestrupTalalay and Gundestrup, 2002a). The safety and health hazards associated with n-butyl acetate now cause us to exclude it from the list of desirable borehole fluids.
The search for safe and ecologically friendly borehole fluids is now a major concern for forthcoming ice-drilling projects. The latest investigations for such a fluid were carried out by Ice Coring and Drilling Services (ICDS), University of Wisconsin, USA, (Reference GerasimoffGerasimoff, 2003) and the Department of Geophysics, University of Copenhagen, Denmark (personal communication from J.P. Steffensen, 2006).
ICDS tested a two-compound fluid consisting of Isopar-K and hydrofluoroether HFE-7100 as densifier. Isopar-K is a high-purity isoparaffinic solvent with low odor and low skin irritation. The properties of Isopar-type solvents are similar to the properties of solvents of the Exxsol D family used previously at the North Greenland Icecore Project (NorthGRIP), European Project for Ice Coring in Antarctica (EPICA) Dome C and Dronning Maud Land (DML, Antarctica), ice-drilling projects. To increase the density of the borehole fluid, Isopar-K was blended with the segregated hydrofluoroether, HFE-7100, produced by 3M Corporation. Ordinarily the segregated HFEs have zero ozone depletion potential, but they exhibit extremely high (low hundreds to about 15 000) global warming potential. The segregated HFEs have low viscosity, low toxicity and no flash point. The density of HFE-7100 is 1520 kgm–3 (at 25˚C). Tests show that the mixture of Isopar-K with HFE-7100 separates into two phases over a limited temperature range of –29 to –37˚C which makes it impractical to use in the drillholes.
The University of Copenhagen investigators chose a mixture of Haltermann ESTISOL 240 and Haltermann COASOL for the laboratory and field tests. These products are di-esters of biological fatty acids. By varying the mixing ratio of these two fluids, densities between 860 and 965 kgm–3 can be obtained. These fluids are characterized by low vapor pressure, almost no odor, low toxicity and good biodegradable properties. The main disadvantage of this mixture is its very high viscosity (~20 cS at –25˚C and ~30 cS at –35˚C (1 cS = 10–6m2 s–1)). To achieve the drill’s optimal lowering/ hoisting rate, a borehole with a larger clearance between the drill and the borehole wall may be required. This will lead to a significant increase in the number of cuttings, shortening of run penetration, decrease of ice production rate and so on.
The field tests revealed another disadvantage of the Haltermann ESTISOL 240 and Haltermann COASOL mixture: although considered a non-hazardous material with very simple handling procedures, the mixture spilled on the floor of the drilling shelter, destroying rubber-soled shoes. It is difficult to consider a fluid with such aggressive properties as a safe and ecologically friendly material.
The foregoing short review indicates that there are no known new alternative fluids suitable for use as drilling fluid. We suggest once again that the low-temperature silicone oils might be considered one of the most promising types of drilling fluid.
General Description
The silicone oils are specified as any of a number of polymers containing silicon atoms connected with carbon atoms over atoms of other elements (oxygen, nitrogen, sulfur, etc.). The silicone oils are hydrophobic and inert substances that are stable to water, air, oxygen, metals, wood, paper and plastics. The use of silicone oil as a drilling fluid was first suggested by Reference Fujita, Yamada, Naruse, Mae, Azuma and FujiiFujita and others (1994) who, however, noted that ‘it has a higher viscosity than other candidates and it is expensive’. Japanese drillers therefore chose to use n-butyl acetate as their borehole fluid.
The most suitable kind of silicone oils for deep ice drilling are the low-molecular (or volatile) dimethyl siloxane oils (DSOs). These differ from other silicone, mineral and synthetic oils in that they have the smallest viscosity changes with temperature variation and also in their volatility properties. The common chemical structure of DSOs is:
where n is the average rate of polymerization (low-molecular DSOs have n < 8).
DSOs are used in industry as hydraulic liquids, lubricants, anti-adhesion coverings, foam breakers, cooling agents, dielectrics, etc. They are also often used as the temperature control fluid in laboratory ice deformation tests (personal
communication from T.H. Jacka, 2008).
Companies that manufacture DSOs use different trade names:
DC-200 by Dow Corning Corp. (USA);
KF96 by Shin-Etsu Chemical Co. (Japan);
MS-200 by Midland Silicones (United Kingdom);
Si by Siss (France);
AK by Bayer (Germany);
Lukosil M by Synthesia Kolin (Czech Republic);
PMS by State Institute of Applied Chemistry (Russia).
For distribution purposes, DSOs are subdivided according to their viscosity (usually in centistokes at 20 or 25˚C), which is considered to be the main working characteristic of the silicone oils. In Russia, DSOs are subdivided additionally into two classes due to content of the branching links expressed by the chemical formula:
These two classes are (1) DSOs with a linear structure (without branching links) and (2) DSOs with a branched structure (containing different amounts of branching links).
DSOs with a branched structure are either not manufactured or not distinguished in other countries. In Russia, DSOs with a branched structure have an additional letter ‘r’ at the end of their trade name (in Cyrillic transliteration the letter ‘p’ from the Russian word razvletvlyennyi which in English means ‘branching’).
The characteristics of DSOs with the linear and branched structures are very similar, and the main differences between oils of these two classes are their low-temperature properties. DSOs with the branched structure do not tend to crystallize at low temperatures down to –110˚C due to the presence of branching links. These silicone oils are used as cooling and damping agents in space apparatuses.
Low-molecular DSOs are clear, water-white, tasteless, odorless and neutral liquids. The properties of low-molecular DSOs with the same structures are slightly different from manufacturer to manufacturer (Tables 1 and 2). Moreover, physical and working characteristics of silicone oils can vary in the range specified by manufacturers. For example, Shin-Etsu Chemical Co. (unpublished information) specifies the viscosity variability in the ranges (at temperature of 25˚C) 1.5+0.15 cS for KF96-1.5cs and 2.0+0.2 cS for KF96-2.0cs.
Table 1. Chemical structure and main properties of low-molecular DSOs with viscosity grade of 1.5 cS (Shin-Etsu Chemical Co. data; Reference Alekseev, Skorokhodov and PovarikhinAlekseev and others, 1997

Table 2. Chemical structure and main properties of low-molecular DSOs with viscosity grade of 2.0 cS (Shin-Etsu Chemical Co. data; Reference Alekseev, Skorokhodov and PovarikhinAlekseev and others, 1997)

Density–Viscosity Properties
The expansion coefficient of low-molecular DSOs is in the range (1.0–1.1)×10–3 K–1. Typical values of density, p (kgm–3), and viscosity, v (cS), as a function of temperature, t (˚C), for some low-molecular DSOs are given in Table 3. The density–temperature relationship of KF96-1.5cS has a linear shape at atmospheric pressure. Our processing of the curve published by Reference Fujita, Yamada, Naruse, Mae, Azuma and FujiiFujita and others (1994) results, for KF96-1.5cs, in the equation
Table 3. Density, p (kgm–3), and viscosity, v (cS), of low-molecular DSOs at atmospheric pressure (Shin-Etsu Chemical Co. data; Reference Alekseev, Skorokhodov and PovarikhinAlekseev and others, 1997)

The experiments carried out at the University of Copenhagen found a similar density–temperature relationship for
KF96-2.0cs in the temperature range –58 to 23˚C (Fig. 1):

Fig. 1 Density of low-molecular DSOs vs temperature.
The densities of these oils are equal to the ice density at –45˚C for KF96-1.5cs and at –27˚C for KF96-2.0cs.
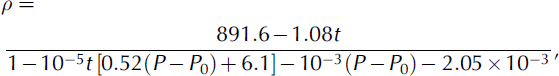
The compressibility of DSOs is high (Table 4). Thus, it is necessary to account for changes in DSO density under hydrostatic pressure in the hole. Processing of the experimental data (personal communication from M. Hazu, 1998) gives the density–temperature–pressure relationship of KF96-2.0cs:
Table 4. Compressibility of PMS-2.0 at room temperature (Reference SobolevskySobolevsky and others, 1985)
where –50 ≤ t ≤ 25˚C, P is the absolute pressure (MPa; 0.1 ≤ P ≤ 35 MPa) and P 0 is the atmospheric pressure (P 0 = 0.1MPa).
Knowing the density–temperature–pressure relationship, it is possible to estimate precisely the difference between the hydrostatic pressure of the fluid and the overburden pressure of ice in order to predict the borehole deformation (Reference Talalay and GundestrupTalalay and Gundestrup, 2002b). The pressure difference estimated theoretically in the borehole filled with type KF96-2.0cs DSO under the temperature and pressure conditions at NorthGRIP and at Vostok station, Antarctica, does not exceed 0.2 MPa at the most critical depths below 2000 m. This is sufficient to prevent borehole closure (Fig. 2). The fluid level is to 60m at NorthGRIP and 100m at Vostok. Due to the high compressibility of KF96-2.0cs, the pressure difference rapidly decreases with depth, and pressure equilibrium is achieved near the borehole bottom. This prognosis confirms the value, from an ice-stress compensation point of view, of using KF96-2.0cs for deep ice drilling.

Fig. 2 Estimated pressure difference in holes filled with KF96-2.0cs type DSO for temperature and pressure conditions at NorthGRIP, Greenland, and Vostok station, Antarctica (liquid level is at 60 m for NorthGRIP and 100 m for Vostok).
The viscosity–temperature relationship of low-molecular DSOs is not linear (Fig. 3). The viscosity of KF96-1.5cs and KF96-2.0cs is about 10 cS at –50˚C, decreasing to near 5 cS at –30˚C.

Fig. 3 Viscosity of low-molecular DSOs vs temperature.
The viscosity, v (cS), of low-molecular KF96-1.5cs type DSOs in the temperature range –58 to –10˚C can be estimated by the equation (from our processing of the curve published by Reference Fujita, Yamada, Naruse, Mae, Azuma and FujiiFujita and others (1994)), as
The tests carried out with KF96-2.0cs type DSO gave a similar approximation in the temperature range –51 to 25˚C (Reference Talalay and GundestrupTalalay and Gundestrup, 2002a):
Due to the long chains, the viscosity of DSOs is highly dependent on pressure, and increases with time. Each 1 MPa of overpressure results in a ~1% increase in the viscosity of the low-molecular DSOs (Reference SobolevskySobolevsky, 1985). Nevertheless, the fluidity of low-molecular DSOs is maintained, even at very high pressures up to 800MPa (Fig. 4).

Fig. 4 Relative change of viscosity of low-molecular DSOs vs pressure (Reference SobolevskySobolevsky, 1985).
The viscosity of KF96-2.0cs predicted for the boreholes under the temperature and pressure conditions of North-GRIP and Vostok is high (Fig. 5) when compared to that for other drill fluids.

Fig. 5 Viscosity of KF96-2.0cs predicted for the boreholes under temperature and pressure conditions at NorthGRIP and Vostok station (liquid level is at 60 m for NorthGRIP and 100 m for Vostok).
While the fluid viscosity determines the travel time of the drill string and, consequently, the total time for drilling, the adverse effects of the higher viscosity of DSOs might be lessened by changing some of the other parameters of the drill’s design. For example, for a hole with a final depth of 3000 m, the optimum range of the drill’s lowering/ hoisting rate might be 0.8–1.0ms–1 (Reference Bobin, Vasiliev, Kudryashov, Stepanov and TalalayBobin and others, 1988). To achieve the desired lowering rate in the more viscous fluid, the clearance between the drill and the borehole wall could be increased. Assuming a borehole with a diameter of 0.13 m, a drill length of 11 m, a drill mass of 130 kg and a fluid density of 930 kgm–3, calculation of the lowering rate for various clearances and fluid viscosities using the methods of Ohishin and others (1990) yields a set of curves (Fig. 6). In this case, a clearance of 8–9mm would provide a lowering rate adequate for Vostok or NorthGRIP.

Fig. 6 Free drill lowering rate vs viscosity at different clearances between drill and hole walls.
The effects of higher-viscosity drill fluids on ice-cutting transport, and also the ability of screens to separate ice cuttings, need examination.
Reactivity, Miscibility and Volatility
DSOs are inert to ice. This has been confirmed by experiments in which ice samples lost no weight over a period of 1 week in contact with KF96-2.0cs (Reference Talalay and GundestrupTalalay and Gundestrup, 2002a). In addition, DSOs do not corrode metals (steel, aluminum, zinc, copper, etc.) or other substances.
DSOs are compatible with most plastics and elastomers. Low-molecular DSOs can extract the plasticizers contained in some types of rubber and plastic, and cause weight and volume loss, with some odor. I have carried out experiments which revealed no visible changes in O-rings and gaskets (ethylene–propylene–diene monomer (EPDM) and nitrile rubber (NBR)) after immersion in KF96-2.0cs for a period of 1 week.
DSOs belong to the typical hydrophobic liquids in which the water wets a liquid/air boundary surface to some extent and, after mixing, the water and the liquid form an emulsion. DSOs are insoluble not only in the water but also in the so-called polar solvents (methanol, ethanol, glycol and glycerine). DSOs are perfectly soluble in the non-polar solvents such as hydrocarbons (gasoline, benzene, toluene), chlorinated hydrocarbons, ethers, esters, and higher alcohols from butanol onward.
DSOs of the different grades can be easily mixed together; this gives additional options to create a borehole fluid with the required density and viscosity properties by the blending of two available standard grades (US Patent 6942820 issued to T. Ihara and others, 13 September 2005).
Low-molecular DSOs evaporate cleanly. DSOs’ use however, does require longer clean-up times than for other borehole fluids. Experiments show that the vaporization rate of KF96-2.0cs is three to four times lower than the rate for solvent Exxsol D60, used as the base fluid at NorthGRIP (Fig. 7). Samples of liquids (10 mL) were poured into a cylindrical glass plate with inner diameter 56 mm (area of free surface 24.6cm2) at room temperature (22.5˚C). The mass loss of the samples was periodically measured with an accuracy of 0.1 g.

Fig. 7 Vaporization rate of solvent Exxsol D-60 and low-molecular KF96-2.0cs type DSO at room temperature.
In the field environment it should not be a problem to wait for the complete evaporation of low-molecular DSOs from the ice-core surface. To shorten clean-up time the contaminated surface can be machined away. Additional testing of DSO compatibility with analytical systems should be completed, but the fact that low-molecular DSOs do not form a film suggests that the core can be processed easily and analyzed with mass spectrometry.
Environmental and Toxicological Properties
Over the past several years, safety and environmental criteria have become more important in the selection of a suitable borehole fluid. Although drilling sites may be outside national boundaries, users should follow recognized standards and recommendations to avoid environmental damage and to maintain a healthy and safe working environment.
A first strategy for environmental control was set out in the Declaration of the United Nations Conference on the Human Environment in Stockholm, Sweden, in 1972. This was confirmed by the Antarctic Treaty Protocol on Environmental Protection, which entered into force on 14 January 1998 following ratification by all Antarctic Treaty Consultative Parties. Article 3 of the protocol declared, ‘The protection of the Antarctic environment and dependent and associated ecosystems . . . shall be fundamental considerations in the planning and conduct of all activities in the Antarctic Treaty area.’ The same environmental requirements were established for the Greenland ice sheet, the northern and northeast parts of which form the largest national park in the world, more than 700 000km2 in area.
All the recent borehole fluids and their components (Exxsol D-series solvents, fluorocarbons, n-butyl acetate) are high-environmental-risk and/or human-hazardous materials (Reference Talalay and GundestrupTalalay and Gundestrup, 2002a). It is difficult to find a chemical that completely meets the technological requirements mentioned above and is harmless to the environment and to human health. Of the millions of existing chemical compounds, the silicone oils are one of the safest and most ecologically friendly materials.
The inertness of DSOs renders these fluids acceptable for use as ingredients in cosmetics, for preventing human skin from chafing and as defoamers for food and beverages. There are no adopted workplace air DSO contaminant levels, specified for a normal 8 hour working day or 40 hour working week, to which all workers may be exposed repeatedly without adverse effect. As a result, there are no recommendations for the control of workplace contaminant concentrations. For example, the US Department of Transportation gives the following certification of DSO health hazards:
Personal protective equipment: Safety goggles.
Symptoms following exposure: Contact of liquid with eyes may cause temporary discomfort. Does not irritate skin. Harmless when ingested.
Treatment of exposure: Except for eye contact, exposures generally do not require treatment. Eyes: flush with water.
TLV-TWA (threshold limit value–time-weighted average): Not listed.
TLV-STEL (TLV–short-term exposure limit): Not listed.
TLV ceiling: Not listed.
Toxicity by ingestion: Currently not available.
Toxicity by inhalation: Currently not available.
Chronic toxicity: None.
Vapor (gas) irritant characteristics: Currently not available.
Liquid or solid characteristics: Currently not available.
Odor threshold: Currently not available.
IDLH (immediately dangerous to life and health) value: Not listed.
OSHA PEL-TWA (US Occupational Safety and Health Administration permissible exposure limits–TWA): Not listed.
OSHA PEL-STEL: Not listed.
OSHA PEL ceiling: Not listed.
EPA AEGL (US Environmental Protection Agency acute exposure guideline): Not listed.
Shin-Etsu Chemical Co., one of the DSOs, manufactories, notes only one potential hazard to human health: in contact with eyes, low-molecular DSOs cause temporary irritation but without permanent harm.
Nashua Corp. indicates that low-molecular DSOs are a questionable carcinogen with experimental neoplastigenic data, and that repeated skin contact may lead to development of dermatitis (Reference GerasimoffGerasimoff, 2003). There is little documentary evidence of the effect of DSOs on the environment. When low-molecular DSOs are discharged in large quantities, aquatic life may be harmed. For bluegill and rainbow trout species, the LC50 (exposure level used in toxicology as a lethal concentration (LC), with the death of 50% of an entire defined experimental animal population) is 10 g L–1.
Ordinary industrial hygiene and satisfactory environmental protection (ventilation of working areas, safety goggles, protection of the open skin, proper organization of the system for fluid storage and dumping, etc.) should easily solve all safety and ecological problems.
Cost
The final choice of the borehole fluid depends on the rational correlation between its cost and other properties. Of the recent and potential borehole fluids, n-butyl acetate is the least expensive (Table 5) and this was one of the main considerations behind its use by US specialists at GISP2 and by Japanese drillers at Dome Fuji in spite of rather high health hazards.
Table 5. Cost of recent and potential borehole fluids

Several researchers are mistaken about the cost of low-molecular DSOs. Reference GerasimoffGerasimoff, 2003 remarked that one of the main disadvantages of using silicone oils is that ‘they are very expensive’. However, the cost of low-molecular DSOs is comparable with the cost of other recent and potential borehole fluids. Whereas the cost of the densifier HCFC-141b has jumped several times during the last few years because of limited stocks, the cost of low-molecular DSOs has decreased over time. For example, the cost of KF96-1.5cs was about US$36 L–1 in 1993 and about US$6.5 L–1 13 years later.
Conclusions
The properties of low-molecular DSOs satisfy all the criteria for use as a deep ice-drilling borehole fluid. DSOs are therefore a very promising candidate for future deep ice-coring operations. The advantages are:
The density of DSOs is right for the compensation of the ice-overburden pressure, and the viscosity is suitable for the output of pumps and for the performance of drill-lowering/hoisting operations.
DSOs are inert to ice, metals and most plastics and elastomers, and have satisfactory volatile properties.
They have no odor and have low toxicity. DSOs are easy to handle, and only the usual commonsense protection is required in the working environment.
Low-molecular DSOs have never been used in ice-core drilling projects. Therefore, any final conclusion as to their suitability for ice deep drilling can only be made after field experiments and after a test borehole has been drilled.
Acknowledgements
I thank Shin-Etsu Chemical Co. Ltd for providing samples of KF96-2.0cs to make it possible to carry out the laboratory tests and for providing data regarding DSO properties. I thank the late N. Gundestrup for his help in test preparations, and V. Chistyakov for fruitful discussions. I also thank D. Lebar of ICDS for constructive and pertinent remarks and for editing the paper.








