Introduction
Appropriate thyroid gland function and activity of thyroid hormones (TH) are considered crucial to sustain the productive performance in domestic animals (growth, milk, hair fibre production) and circulating TH can be considered as indicators of the metabolic and nutritional status of the animals (Riis and Madsen, Reference Riis and Madsen1985; Todini et al., Reference Todini, Malfatti, Valbonesi, Trabalza-Marinucci and Debenedetti2007). Changes of blood TH concentrations are an indirect measure of the changes in thyroid gland activity. Many papers report marked seasonal variation in thyroid activity and in blood TH concentration. These hormone variations are particularly important in the free-ranging and grazing animals, whose main physiological functions (feed intake, reproduction, hair growth) are markedly seasonal. This is the case of small ruminants traditionally reared. Such variations in hormone concentration, in fact, allow the animals to adapt their metabolic balance to different environmental conditions, variations in nutrient requirements and availability, and to homeoretic changes during different physiological stages.
The present paper aims to review and summarise literature data about actions specifically described in domestic small ruminants and the effects that several factors may exert on thyroid activity and circulating TH. Endogenous factors (breed, age, gender, physiological state), environmental factors (climate, season) and nutrition are considered. Many other particular conditions are well known to alter thyroid functions in small ruminants, but they are not discussed in the present paper as they are not physiological: illness, iodine excess or deficiency, ingestion of goitrogenic substances, phytoestrogens and other endocrine-disrupting compounds, exogenous hormones or drugs intake. The values of blood hormone concentrations are characterised by an extreme variability, which is of course very meaningful in each particular study. On the other hand, values reported in different papers are not comparable due to the very large differences of the experimental animals and conditions, as well as assay methods. For this reason, the author’s choice was not to report the absolute numerics of hormone values in the text.
Overview of thyroid hormone physiology
TH, tetra-iodothyronine or thyroxine (T4) and 3-5-3′-tri-iodothyronine (T3), are iodinated derivatives from the amino acid tyrosine. T4 can be deiodinated to the biologically active hormone T3 by a 5′-deiodinase enzyme (outer-ring deiodination), and to the inactive reverse T3 (rT3) by the enzyme 5-deiodinase (inner-ring deiodination) (Utiger, Reference Utiger1995). Thyroid gland of adult sheep contains about 90.4%, 8.8% and 0.7% of T4, T3 and rT3, respectively, and T4 is the main secretory product (about 77%) (Chopra et al., Reference Chopra, Sack and Fisher1975). In adult sheep more than 99.9% of T4 and 99.5% of T3 circulate in blood bound to plasma proteins (Chopra et al., Reference Chopra, Sack and Fisher1975). Only the free hormone is responsible for the biological activity and protein-bound hormones function as a promptly utilisable storage, delaying the effects of decreased thyroid secretion, as well as buffer against sudden increases in thyroid’s secretory activity (Bartalena, Reference Bartalena1990; Utiger, Reference Utiger1995).
Small amounts of the active hormone T3 come from the thyroid, but in adult sheep at least 50% of serum T3 and 97% of serum rT3 derive from monodeiodination of T4 in peripheral tissues (Fisher et al., Reference Fisher, Chopra and Dussault1972; Chopra et al., Reference Chopra, Sack and Fisher1975). Deiodination can occur in most if not all tissues, but the liver and the kidney show the highest deiodinating activity. Iodothyronine deiodinase enzymes are selenoproteins and show structural differences and different tissue distribution between various species (Santini et al., Reference Santini, Chopra, Hurd and Teco1992; Nicol et al., Reference Nicol, Lefranc, Arthur and Trayhurn1994; Chadio et al., Reference Chadio, Kotsampasi, Menegatos, Zervas and Kalogiannis2006). Type I is predominantly expressed in the liver and kidney; it is inhibited by propylthiouracil (PTU) and stimulated by T3. The type II enzyme is predominant in the brain, pituitary, skin, skeletal muscle, brown adipose tissue; it is not sensitive to PTU, but it is inhibited by rT3 and T4 (Kohrle, Reference Kohrle1999). Type III monodeiodinase is a 5-deiodinase, which catalyses the transformation of T3 to 3-3′-diiodothyronine (T2) and of T4 to rT3. The latter does not bind to the nuclear receptor and is considered biologically inactive, but it is a powerful inhibitor of type II deiodinase (Kaiser et al., Reference Kaiser, Goumaz and Burger1986) and decreases oxygen consumption and ATP/ADP ratio (Okamoto and Leibfritz, Reference Okamoto and Leibfritz1997). Type III is widely distributed throughout the body, playing an important role in regulating TH homeostasis and bioavailability (Bianco et al., Reference Bianco, Salvatore, Gereben, Berry and Larsen2002; Bianco and Kim, Reference Bianco and Kim2006). It is particularly expressed in the placenta, in the pregnant uterus and in foetal tissues, limiting TH bioactivity and playing a critical role in the development and maturation of the thyroid axis of the foetus and newborn animal (Galton, Reference Galton2005; Hernandez et al., Reference Hernandez, Martinez, Fiering, Galton and St Germain2006). The functions and regulation of the different deiodinase activities are also a mean for allowing the organism to adapt to changing states such as iodine deficiency or chronic illness (Wartofsky and Burman, Reference Wartofsky and Burman1982; Chopra et al., Reference Chopra, Huang, Beredo, Solomon, Chua, Teco and Mead1985). Earlier, diiodothyronines also were considered inactive metabolites, but recently their thermogenic actions have been highlighted (Moreno et al., Reference Moreno, Lombardi, Beneduce, Silvestri, Pinna, Goglia and Lanni2002).
TH are mostly inactivated by glucuronidation in the liver and secretion into bile, or by sulphation and deiodination in the liver or kidney (Chopra et al., Reference Chopra, Solomon, Chopra, Wu, Fisher and Nakamura1978). Oxidative deamination and decarboxylation occurring in the kidney, liver and muscle, form acid metabolites, which maintain a certain biological activity, but do not contribute to the hormone action in euthyroid subjects because they are produced in very small amounts (Greenspan, Reference Greenspan2001). Decarboxylated derivatives of iodothyronines, such as monoiodothyronamine and thyronamine, actually represent a very interesting field of investigation, because they may have some biological actions, even different from those of TH (Wu et al., Reference Wu, Green, Huang, Hays and Chopra2005).
Thyroid cell growth and all the steps in the synthesis and secretion of TH are stimulated by the pituitary glycoprotein thyrotropin (TSH). TSH synthesis and release are in turn stimulated by the hypothalamic tripeptide TSH-releasing hormone (TRH). The hypothalamus controls the pituitary thyrotrophs also by inhibiting factors (somatostatin, dopamine). Increased plasma levels of TH exert a negative feedback control on both the pituitary and the hypothalamus (Utiger, Reference Utiger1995). Many factors are able to affect thyroid activity and hormone concentrations in blood, acting at the level of hypothalamus, pituitary and/or thyroid gland, as well as on peripheral monodeiodination (Figure 1). In addition, growth factors, prostaglandins, cytokines, by means of paracrine and/or autocrine actions, may modify thyroid cell growth and activity (Greenspan, Reference Greenspan2001).

Figure 1 Schematic representation of the regulation of thyroid gland and thyroid hormones activity.
TH acts on many different target tissues, stimulating oxygen utilisation and heat production in every cell of the body. The overall effects are to increase the basal metabolic rate, to make more glucose available to cells, to stimulate protein synthesis, to increase lipid metabolism and to stimulate cardiac and neural functions (Capen and Martin, Reference Capen and Martin1989). Peculiar actions consist in cell and tissue differentiation. TH are the primary endocrine stimulators of non-shivering (‘facultative’ or ‘adaptive’) thermogenesis, thus regulating body temperature (Silva, Reference Silva2005). One main mechanism of this function should be the stimulation of expression and activity of uncoupling proteins (UCPs), which uncouple re-oxidation of reduced coenymes to ADP phosphorylation, hence producing heat (Collin et al., Reference Collin, Cassy, Buyse, Decuypere and Damon2005). UCPs have been found in various tissues, also in ovine species (Darby et al., Reference Darby, Clarke, Lomax and Symonds1996; Mostyn et al., Reference Mostyn, Wilson, Dandrea, Yakubu, Budge, Alves-Guerra, Pecqueur, Miroux, Symonds and Stephenson2003). Most of the physiological actions of TH are mediated by the binding to nuclear receptors. Recently, several membrane transporters for cellular entry have been identified and they are now considered among the factors on which TH biological activity could depend (Hennemann et al., Reference Hennemann, Docter, Friesema, de Jong, Krenning and Visser2001; Friesema et al., Reference Friesema, Jansen, Milici and Visser2005). As it is the case of steroid hormones some actions of TH are rapid and non-genomic (Davis et al., Reference Davis, Tillman, Davis and Wehling2002; Hiroi et al., Reference Hiroi, Kim, Ying, Furuya, Huang, Simoncini, Noma, Ueki, Nguyen, Scanlan, Moskowitz, Cheng and Liao2006) due to actions on mitochondria and cell membranes on which binding proteins have been identified (Wrutniak-Cabello et al., Reference Wrutniak-Cabello, Casas and Cabello2001; Davis et al., Reference Davis, Davis and Cody2005).
Seasonality of reproduction
In ovine species, a notable interest has been excited by the involvement of TH in seasonal reproduction (Karsch et al., Reference Karsch, Dahl, Hachigian and Thrun1995). In fact, TH play an important function in the expression of endogenous seasonal rhythms of neuroendocrine reproductive activity in sheep, as in many species of birds (Nicholls et al., Reference Nicholls, Follett, Goldsmith and Pearson1988b). Thyroidectomised ewes began their sexual season at the same time as intact animals, but continued to cycle when the intact ewes enter seasonal anoestrus (Nicholls et al., Reference Nicholls, Goldsmith and Dawson1988a; Maurenbrecher and Barrell, Reference Maurenbrecher and Barrell2003). Similar but less-pronounced effects have been obtained in sheep rendered hypothyroid, in which the end of the reproductive season occurred later than in controls (Follett and Potts, Reference Follett and Potts1990; Hernandez et al., Reference Hernandez, Hallford and Wells2003). TH are necessary during a limited period late in the breeding season to permit transition to seasonal anoestrus (Thrun et al., Reference Thrun, Dahl, Evans and Karsch1996 and Reference Thrun, Dahl, Evans and Karsch1997a), acting primarily within the brain to promote inhibition of neuroendocrine reproductive function (Viguié et al., Reference Viguié, Battaglia, Krasa, Thrun and Karsch1999). TH permit the increase of the responsiveness to the oestradiol negative feeback, but are also required for steroid-independent seasonal cycles in luteinising hormone pulse frequency (Anderson et al., Reference Anderson, Connors, Hardy, Valent and Goodman2002). This permissive role of TH seemed limited to changes related to transition to seasonal anoestrus, since thyroidectomy during anoestrus did not affect the onset of the subsequent breeding season (Thrun et al., Reference Thrun, Dahl, Evans and Karsch1997b). Anyway, TH may be required for the long-term expression and maintenance of the endogenous seasonal reproductive rhythm (Billings et al., Reference Billings, Viguié, Karsch, Goodman, Connors and Anderson2002).
In male sheep, thyroidectomy abolished seasonal cycles of gonadotropin secretion and testicular size (Parkinson and Follett, Reference Parkinson and Follett1994; Parkinson et al., Reference Parkinson, Douthwaite and Follett1995).
The anatomical substrate for TH action on seasonal reproduction may be provided by the finding of TH receptor in GnRH and other neurotransmitters–containing neurons (Jansen et al., Reference Jansen, Lubbers, Macchia, DeGroot and Lehman1997). Recently, it has been found that photoperiod regulates the expression of type II deiodinase gene in the mediobasal hypothalamus of the Saanen goat, hence seasonally affecting the bioavailability of TH for the reproductive neuroendocrine axis (Yasuo et al., Reference Yasuo, Nakao, Ohkura, Iigo, Hagiwara, Goto, Ando, Yamamura, Watanabe, Watanabe, Oda, Maeda, Lincoln, Okamura, Ebihara and Yoshimura2006).
To our knowledge, there is only one report about the requirement of TH in seasonal reproduction in goat species, and these results are in contrast with the above-mentioned numerous investigations carried on in sheep: Cashmere goats thyroidectomised in late breeding season advanced the onset of seasonal anoestrus (Walkden-Brown et al., 1996). Furthermore, T3 at the goat testis level induces the synthesis of a soluble protein in Leydig cells, which in turn stimulates androgen release (Jana and Bhattacharya, Reference Jana and Bhattacharya1994; Jana et al., Reference Jana, Halder and Bhattacharya1996).
Hair fibre growth
At the skin level, the availability of bioactive TH may depend not only on the circulating hormone levels but also on the local synthesis of T3. Type II and III, but not type I, deiodinase activity was detected in skin samples from cashmere goats (Villar et al., Reference Villar, Rhind, Dicks, McMillen, Nicol and Arthur1998 and Reference Villar, Nicol, Arthur, Dicks, Cannavan, Kennedy and Rhind2000b) and showed marked individual variability between animals and seasonal changes. Type II deiodinase enzyme was higher during winter short-day photoperiod and lower during periods of long daylength, whereas type III showed an opposite pattern. Manipulations of circulating prolactin affected further the seasonal changes in the ratios of type II and type III deiodinase enzymes, and this was associated with differences in follicle activity and cashmere moult (Rhind et al., Reference Rhind, Kyle and Duff2004). In Soay sheep, showing marked seasonal variations in hair growth rate, the quiescent period corresponded to the seasonal physiological decline in plasma TH concentrations (Lincoln et al., Reference Lincoln, Klandorf and Anderson1980). Studies correlating seasonal changes of plasma TH and cashmere growth cycle failed to ascertain the putative regulatory role of TH (Kloren et al., Reference Kloren, Norton and Waters1993) and contrasting results have been reported (Rhind and McMillen, Reference Rhind and McMillen1995; Merchant and Riach, Reference Merchant and Riach2002; Celi et al., Reference Celi, Seren, Celi, Parmeggiani and Di Trana2003; Rhind and Kyle, Reference Rhind and Kyle2004). To clarify the role of TH on hair fibre production, many investigations have been carried out on manipulating TH availability for hair growth: on the whole, also the results of such papers were often contradictory (Ryder, Reference Ryder1979; Maddocks et al., Reference Maddocks, Chandrasekhar and Setchell1985; Hynd, Reference Hynd1994; Rhind and McMillen, Reference Rhind and McMillen1996). It seems that the sensitivity to TH failure or excess may be dependent on breed, season and interactions with other regulatory factors. TH action may be permissive rather than inductive, i.e. they might be present above certain threshold levels. Very important should be the interactions with other factors: firstly prolactin (Villar et al., Reference Villar, McMillen, Dicks and Rhind2000a; Rhind et al., Reference Rhind, Kyle and Duff2004), as well as the local actions of insulin (Puchala et al., Reference Puchala, Pierzynowski and Sahlu1998) and growth factors, such as EGF (Hoath et al., Reference Hoath, Laksmanan, Scott and Fisher1983). The putative effects of TH on hair fibre diameter are very interesting from a commercial and technological viewpoint. In an earlier study, it was reported that exogenous T4 administration to intact sheep induced increased wool growth, in terms of increased fibre length, without affecting the diameter (Hart, Reference Hart1957). T4, but not T3, reduced fibre diameter in sheep supplemented with selenium (Donald et al., Reference Donald, Langlands, Bowles and Smith1994) but T4 administration failed to avoid the increase in wool diameter following increased feed intake (Lee et al., Reference Lee, Thornberry and Williams2001). Angora goats rendered hyperthyroid by daily subcutaneous injections of T4 showed increased mohair growth, with higher fibre length and lower fibre diameter (Puchala et al., Reference Puchala, Prieto, Banskalieva, Goetsch, Lachica and Sahlu2001). In Angora kids supplemented with energy and protein (horse bean), the higher plasma TH were associated with increased fibre length, decreased fibre diameter and higher percentage of active secondary follicles than controls (Todini et al., Reference Todini, Malfatti, Barbato, Trabalza-Marinucci, Acuti, Antonini and Debenedetti2005). Anyway, further investigations are needed in order to clarify the role of TH in hair fibre production. This role should be rather different between animals showing a marked seasonality and clear moulting cycles (such as cashmere goats) and animals whose hair growth is more or less continuous throughout the year (Angora goats, Merino sheep).
Foetal life
In foetal sheep, during the last one-third period of gestation, serum T4 concentrations were slightly higher or comparable with those in adult sheep, while foetal serum T3 were much lower and rT3 much higher. The elevated rT3 concentrations in foetal sheep serum decreased progressively after birth and reached comparable levels with those in adults, within few days of life (Chopra et al., Reference Chopra, Sack and Fisher1975). An opposite trend was described for T3 concentrations (Nathanielsz et al., Reference Nathanielsz, Silver and Comline1973; Klein et al., Reference Klein, Oddie and Fisher1978). These differences in serum hormone concentrations have been related to differences in peripheral deiodinase activity as the relative thyroidal content of T4 and T3 was similar in foetal and adult sheep (Chopra et al., Reference Chopra, Sack and Fisher1975). In fact, type I deiodinase activity in the liver and kidney of foetus up to the fourth month was lower than that in pre-term foetus or in the newborn (Wu et al., Reference Wu, Polk, Wong, Reviczky, Vu and Fisher1992). Low foetal T3 levels are maintained also by sulphation and deiodination (Wu et al., Reference Wu, Polk, Huang, Green, Thai and Fisher2006). In the foetus, low T3 levels allow anabolic processes to prevail, despite the high rate of foetal T4 secretion, which resulted eight-fold than maternal one during the last one-third period of gestation (Dussault et al., Reference Dussault, Hobel and Fisher1971). The pre-partum cortisol surge increased hepatic renal and perirenal adipose tissue type I deiodinase, and reduced renal and placental type III deiodinase activities (Forhead et al., Reference Forhead, Curtis, Kaptein, Visser and Fowden2006). The increased availability of active T3 is important for the latter phases of tissue differentiation. The functional development of brown adipose tissue allows to optimise non-shivering thermogenesis, thus permitting an adequate thermoregulation in the newborn (Schermer et al., Reference Schermer, Bird, Lomax, Sheperd and Symonds1996). Therefore, UCP1, induced by TH, is of primary importance for the transition from foetal to neonatal life, when cellular energy and thermoregulatory requirements are at maximal rates (Symonds et al., Reference Symonds, Mostyn, Pearce, Budge and Stephenson2003). When the pre-partum rise of cortisol occurs, TH may also influence the growth and development of foetal liver and skeletal muscle, modulating the local activity of the somatotropic axis, i.e. the local expression of growth hormone receptor and insulin-like growth factors (Forhead et al., Reference Forhead, Li, Gilmour and Fowden1998, Reference Forhead, Li, Saunders, Dauncey, Gilmour and Fowden2000 and Reference Forhead, Li, Gilmour, Dauncey and Fowden2002). At the same time TH are essential for foetal glucogenesis (Fowden et al., Reference Fowden, Mapstone and Forhead2001), allowing the pre-partum rise in glucose-6-phosphatase and phosphoenolpyruvate carboxykinase activity in the foetal liver and kidney Forhead et al., Reference Forhead, Poore, Mapstone and Fowden2003).
Age effects: birth, neonatal period and growth. Gender effects
The pre-partum cortisol rise is accompanied by an increase in foetal T3 and a decrease in rT3 concentrations (Sensky et al., Reference Sensky, Roy, Barnes and Heath1994). This pattern should be maintained throughout the early postnatal life (Nathanielsz et al., Reference Nathanielsz, Silver and Comline1973; Klein et al., Reference Klein, Oddie and Fisher1978). Plasma free T3 (fT3) in neonatal lambs increased parallel to total T3 (Cabello and Wrutniak, Reference Cabello and Wrutniak1986), whereas the neonatal increase of free T4 (fT4) concentrations was greater and longer lasting than total T4 (Cabello and Wrutniak, Reference Cabello and Wrutniak1990). In fact, neonatal plasma T3 and fT4 rises followed that of TSH concentrations, lasting for 24 h after birth, but T4 levels declined before (after 2 h of life), when TSH levels were still elevated (Cabello and Wrutniak, Reference Cabello and Wrutniak1990). Therefore, the thyroid gland seems unable to respond, in terms of T4 secretion, to a prolonged stimulation by TSH, probably because a depletion of hormonal stores in the gland occurs during the first minutes of life (Slebodzinski, Reference Slebodzinski1972). It is likely that during the first hours of life the thyroid gland can respond to other stimulating factors: small increases of plasma TH followed exogenous prolactin administration in neonatal lamb, but not in growing lambs and ewes (Peeters et al., Reference Peeters, Buys, Vanmontfort, van Isterdael, Decuypere and Kuhn1992). Plasma rT3 levels during the first 48 h of life progressively decreased in suckling lambs, but increased in bottle-fed lambs (Cabello and Wrutniak, Reference Cabello and Wrutniak1986 and Reference Cabello and Wrutniak1990). Plasma T4 concentrations were higher in single lambs than in twins at birth (Assane and Sere, Reference Assane and Sere1990). Plasma TH levels highly correlated with lambs’ birth-weight (Dwyer and Morgan, Reference Dwyer and Morgan2006) and were lower in lambs separated from their mothers just after parturition than in those maintained with their mothers (Firat et al., Reference Firat, Ozpinar, Serpek and Haliloglu2005). Neonatal lambs had higher levels of T3 and T4 compared with growing lambs and ewes (Peeters et al., Reference Peeters, Buys, Vanmontfort, van Isterdael, Decuypere and Kuhn1992). Growing goat kids displayed higher TH levels than adults (Colavita et al., Reference Colavita, Debenedetti, Ferri, Lisi and Lucaroni1983) and the lowest values were found in elderly animals (Table 1; Lucaroni et al., Reference Lucaroni, Todini, Malfatti and Debenedetti1989). Age-related differences were particularly evident during the hot season, especially for T3 blood concentrations (Lucaroni et al., Reference Lucaroni, Todini, Malfatti and Debenedetti1989).
Table 1 Serum thyroid hormone concentrations (mean ± s.d.) in goats (local Umbrian population) at different ages (data grouped from samplings at different seasons), adapted from Lucaroni et al. (1989)
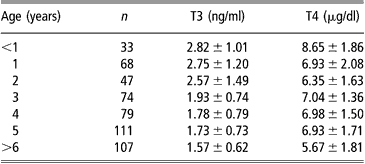
In young animals, there is no sex-dependent differences in blood TH concentrations, whereas in adult goats mean plasma TH levels were higher (significantly for T4) in does than in bucks (Table 2; Todini et al., Reference Todini, Lucaroni, Malfatti, Debenedetti and Costarelli1992). In young cashmere goats, T3 levels were lower in males than in females after 8 months of age, while T4 was not affected by sex (Celi et al., Reference Celi, Seren, Celi, Parmeggiani and Di Trana2003). Sex-related differences are reported in others mammals and are referred to several actions by sexual steroid hormones: differences in total T4 levels can be explained by oestrogen-reduced catabolism of thyroxine-binding globulin (TBG) (Ain et al., Reference Ain, Mori and Refetoff1987), or androgen inhibition of the synthesis of TBG by the liver (Federman et al., Reference Federman, Robbins and Rall1958). Moreover, androgens inhibit TSH secretion by the pituitary (Christianson et al., Reference Christianson, Roti, Vagenakis and Braverman1981).
Table 2 Plasma thyroid hormone concentrations (mean ± s.d.) in 16 adult does and 8 adult bucks (dairy Mediterranean breeds), maintained sex-separated and fed a qualitatively constant diet throughout the year (weekly samplings). Monthly mean, minimal and maximal environmental temperatures are also indicated (adapted from Todini et al. (1992)).

Breed effects
To our knowledge, there are no published data on goat breed differences. At birth, Blackface lambs had higher T3 and T4 levels than Suffolk lambs and this was correlated with higher body temperature and better thermoregulatory ability (Dwyer and Morgan, Reference Dwyer and Morgan2006). Merino lambs aged 2 to 3 days, submitted to cold stress, showed a stronger increase of TH levels compared with Romney-Marsh lambs (Doubek et al., Reference Doubek, Slosarkova, Fleischer, Malà and Skrivanek2003). Lamb breeds that are usually reared under extensive conditions (hill regions) have an improved thermoregulation than those reared intensively in lowland: this is partly related to birthcoat characteristics, accompanied by higher TH concentrations (important for endogenous heat production and hair growth) in hill than lowland lambs (Dwyer and Lawrence, Reference Dwyer and Lawrence2005). Assaf ewes had higher serum T4 concentrations than Rasa Aragonesa and Merino ewes, which was associated with differences in wool growth rate (Abecia et al., Reference Abecia, Valares and Forcada2005). Higher plasma T4 levels in Suffolk ewes than Gulf Coast native ewes in the US were shown to be positively related to larger body size and enhanced growth potential (Williams et al., Reference Williams, Calmes, Fernandez, Stanley, Lovejoy, Bateman, Gentry, Gantt and Harding2004). Higher levels of T3 and T4 in ram lambs have been associated with higher prolificacy of the Outaouais breed compared with the Suffolk breed (lower prolificacy) (Fallah-Rad and Connor, Reference Fallah-Rad and Connor1999). The decline in serum T4 levels induced by feed restriction was greater in crossbreed ewes than in native Indian sheep (Naqvi and Rai, Reference Naqvi and Rai1991).
Changes during oestrus, pregnancy, peri-parturient period and lactation
During induced or spontaneous oestrus in goats, a rise in plasma total T4 (Colavita and Malfatti, Reference Colavita and Malfatti1989) and fT4 (Blaszczyk et al., Reference Blaszczyk, Udala and Gaczrzewicz2004) levels has been observed. In ewes, plasma T4 levels were higher during oestrus and lower during the luteal phase, T3 concentrations were higher during the luteal phase, while the concentrations of rT3 were not associated with the oestrous cycle (Peeters et al., Reference Peeters, Buys, Pauwels, Kuhn, Decuypere, Siau and Van Isterdael1989).
During pregnancy, thyroid activity and circulating hormone levels are reported to increase in all the investigated mammalian species. Several mechanisms have been claimed to explain these observations: increased binding protein concentrations in plasma, secretion of thyrotropic factors by the placenta, enhanced responsiveness of pituitary TSH secretion to hypothalamic TRH and changes in maternal TH catabolism (De Leo et al., Reference De Leo, la Marca, Lanzetta and Morgante1998; Glinoer, Reference Glinoer2001). Towards the end of pregnancy, the goat foetus(es) should play a competitive role (higher thyroid activity, iodine affinity and uptake than maternal ones), so that a decrease in maternal plasma fT4 concentrations has been observed (McDonald et al., Reference McDonald, Stocks, Connell and Hoey1988). Plasma T3 and T4 levels in goats at mid-pregnancy rised compared with the low levels observed just before oestrus and mating. Then, during the second half of pregnancy, maternal hormone levels progressively decrease, probably because of the negative energy balance (Todini et al., Reference Todini, Malfatti, Valbonesi, Trabalza-Marinucci and Debenedetti2007). This is supported by the lower maternal serum TH levels (more marked and significant for T4) observed in twin-bearing does, that are often characterised by negative energy balance, compared with aborted and single-bearing does (whose energy balance is usually less negative) (Manalu et al., Reference Manalu, Sumaryadi and Kusumorini1997). Very similar findings are reported for ewes. Plasma T4 concentration was highest during early pregnancy and decreased gradually, reaching lowest values during late pregnancy and post partum (Assane and Sere, Reference Assane and Sere1990; Okab et al., Reference Okab, Elebanna, Mekkawy, Hassan, Elnouty and Salem1993; Yildiz et al., Reference Yildiz, Balikci and Gurdogan2005). Like in goats, maternal T3 and T4 in twin pregnancy were lower compared with single-bearing sheep (Yildiz et al., Reference Yildiz, Balikci and Gurdogan2005), especially at the end of pregnancy (Assane and Sere, Reference Assane and Sere1990).
In goats, maternal plasma T3 levels remained rather steady around parturition, while T4 concentrations markedly decreased and remained low until day 10 post partum (Lucaroni and Todini, Reference Lucaroni and Todini1989). Khan and Ludri (Reference Khan and Ludri2002b) reported that both TH concentrations did not change from day 20 before parturition until the day of kidding, when they reached a minimal level, followed by an increase until day 20 post partum. In ewes, plasma TH concentrations were lower post partum than during pregnancy (Okab et al., Reference Okab, Elebanna, Mekkawy, Hassan, Elnouty and Salem1993), tended to decrease from 36 h to 21 days post partum and thereafter constantly rose until day 51 post partum (Bekeova et al., Reference Bekeova, Elecko, Krajnicakova, Hendrichovsky and Maracek1991).
Blood TH levels were low at the beginning of lactation, afterwards gradually rose in does (Riis and Madsen, Reference Riis and Madsen1985; Emre and Garmo, Reference Emre and Garmo1985) and in ewes (Mitin et al., Reference Mitin, Mikulec and Karadjole1986). Administration of TH is known to stimulate lactation in many species (Tucker, Reference Tucker1994 and Reference Tucker2000) but an inverse relationship between blood hormone concentration and milk yield has been observed in goats (Riis and Madsen, Reference Riis and Madsen1985), at least during the first phases of lactation. In ewes, during late lactation, the increase of T4 concentration in blood seems related to the decrease of milk production (Bass, Reference Bass1989).
Within the first 20 days post partum, in twin-bearing does, plasma TH levels were significantly lower compared with single-bearing does (Khan and Ludri, Reference Khan and Ludri2002a), but throughout lactation very slight or no differences between single and twin-suckling ewes were found (Bass, Reference Bass1989; Rhind et al., Reference Rhind, Bass, Doney and Hunter1991). Taken together, these findings may support the meaning of blood TH levels as indicators of the energy balance, also in lactating animals.
Circadian rhythms
Circadian changes in hormone secretion are probably associated with the rhythms of environmental temperature and light, as well as with feed intake and metabolism, which in turn are related to the alternance activity/rest throughout the day. Moreover, overlapping effects by season and physiological state are expected. Because many factors can influence T4 and T3 levels and because interactions between these factors are likely, the few data available in the literature on such topics are rather discordant.
Blood samplings at 4-h intervals in late spring did not permit to find significant circadian differences in TH concentrations in lactating (milked or suckled) goats, but the maximal levels were observed during the night (Lucaroni et al., Reference Lucaroni, Todini, Malfatti and Debenedetti1989). In ewes sampled twice a day, the differences between morning and afternoon were not univocal, depending on the season (Ashutosh et al., Reference Ashutosh, Dhanda and Kundu2001). In ewes sampled at 2-h intervals, lowest blood hormone levels were found in the afternoon, concentrations then increased progressively during the night, and reached the highest levels in the morning (Velasquez et al., Reference Velasquez, Souza, Oba and Ramos1997). In winter, T3 and T4 concentrations reached maximal levels in early morning, probably because of a delayed response to cold stress to which the animals were exposed by night; furthermore, the circadian variations in winter decreased with the increase in wool length (Salem et al., Reference Salem, Elsherbiny, Khalil and Yousef1991). Combining the results obtained from samplings carried out every 2 months for 1 year, rams showed the highest TH concentrations during the afternoon and the lowest in the early morning (Souza et al., Reference Souza, Bicudo, Uribe-Velasquez and Ramos2002).
Season effects
A major exogenous regulator of thyroid gland activity is the environmental temperature (Dickson, Reference Dickson1993), so an inverse relationship between ambient temperature and blood TH concentrations has been found in sheep (Valtorta et al., Reference Valtorta, Hahn and Johnson1982; Webster et al., Reference Webster, Moenter, Woodfill and Karsh1991; Starling et al., Reference Starling, da Silva, Negrao, Maia and Bueno2005) and goats (Colavita et al., Reference Colavita, Debenedetti, Ferri, Lisi and Lucaroni1983; Todini et al., Reference Todini, Lucaroni, Malfatti, Debenedetti and Costarelli1992).
During heat stress, blood T3 and T4 concentrations, as well as metabolic rate, feed intake, growth and milk production were decreased (Valtorta et al., Reference Valtorta, Hahn and Johnson1982; Silanikove, Reference Silanikove2000). On the other hand, cold stress in ewes (Hocquette et al., Reference Hocquette, Vermorel, Bouix, Anglaret, Donnat, Leoty, Meyer and Souchet1992) ram lambs (Ekpe and Christopherson, Reference Ekpe and Christopherson2000; Doubek et al., Reference Doubek, Slosarkova, Fleischer, Malà and Skrivanek2003) and shearing (Morris et al., Reference Morris, McCutcheon and Revell2000; Merchant and Riach, Reference Merchant and Riach2002) induced increases in blood TH levels. The seasonal pattern of blood TH levels often showed maximal values during winter (cold months) and minimal during summer (hot months) (Salem et al., Reference Salem, Elsherbiny, Khalil and Yousef1991; Webster et al., Reference Webster, Moenter, Woodfill and Karsh1991; Okab et al., Reference Okab, Elebanna, Mekkawy, Hassan, Elnouty and Salem1993; Menegatos et al., Reference Menegatos, Goulas and Kalogiannis2006). However, contrasting results have been reported (Kloren et al., Reference Kloren, Norton and Waters1993; Rhind et al., Reference Rhind, McMillen, Duff, Hirst and Wright1998; Ashutosh et al., Reference Ashutosh, Dhanda and Kundu2001; Yokus et al., Reference Yokus, Cakir, Kanay, Gulten and Uysal2006). In the Sahel desert, plasma T3 and T4 levels did not change significantly from the beginning of the cool season (December) until the end of the dry warm season (May), but a highly significant rise of both hormones was observed at the onset of the humid warm season (June) (Assane and Sere, Reference Assane and Sere1990). It can be supposed that an enhanced thyroid activity during the humid warm season in such environments is functional for the animals facing the increased availability of food (quantity and quality), following the seasons characterised by food shortage.
Blood TH concentrations were high in spring (increasing daylength) and low in autumn (decreasing daylength), which was not fully explained by the changes in environmental temperature (Figure 2; Buys et al., Reference Buys, Peeters, De Clerck, Van Isterdael, Kuhn and Decuypere1990; Todini et al., Reference Todini, Lucaroni, Malfatti, Debenedetti and Costarelli1992; Rhind and McMillen, Reference Rhind and McMillen1995: Clariget et al., Reference Clariget, Forsberg and Rodriguez-Martinez1998; Rhind et al., Reference Rhind, McMillen, Duff, Kyle and Wright2000; Taha et al., Reference Taha, Abdel-Gawad and Ayoub2000; Villar et al, Reference Villar, McMillen, Dicks and Rhind2000a; Merchant and Riach, Reference Merchant and Riach2002; Souza et al., Reference Souza, Bicudo, Uribe-Velasquez and Ramos2002; Blaszczyk et al., Reference Blaszczyk, Udala and Gaczrzewicz2004; Zamiri and Khodaei, Reference Zamiri and Khodaei2005; Menegatos et al., Reference Menegatos, Goulas and Kalogiannis2006; Todini et al., Reference Todini, Delgadillo, Debenedetti and Chemineau2006). It seems that when the temperature ranges are not extreme (mild climate, indoor housing, shelter in the night time), the effect of photoperiod and season-dependent TH profiles (mainly related to the daylength changes) are present.

Figure 2 Circannual profiles of mean plasma T3 (3-5-3′-triiodothyronine) and T4 (thyroxine) in 20 female goats (local Umbrian population), mean environmental temperature, daylength and physiological state (modified from Lucaroni et al. (Reference Lucaroni, Todini, Malfatti and Debenedetti1989)).
In Alpine and Saanen bucks exposed to artificial photoperiodic cycles, alternating 1 or 2 months of long days (LD: 16 h light and 8 h dark) to 1 or 2 months of short days (SD: 16 h dark and 8 h light), plasma T3 levels rapidly followed the photoperiodic changes, increasing during LD and decreasing during SD. The effects of daylength changes on plasma T4 concentrations were seen after a delay of several weeks and the T3:T4 ratio showed very marked variations, increasing during LD and decreasing during SD (Todini et al., Reference Todini, Delgadillo, Debenedetti and Chemineau2006). Similar results were obtained by Lincoln et al. (Reference Lincoln, Klandorf and Anderson1980) in rams submitted to an alternance of 16 weeks of SD and 16 weeks of LD. The mechanisms of the photoperiodic effects on peripheral TH are far from being elucidated. Additional data on actions of the photoperiod in the brain are scanty in small ruminants: TRH from hypothalamic perfusate samples of ewes only tended to be significantly higher during LD than during SD (Leshin and Jackson, Reference Leshin and Jackson1987). Long days suppressed the expression of monodeiodinase gene in the hypothalamus of goats, thus limiting the local bioavailability of TH, which should be related to the role of the thyroid gland in seasonal reproduction (Yasuo et al., Reference Yasuo, Nakao, Ohkura, Iigo, Hagiwara, Goto, Ando, Yamamura, Watanabe, Watanabe, Oda, Maeda, Lincoln, Okamura, Ebihara and Yoshimura2006).
On the basis of the above-quoted studies, it is not possible to discriminate between the relative role of temperature and photoperiod on the seasonality of thyroid activity, in different environmental conditions. Moreover, when the feed intake is markedly seasonal, it becomes a major factor modifying the seasonal pattern of blood TH profiles.
Nutrition effects
T3 directly stimulates feed intake at the hypothalamic level (Kong et al., Reference Kong, Martin, Smith, Gardiner, Connoley, Stephens, Dhillo, Ghatei, Small and Bloom2004), while on the other hand, the quantity and quality of food eaten is a major factor determining plasma concentrations of TH (Dauncey, Reference Dauncey1990). Blood TH levels are considered to be good indicators of the nutritional status of an animal (Riis and Madsen, Reference Riis and Madsen1985) and were correlated with feed intake in ruminant species, including those that exhibit very marked seasonal cyclicity in feed intake, body weight and reproductive activity, e.g. deers (Ryg and Langvatn, Reference Ryg and Langvatn1982; Chao and Brown, Reference Chao and Brown1984; Rhind et al., Reference Rhind, McMillen, Duff, Hirst and Wright1998).
Circulating TH concentrations seem better correlated with feed intake than adiposity status (McCann et al., Reference McCann, Bergman and Beermann1992; Caldeira et al., Reference Caldeira, Belo, Santos, Vazques and Portugal2007a and Reference Caldeira, Belo, Santos, Vazques and Portugalb).
Energy deprivation decreased concentrations of T3 and fT3 in adult sheep, while subsequent overnutrition increased them. Plasma total T3 concentrations significantly correlated with energy and nitrogen balances. Plasma rT3 levels showed an opposite pattern, increasing during energy deprivation and decreasing during overnutrition (Blum et al., Reference Blum, Gingins, Vitins and Bickel1980). Concentrate supplementation induced an increase of plasma T4 levels in lactating ewes (Shetaewi and Ross, Reference Shetaewi and Ross1991) and plasma T3 concentrations was higher in rams with high amounts of ingested energy and protein (Zhang et al., Reference Zhang, Blache, Blackberry and Martin2004). Following feed restriction or food deprivation, plasma TH concentrations were reduced in sheep (Naqvi and Rai, Reference Naqvi and Rai1991; Wronska-Fortuna et al., Reference Wronska-Fortuna, Sechman, Niezgoda and Bobek1993; Wester et al., Reference Wester, Britton, Klopfenstein, Ham, Hickok and Krehbiel1995; Ekpe and Christopherson, Reference Ekpe and Christopherson2000; Abecia et al., Reference Abecia, Zuniga and Forcada2001; Rae et al., Reference Rae, Rhind, Miller and Brooks2002). Feed-restricted animals also showed an earlier and more marked decline in plasma TH concentrations during the late summer/early autumn, compared with ad libitum fed animals (Rhind et al., Reference Rhind, McMillen, Duff, Hirst and Wright1998 and Reference Rhind, McMillen, Duff, Kyle and Wright2000).
Lactating Angora does and their kids supplemented with energy and protein (horse bean) had higher plasma TH concentrations than controls (Todini et al., Reference Todini, Malfatti, Barbato, Trabalza-Marinucci, Acuti, Antonini and Debenedetti2005). Goats with a slightly higher energy intake showed higher plasma TH concentrations during the second half of gestation, and the decrease of plasma TH in mid- and late gestation was attenuated and delayed (Todini et al., Reference Todini, Malfatti, Valbonesi, Trabalza-Marinucci and Debenedetti2007). These effects suggested that energy balance could play a major role in affecting the decrease in plasma TH levels usually observed at the end of gestation in small ruminants (see above). Furthermore, in the higher energy diet-fed goats, the variations of circulating T4 during different physiological states were not significant (Todini et al., Reference Todini, Malfatti, Valbonesi, Trabalza-Marinucci and Debenedetti2007). Recently, no significant difference in the rates of type II and type III deiodinase activity in the skin or in blood TH concentrations was found between cashmere goats maintained at a different plane of nutrition (Rhind et al., Reference Rhind, Kyle, Riach and Duff2006).
Selenium is present in deiodinase enzymes, and other selenoproteins play a protective role for the thyrocytes against damage by hydrogen peroxide produced for TH biosynthesis (Kohrle et al., Reference Kohrle, Jakob, Contempré and Dumont2005). Oral iodine and selenium supplements increased blood concentrations of TH in sheep, and selenium supplementation alone increased plasma T3 concentrations and decreased T4 concentrations (Bik, Reference Bik2003). Following selenium supplementation, type I deiodinase activity decreased in the liver and increased in the pituitary, while pituitary type II deiodinase was unaffected, indicating that enzyme activity is homeostatically controlled when a sufficient amount of selenium is present, in order to ensure TH homeostasis (Chadio et al., Reference Chadio, Kotsampasi, Menegatos, Zervas and Kalogiannis2006).
Conclusion
Changes of blood TH concentrations are an indirect measure of the changes in thyroid gland and extrathyreoidal deiodination activity. Many factors act simultaneously modulating thyroid gland activity and/or peripheral monodeiodination. Besides endogenous and environmental climatic factors, nutrition plays a primary role on thyroid gland activity and on blood TH concentrations. The physiological range of the endocrine responses to different conditions is very large, thus reference values are very difficult to obtain. Assay results must be carefully evaluated, not only for diagnostic and clinical purposes but also to evaluate the physiological states and responses of the animals. The systemic actions of TH justify their pivotal role in the mechanisms permitting the animals to adapt to the surrounding environment. New insights are gathered from investigations on the regulation of monodeiodinase activity, hence of TH bioavailability, in the central nervous system and at the peripheral level. Little is known about TH receptor expression and activity or about the targets at molecular levels, even in humans and rodents. The field of the non-genomic, rapid TH actions needs further research. Knowledge on such topics will possibly allow the monitoring and manipulation of thyroid physiology, in order to improve animal health, welfare and production (meat, milk, hair fibre).
Acknowledgements
The author wishes to thank Professor Alessandro Debenedetti and Professor Alessandro Malfatti for their guidance and encouragement, Dr Alessia Zicavo and Mr Diego Todini for their patient support.






