Introduction
A balanced supply of amino acids and energy-yielding nutrients is required for optimal protein deposition in growing animals. The relationship between digestible energy supply and protein deposition was described (Campbell and Taverner, Reference Campbell and Taverner1988; Gerrits et al., Reference Gerrits, Tolman, Schrama, Tamminga, Bosch and Verstegen1996; Eits et al., Reference Eits, Kwakkel, Verstegen, Stoutjesdijk and De Greef2002) and included in feed evaluation systems and mechanistic growth simulation models for many animal species. Requirements for protein and energy are expressed as daily means in current feed evaluation systems, and a lack of concurrent availability of amino acids and protein-free energy within a day is usually assumed not to hamper protein utilisation.
The post-absorptive availability of amino acids is, however, not always in synchrony with that of non-protein energy (i.e. mainly glucose). In this perspective, nutrient asynchrony is defined as a (partial) separation of amino acid and glucose availability in time. Asynchronous nutrient absorption patterns can be induced by either a separated supply of protein and carbohydrates (Barja et al., Reference Barja, Araya, Munoz, Vega, Arteaga and Tagle1972; Arnal et al., Reference Arnal, Mosoni, Boirie, Houlier, Morin, Verdier, Ritz, Antoine, Prugnaud, Beaufrère and Patureau-Mirand2000) or by formulating diets using ingredients with different kinetics of digestion and absorption (Metges et al., Reference Metges, El-Khoury, Selvaraj, Tsay, Atkinson, Regan, Bequette and Young2000; Englyst et al., Reference Englyst, Vinoy, Englyst and Lang2003; Mosoni and Patureau-Mirand, Reference Mosoni and Patureau-Mirand2003). Moreover, the effects of the kinetics of absorption of dietary ingredients can vary between species, because the digestive system differs and particular ingredients are used in practical diets for various species. In pre-ruminant calves, for example, skimmed milk protein, coagulates in the abomasum, resulting in a delayed amino acid absorption compared to glucose (Longenbach and Heinrichs, Reference Longenbach and Heinrichs1997). Delayed portal glucose appearance in pigs fed resistant starches compared with those fed pregelatinised starch also changes the synchrony between glucose and amino acids (Cummings and Englyst, Reference Cummings and Englyst1995; Van der Meulen et al., Reference Van der Meulen, Bakker, Smits and De Visser1997).
The effects of nutrient asynchrony have to some extent been addressed in early literature (e.g. Munro, Reference Munro, Munro and Allison1964), generally reporting a decrease in the efficiency of protein utilisation (Larson and Chaikoff, Reference Larson and Chaikoff1937; Cuthbertson and Munro, Reference Cuthbertson and Munro1939; Cuthbertson et al., Reference Cuthbertson, McCutcheon and Munro1940). These studies, however, included only non-growing animals and humans. In growing pigs, a partial separation of protein and carbohydrate intake within a day did not result in significant changes in protein utilisation (Eggert et al., Reference Eggert, Brinegar and Anderson1953; Yeo and Chamberlain, Reference Yeo and Chamberlain1966), whereas nutrient asynchrony increased urea production in lambs (Randles, Reference Randles2001). The consequences of a (virtually) complete nutrient asynchrony on protein and energy metabolism, however, remain to be determined in growing animals. Possibly, there may even be benefits of inducing nutrient asynchrony, because it was recently shown in milk-fed calves that protein utilisation was unaffected, but that fat deposition increased substantially, with increasing nutrient asynchrony (Van den Borne et al., Reference Van den Borne, Verstegen, Alferink, Van Ass and Gerrits2006). Therefore, effects of a (virtually) complete separation need to be studied for quantifying the potential impact of nutrient asynchrony on protein utilisation in growing animals.
In this study, it is hypothesised that the efficiency of protein utilisation for growth in pigs may decrease with a virtually complete separation of dietary protein and carbohydrate intake. The net effect on fat deposition will depend on the balance of hypothesised increases and decreases in fat deposition after the carbohydrate diet and protein diet respectively. In this study, effects of synchronising amino acid and glucose availability on protein and energy metabolism were therefore quantified.
Material and methods
Animals, treatments and diets
Ten crossbred barrows ([Finnish Landrace × Great Yorkshire] × Tybor-G) of approximately 50 kg live weight were used in five replicates of two pigs each. Each replicate consisted of two experimental periods of 7 days each. Within each experimental period, pigs were assigned to one of two treatments in a change-over design: a synchronous supply of protein and starch (SYN) or an asynchronous supply of protein and starch (ASYN) (Table 1). The daily supply of nutrients and ingredients was similar for both treatments. A contrast was created in the distribution of the nutrient intake over the two daily meals (at 0800 h and at 1600 h). Pigs assigned to SYN received a synchronised diet at 0800 h and at 1600 h, involving an identical composition of both meals. For ASYN, protein and starch intake were as much as possible separated between the two daily meals. This meant that pigs assigned to ASYN were to receive 95% of the daily protein and 0% of the daily starch at 0800 h and 5% of the daily protein and 100% of the daily starch at 1600 h (Table 1).
Table 1 Experimental design; distribution of the total nutrient intake over two daily meals for synchronously and asynchronously fed pigs†

† Based on calculated values.
The protein and carbohydrate diets were formulated separately (i.e. basal diets) (Table 2) and the synchronous diet was formulated by mixing these two in a ratio of 1:2.6. The gross energy intakes at 0800 and 1600 h were equal between treatments and contributed 33 and 67% to the daily gross energy intake, respectively. The basal diets were formulated to differ in 13C natural abundance (1.0952 atom % and 1.0814 atom % for the protein and the carbohydrate diet, respectively). The maize starch and maize oil were the only naturally 13C enriched ingredients. Measuring 13C enrichment of CO2 in the expired air allowed an improved specification of the contribution of different nutrients to total nutrient oxidation.
Table 2 Ingredient and nutrient composition of the basal diets†

† Basal diets were used as single diets for the asynchronous treatment (ASYN). For the synchronous treatment (SYN), the protein and carbohydrate diet were mixed in a ratio of 1 to 2.6.
‡ Diatomaceous shell powder, SiO2, added as an indigestible marker.
§ Provided per kg of the experimental diet: 5000 IU retinol; 1000 IU cholecalciferol; 7.5 mg α-tocopherol; 15 μg cyanocobalamin; 0.4 mg phylloquinone; 3.5 mg riboflavin; 20 mg niacinamid; 5 mg d-pantothenic acid; 200 mg choline chloride; 1 mg CoSO4.7H2O; 0.5 mg KI; 0.06 mg organic Se; 400 mg FeSO4.7H2O; 80 mg CuSO4. 5H2O; 70 mg MnO2; 200 mg ZnSO4.H2O.
‖ Analysed content, unless indicated otherwise. Sugars were mono- and disaccharides as glucose units. NSP = non-starch polysaccharide content which was calculated by subtracting the crude protein, crude fat, starch, sugars and ash content from the dry matter content.
Pigs were fed according to their metabolic body weight (BW0.75) at 2.1 times the digestible energy requirements for maintenance, which were assumed to be 480 kJ/kg BW0.75 per d. The conversion from gross energy (Tables 1 and 2) to digestible energy was performed according to the digestibility coefficients in the Dutch feed evaluation table (Centraal Veevoeder Bureau (CVB), 2000). Feed intake was adjusted daily for a projected average daily gain of 500 g. At the start of the adaptation period, pigs were switched to the experimental diets within two days. Feed was provided as mash and was mixed with water (3 l/kg feed) immediately prior to feeding (viz. no soaking time).
Each experimental period was preceded by a 7-day adaptation period on the experimental diets and feeding strategy. During the adaptation and experimental periods, pigs were individually housed on a tenderfoot floor (150 × 60 cm) in one of two identical, size-adjustable climatic respiration chambers. To collect faeces quantitatively, plastic bags were attached to the rear end of the pigs as described by Van Kleef et al. (Reference Van Kleef, Deuring and Van Leeuwen1994). The ambient temperature was kept at 20°C, relative humidity was maintained at 65%, and air velocity was < 0.2 m/s. Pigs were exposed to 12 h of light (300 lux from 0700 to 1900 h) and 12 h of darkness (10 lux). The experiment was approved by the Ethical Committee of Wageningen University.
Measurements
Pigs were weighed at the start and end of each experimental period. Gaseous exchange (carbon dioxide, oxygen and methane) was measured in pigs in 9-min intervals as described by Verstegen et al. (Reference Verstegen, Van der Hel, Brandsma, Henken, Bransen, Verstegen and Henken1987). Physical activity of pigs was recorded by a radar-Doppler device (Wenk and Van Es, Reference Wenk and Van Es1976). On days 2 and 6 of each experimental period, air was sampled in 30-min intervals during a 24-h period from the respiration chambers in evacuated tubes (Vacutainer, Becton Dickinson, Rutherford, NJ, USA) for analysis of the 13C enrichment in CO2 using a Delta C continuous-flow isotope ratio mass spectrometer (Finnigan MAT, Bremen, Germany).
Feed was sampled during each experimental period and refusals were collected at each feeding. Feed and refusal samples were stored at − 20°C until analysed. Faeces were collected quantitatively in plastic bags that were connected to the pigs and stored at − 20°C until analysed. Urine was collected via two funnels below the tenderfoot floor in two buckets containing 125 ml of 4.5 mol/l sulphuric acid each. The total amount of urine was registered and mixed at the end of each experimental period, and a sample was taken for analysis. Aerial NH3 was quantitatively trapped in 4.5 mol/l sulphuric acid and in water that condensed on the heat exchanger (Verstegen et al., Reference Verstegen, Van der Hel, Brandsma, Henken, Bransen, Verstegen and Henken1987).
For determination of the dry matter content, feed, feed refusals and fresh faeces were freeze-dried according to ISO 6496 (International Organization for Standardization (ISO), 1998b). Subsequently, faeces and feed were ground to pass a 1-mm screen and kept for analyses. Air-dry faeces and feed were dried in a forced air oven at 103°C to a constant weight according to ISO 6496 (ISO, 1998b). Kjeldahl nitrogen content was measured according to ISO 5983 (ISO, 1997) in fresh feed, feed refusals, faeces, urine and in 
Experimental diets were ground in a ball mill (Retsch MM 2000, Retsch GmbH & Co., Haan, Germany) and 13C natural abundance was measured after combustion in an elemental analyser using the continuous flow isotope ratio mass spectrometer. All analyses were carried out in duplicate, except nitrogen content in urine which was determined in triplicate.
Calculations
For each experimental period, the metabolisable energy intake (ME) was calculated per chamber as the difference between digestible energy intake and the sum of urinary energy loss and methane production. From the gaseous exchange, heat production (Htot) was calculated according to the formula of Brouwer (Reference Brouwer and Blaxter1965). For each pig within a balance period, the energy costs per unit of physical activity were estimated by regression of physical activity against Htot. Activity related heat production (Hact) was calculated (Heetkamp et al., Reference Heetkamp, Schrama, de Jong, Swinkels, Schouten and Bosch1995). Heat production not related to physical activity was derived by subtracting Hact from Htot. Energy retention was calculated by subtracting Htot from ME intake. Nitrogen (N) retention was calculated as the difference between N intake and N output in faeces and urine. Aerial NH3 and 
Statistical analysis
Faecal apparent digestibility and nitrogen and energy balance traits were analysed for the effect of treatment (SYN or ASYN) by anova using the following model:
where Y ij = dependent variable; μ = average intercept; T i = effect of treatment i (i = 1, 2); P j = effect of pig (j = 1,…, 4 or 10); and εij = error term. Due to technical problems in the measurements of gas exchange, data from four pigs were used to estimate energy metabolism variables. Data from ten pigs were used for other variables. Therefore, effects on energy balance are not presented in the results, but briefly mentioned in the discussion section. For the analysis of all other dependent variables, all ten pigs could be used. Effects of the sequence of both treatments were separately tested but were not significant and are therefore not included in the model. Hourly means were calculated for Htot, Hact, RQ and 13C enrichment of expired CO2 and treatment effects were tested for each hour separately. The SAS software package version 9.1 (Statistical Analysis Systems Institute Inc., Cary, NC, USA) was used in all statistical evaluations. Results are presented as least-square means with their s.e.
Results
Average daily gain did not differ (P>0.10) between treatments and averaged 515 (s.e. 26.8) g. The feed refusals, as a percentage of the feed supply, were generally low, but were twofold higher for the carbohydrate diet (5.6%) than for the protein diet (2.8%) in asynchronously fed pigs.
Digestibility
Apparent digestibility of dry matter, organic matter and energy was lower (P = 0.019) in ASYN pigs compared with SYN pigs (Table 3). Fat, ash and crude protein digestibility was similar in ASYN and SYN pigs. Starch was not detected in faeces of pigs fed experimental diets. Digestibility of dietary non-starch polysaccharides (NSP) was higher (P < 0.001) in ASYN pigs than in SYN pigs.
Table 3 Effects of synchronising amino acid and glucose availability on animal performance and on apparent nutrient digestibility in pigs†

† Values are least-square means, n = 10 for each treatment.
‡ NSP = non-starch polysaccharides; NSP content in feed and faeces was calculated by subtracting the crude protein, crude fat, starch, sugars and ash content from the dry matter content.
Protein metabolism
N intake was 5% higher for ASYN than for SYN (P = 0.003) (Table 4). Urinary N loss was higher (P < 0.001) and N retention was lower (P = 0.017) in ASYN pigs than in SYN pigs. Nitrogen balance (expressed as a percent of digestible N intake) was higher (P < 0.001) in SYN pigs (56.7%) compared with ASYN pigs (47.1%).
Table 4 Effects of synchronising amino acid and glucose availability on protein metabolism in growing pigs†
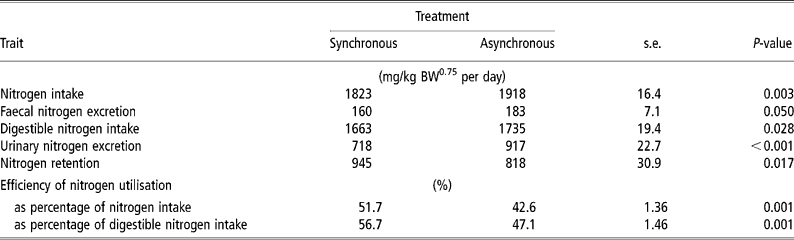
† Values are least-square means, n = 10 for each treatment.
Within-day substrate oxidation
The circadian rhythm in RQ showed a considerably larger amplitude for ASYN (0.28 units) than for SYN (0.17 units) (P < 0.05; Figure 1a). The RQ did not increase after a protein diet, whereas a synchronised diet and a carbohydrate diet resulted in an increase in RQ. The 13C enrichment of CO2 in the expired air was relatively constant (1.0915–1.0929 atom %) during the day in synchronously fed pigs (Figure 1b) and always exceeded the natural 13C abundance of the synchronised diet (1.0910 atom %). In asynchronously fed pigs, however, the pattern of 13C enrichment of CO2 within the day clearly reflected the natural 13C abundance of the diet supplied.

Figure 1 Effects of synchronous (●, ■) or asynchronous (○, □) availability of amino acids and glucose in growing pigs on the circadian rhythms of (a) the respiratory quotient; and (b) the 13C enrichment of the expired CO2. Stars indicate significance (P < 0.05). Results are expressed as least-square means ± s.e., n = 4 for each treatment. Arrows represent feeding times. Asynchronously fed pigs received a protein diet in the morning and a carbohydrate diet in the afternoon. Horizontal lines in (b) represent 13C enrichment of the carbohydrate diet 1.0952 atom %), the protein diet (- - - -; 1.0814 atom %) and the synchronised diet (– — – —; 1.0910 atom %).
Discussion
Digestibility
Apparent digestibility of dry matter, organic matter and energy was higher for SYN than for ASYN, which mainly originated from the increased digestibility of NSP and from the numerically increased digestibility of protein and fat. The increase in apparent digestibility of NSP for SYN compared with ASYN is related to an increase in fermentation and corresponds with a higher methane production (Table 4). This is in accordance with a higher methane production in synchronously than in asynchronously fed sows (Müller and Kirchgessner, Reference Müller and Kirchgessner1996). It is speculated that the large circadian fluctuations in the substrate supply for ASYN may have changed the ratio between available carbon and nitrogen for the microflora. This may have required continuous adaptation of the microflora to their environment and decreased the ability of (large) intestinal microflora to degrade NSP. Protein digestibility was not affected by treatment and exceeded 90%, which is in accordance with tabulated values for soy protein isolate and potato protein (CVB, 2000).
Protein metabolism
The higher nitrogen intake for ASYN than for SYN was caused by a higher measured protein content in the protein diet than calculated (672 g/kg v. 620 g/kg) in combination with slightly more feed refusals for the carbohydrate diet than for the protein diet in asynchronously fed pigs. Despite the slightly higher N intake for ASYN than for SYN, nitrogen retention and the efficiency of utilisation of digestible protein for protein gain were substantially lower for ASYN than for SYN. This does not correspond with studies in growing pigs where a partial within-day separation of corn (Eggert et al., Reference Eggert, Brinegar and Anderson1953) or barley (Yeo and Chamberlain, Reference Yeo and Chamberlain1966) intake from the dietary protein intake did not affect nitrogen deposition. Our results are, however, in accordance with most studies on growing subjects in other species. Protein retention decreased in children (Barja et al., Reference Barja, Araya, Munoz, Vega, Arteaga and Tagle1972) and growing rats (Bancroft et al., Reference Bancroft, Geiger and Hagerty1951) when protein and carbohydrate intake were partially separated. A lower nitrogen retention was also found in growing rats after imposing nutrient asynchrony by feeding (Geiger et al., Reference Geiger, Bancroft and Hagerty1950) or intravenous infusion (Martins et al., Reference Martins, Sandberg, Ekman and Lindmark1985). In growing, ruminant lambs, an asynchronous abomasal infusion of amino acids and energy (i.e. triglycerides of acetate and butyrate) decreased protein retention as indicated by an increase in urea production (Randles, Reference Randles2001). On the other hand, Cuthbertson et al. (Reference Cuthbertson, McCutcheon and Munro1940) did not find an effect of nutrient asynchrony on nitrogen balance in growing rats, but the growth rates observed were very low (1 g/d) and it was argued that there may have been a dietary deficiency in that study (Geiger, Reference Geiger1948). In milk-fed calves, nutrient asynchrony did not decrease protein retention (Van den Borne et al., Reference Van den Borne, Verstegen, Alferink, Van Ass and Gerrits2006).
In non-growing subjects, protein utilization generally decreases with nutrient asynchrony. In adult dogs, nitrogen balance decreased when carbohydrates were given more than 4 hours before or after a high protein diet (Larson and Chaikoff, Reference Larson and Chaikoff1937). Similar results were described when nutrient asynchrony was imposed in adult man (Cuthbertson and Munro, Reference Cuthbertson and Munro1939) and adult rats (Cuthbertson et al., Reference Cuthbertson, McCutcheon and Munro1940; Munro, Reference Munro1949; Van Dam-Bakker et al., Reference Van Dam-Bakker, De Groot and Luyken1958). In non-growing sows, however, separation of protein and carbohydrate intake for 33, or even for 48 h, did not depress nitrogen balance (Müller and Kirchgessner, Reference Müller and Kirchgessner1996, Kirchgessner and Müller, Reference Kirchgessner, Müller, McCracken, Unsworth and Wylie1998). In 26-year-old women, a partial separation of protein and carbohydrate intake did not clearly affect protein retention, but it decreased numerically with increasing nutrient asynchrony (Arnal et al., Reference Arnal, Mosoni, Boirie, Houlier, Morin, Verdier, Ritz, Antoine, Prugnaud, Beaufrère and Patureau-Mirand2000). Surprisingly, in 68-year-old women at a high protein intake, protein utilisation increased with increasing nutrient asynchrony (Arnal et al., Reference Arnal, Mosoni, Boirie, Houlier, Morin, Verdier, Ritz, Antoine, Prugnaud, Beaufrère and Patureau-Mirand1999). This may be caused by an age-related impairment of the response of both protein synthesis and protein breakdown to feeding as described in adult (11 months old) and old (23 months old) rats (Arnal et al., Reference Arnal, Mosoni, Dardevet, Ribeyre, Bayle, Prugnaud and Patureau-Mirand2002). As a consequence, an asynchronous nutrient intake was suggested to stimulate protein retention in old but not in adult rats (Arnal et al., Reference Arnal, Mosoni, Dardevet, Ribeyre, Bayle, Prugnaud and Patureau-Mirand2002). The effect of nutrient asynchrony on N balance may therefore be influenced by both the degree of nutrient synchrony and the stage of maturity of the animals.
Regulation of protein utilisation by nutrient synchrony
The increased urinary nitrogen losses for ASYN compared with SYN have likely resulted from an increased amino acid oxidation after the protein diet. The decreasing RQ after a protein diet (Figure 1a) suggests an increased oxidation of fat (RQ = 0.70) and amino acids (RQ = 0.81) due to a lack of glucose (RQ = 1) availability. Similar changes in the RQ after carbohydrate and protein diets in growing pigs were described by Charlet-Lery and Morel (Reference Charlet-Lery and Morel1977). The increased amino acid oxidation after the protein diet could be related to the circadian rhythm of the 13C enrichment of expired CO2, which was nearly invariable for SYN but showed clear fluctuations for ASYN (Figure 1b). Although firm evidence can only be obtained from direct measurement of the amino acid oxidation flux, the decrease in 13C enrichment for ASYN during daytime strongly suggests that the contribution of amino acid oxidation to total substrate oxidation was substantially increased during this period. The 13C enrichment of body protein, glycogen and adipose tissues were not measured and the contribution of their oxidation to total substrate oxidation could not be quantified. It can, however, quite safely be assumed that the enrichment of the protein diet is reflected in body protein, because pigs were fed exclusively proteins from C3 plants during the growing period prior to the study. The 13C enrichment of body fat can be assumed to be only slightly lower than the natural 13C abundance of the carbohydrate diet, because fat deposition mainly originates from dietary glucose (high natural 13C abundance) and dietary fat (intermediary natural 13C abundance). It is therefore concluded from the circadian rhythms of RQ and CO2 enrichment that amino acids are oxidized in absence of glucose. Several potential mechanisms for the increased amino acid oxidation with decreasing nutrient synchrony can be suggested.
First, amino acids may be used to provide ATP for maintenance and for protein deposition or for gluconeogenesis in the absence of glucose from dietary origin. Second, glucose may interact with amino acid metabolism via endocrine responses. To increase the retention of dietary amino acids and inhibit gluconeogenesis, insulin secretion should be in synchrony with the post-absorptive supply of amino acids (Fuller et al., Reference Fuller, Weekes, Cadenhead and Bruce1977; Barthel and Schmoll, Reference Barthel and Schmoll2003). Third, the presence of dietary carbohydrates may affect amino acid metabolism in the intestinal tissues with concurrent implications for the kinetics of amino acid availability to extra-intestinal tissues (Mariotti et al., Reference Mariotti, Huneau, Mahé and Tomé2000; Soeters et al., Reference Soeters, Dejong and Deutz2001; Bos et al., Reference Bos, Metges, Gaudichon, Petzke, Pueyo, Morens, Everwand, Benamouzig and Tomé2003).
Energy metabolism
Unfortunately, data on energy partitioning could not be used for all pigs, because problems with the measurement of gas exchange occurred. However, the efficiency of digestible protein utilisation corresponded between the eight observations included in the data on energy balance and all 20 observations; 56.6 v. 56.7% for SYN and 46.9 v. 47.1% for ASYN respectively. This suggests that net protein utilisation was similar between the observations from the total study and observations that could be used for calculation of energy balance (Table 5). For the latter, gross energy intake did not differ between treatments, but DE intake was 50 kJ/kg BW0.75 per day lower for ASYN than for SYN. The 61 kJ/kg BW0.75 per day lower ME intake combined with an 11 kJ/kg BW0.75 per day lower heat production for ASYN than for SYN resulted in a tendency for a lower energy retention ( − 50 kJ/kg BW0.75 per day) for ASYN than for SYN. Despite the 61 kJ/kg BW0.75 per day lower ME intake for ASYN than for SYN, Htot was only 11 kJ/kg BW0.75 per day lower for ASYN than for SYN. If Hact was excluded, the corrected heat production was numerically even higher for ASYN (547 kJ/kg BW0.75 per day) than for SYN (542 kJ/kg BW0.75 per day). This indicates that a substantial part of the increased quantity of deaminated amino acids for ASYN when compared with SYN was oxidised and not deposited as fat.
Table 5 Effects of synchronising amino acid and glucose availability on energy metabolism in growing pigs†

† Values are least-square means, n = 4 for each treatment.
‡ Δ RQ: the amplitude (maximum hourly mean minus minimum hourly mean) of the RQ.
From Figure 1a, it is clear that there was considerable variation in RQ between treatments within the day. An RQ greater than 1 indicates conversion of glucose into fat. The RQ exceeded unity after the 1600 h meal for 11 h for ASYN and for only 5 h/day for SYN. A low RQ, on the other hand, suggests increased rates of fatty acid (and amino acid) oxidation in the morning and early afternoon for ASYN when compared with SYN (Figure 1a), providing support for the numerically (17%) reduced fat deposition for ASYN.
In conclusion, a virtually complete separation of protein and carbohydrate intake within a day decreased the faecal apparent digestibility of dry matter, organic matter, energy and NSP. The efficiency of digestible protein utilisation for protein retention decreased from 57% to 47% with decreasing nutrient synchrony in growing pigs. The energy yield from the increased amino acid degradation was largely lost as heat. Within the day, more prolonged periods of de novo fatty acid synthesis occurred for ASYN than for SYN, combined with increased rates of fatty acid oxidation for ASYN than for SYN during the remainder of the day.
Acknowledgements
The authors thank Karin Frijters, Marjolein de Haan, Koos van der Linden, Tamme Zandstra and the personnel of the experimental farm ‘De Haar’ for their contribution to the experiment. The laboratory staff of the Animal Nutrition Group is gratefully acknowledged for their help in chemical analyses.








