Implications
It has been understood for many years that dairy cows experience a temporary reduction in immune function soon after calving, which contributes to their increased risk of reproductive tract disease and mastitis. There are numerous metabolic changes related to energy and calcium supply in support of lactation which occur at the same time and which impair the function of neutrophils, immune cells that are critically important in the early stages of defence against several common diseases of dairy cows in early lactation. Research is needed to more holistically evaluate how neutrophil function is influenced in dairy cows around calving.
Introduction
Dairy cows experience massive metabolic demands to support lactation. They adapt their metabolism to do so, including uncoupling of the somatotropic axis (Baumgard et al., Reference Baumgard, Collier and Bauman2017) with peripheral insulin resistance and increased lipolysis to fuel milk production. However, cows are challenged by a transient decrease in feed intake resulting in negative energy and protein balance in early lactation, and short duration but substantial hypocalcemia around calving. Concurrently, there is impairment of innate immune function and regulation of inflammation which is central to the development of mastitis (Ballou et al., Reference Ballou2012) and uterine diseases (Sheldon et al., Reference Sheldon, Cronin and Bromfield2019). The mechanisms linking these changes and metabolic challenges are only partially understood.
Markers of aspects of adaptation to negative energy balance (e.g., serum concentrations of non-esterified fatty acids (NEFA) and β-hydroxybutyrate (BHB)) are associated with the risk of many metabolic and infectious diseases, in part through their associations with suppressed immune function and excessive inflammation (Ingvartsen and Moyes, Reference Ingvartsen and Moyes2013). Approximately 35% of peripartum cows have NEFA and 45% have BHB above thresholds associated with metabolic disease or compromised production or reproduction (McArt et al., Reference McArt, Nydam, Oetzel, Overton and Ospina2013). In a large dataset, 44% of cows had at least one disease condition in early lactation, and of these, 39% had two or more separate diseases (Santos et al., Reference Santos, Bisinotto, Ribeiro, Lima, Greco, Staples and Thatcher2010). Impaired innate immune function appears to have an important place in this web of metabolic health and disease.
This paper provides a brief narrative review of selected important determinants of health in dairy cows in the transition period around calving. Specifically, the focus is on management and social stressors, markers of adaptation to negative energy balance and hypocalcemia, and their associations with neutrophil function.
Overview of neutrophil function and its assessment in peripartum dairy cows
General neutrophil biology
Neutrophils are differentiated and proliferated in the bone marrow under the influence of granulocyte colony-stimulating factor (G-CSF), a process that appears to take about 7 to 10 days from myeloblast to release of a neutrophil into circulation (Paape et al., Reference Paape, Bannerman, Zhao and Lee2003; Kubes, Reference Kubes2018). The turnover and production of neutrophils are impressive, estimated at 109 cells/kg body weight per day (Kubes, Reference Kubes2018) or >1011 cells per day in cattle, which can increase 10-fold during acute inflammation or administration of G-CSF (Van Schyndel et al., Reference Van Schyndel, Carrier, Bogado Pascottini and LeBlanc2018). The process and regulation of granulopoiesis have recently been reviewed in detail (Yvan-Charvet and Ng, Reference Yvan-Charvet and Ng2019), highlighting differences in the process between homeostatic and acute response states, including diurnal patterns, at least in mice.
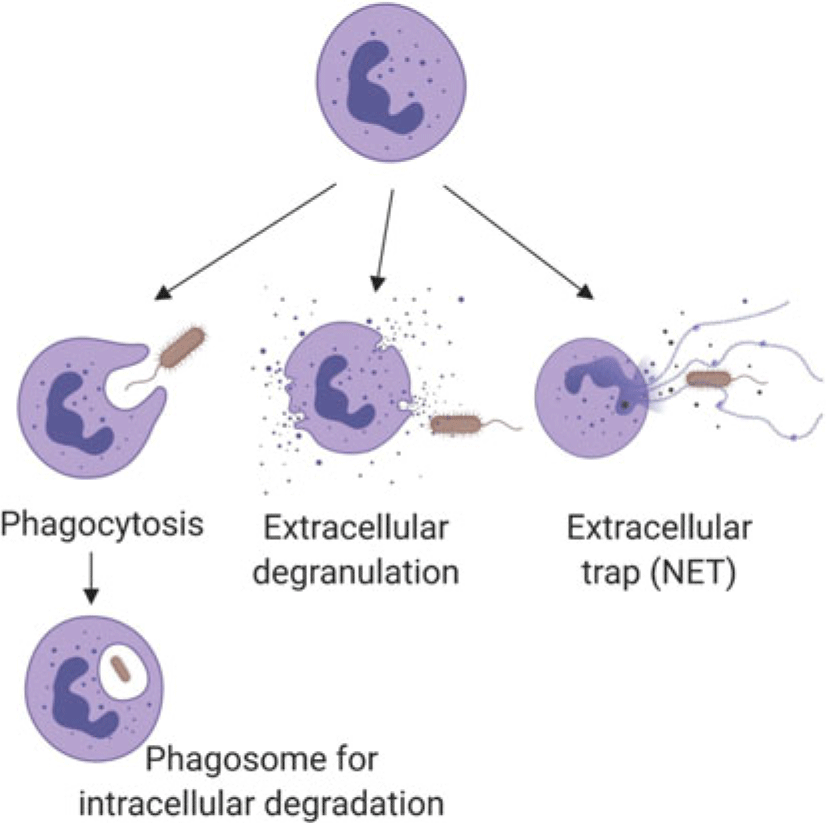
Neutrophils marginate, and adhere to and ‘crawl’ along endothelial cells, then perform diapedesis to move from blood vessels into tissue (Kolaczkowska and Kubes, Reference Kolaczkowska and Kubes2013). While the basic process is well established (Liew and Kubes, Reference Liew and Kubes2019), neutrophil recruitment appears to have tissue-specific features (Margraf et al., Reference Margraf, Ley and Zarbock2019), which have been described using intravital microscopy (among other techniques). The method is informative but invasive. Details of tissue-specific recruitment or function have not been described for the mammary gland or uterus, which are of the greatest interest for dairy cows. Once in tissues, neutrophils interact with damaged cells or bacteria to remove foreign cells through a variety of mechanisms (Figure 1) including phagocytosis and intracellular digestion by oxidation (e.g., oxidative burst in lysosomes), extracellular release of oxidants from neutrophil granules (e.g., myeloperoxidase; Figure 1), or casting neutrophil extracellular traps (NETs) of DNA (Nauseef and Borregaard, Reference Nauseef and Borregaard2014; Liew and Kubes, Reference Liew and Kubes2019). Neutrophils may die by apoptosis or necrosis. The former is programmed cell death with controlled clearance of the potentially destructive residual cargo of the neutrophil by macrophages, whereas the latter features rupture of the cell contributing to damage to nearby cells and additional inflammation (Paape et al., Reference Paape, Bannerman, Zhao and Lee2003). Recent data indicate that apoptotic neutrophils return from sites of action to be cleared from the bone marrow or resident macrophages in the liver or spleen, which likely helps to modulate further neutrophil responses, including shifting towards resolution of inflammation and healing (Kolaczkowska and Kubes, Reference Kolaczkowska and Kubes2013).
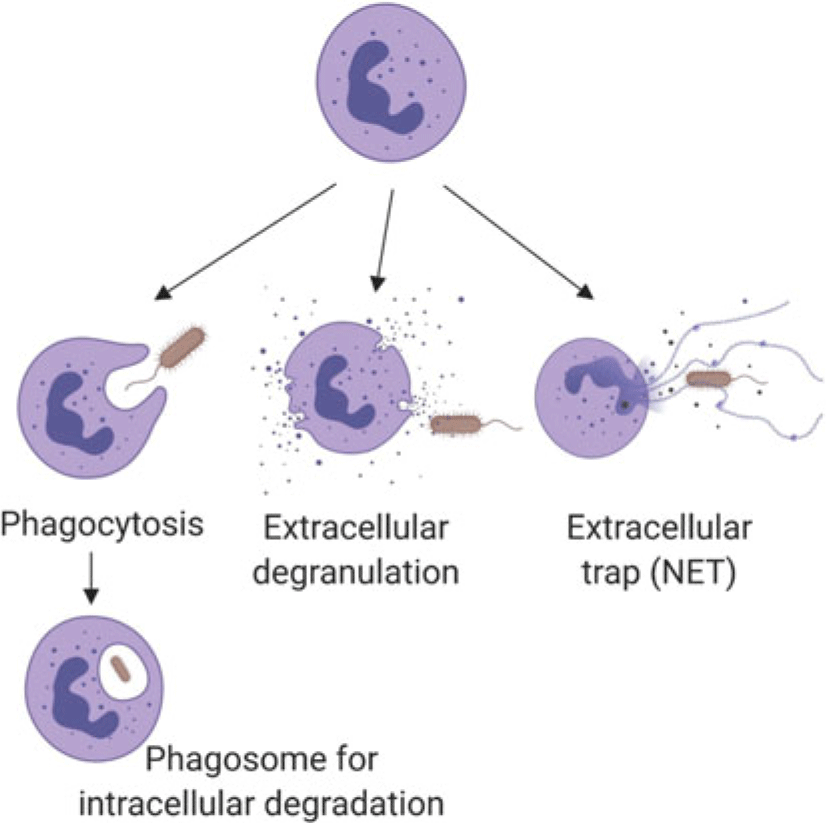
Figure 1 (Colour online)Schematic summary of the mechanisms by which neutrophils kill pathogens, such as bacteria as illustrated here. Neutrophil granules contain pro-inflammatory proteins including myeloperoxidase, lactoferrin, gelatinase and matrix metalloproteinase 9. Following phagocytosis, encapsulated pathogens are killed intracellularly by reactive oxygen species or proteins from granules that fuse with the phagosome. Neutrophil extracellular traps consist of extruded DNA and cargo from extracellular granules. (Figure created with BioRender.com.)
It is suggested that distinct subsets of neutrophils may exist, characterised by a pro-inflammatory or a pro-angiogenic phenotype and their propensity to form NETs, although it is not clear whether these are specific developmental lineages, different stages of maturity of neutrophils or the result of plasticity of function based on specific chemokines at the site of action (Kubes, Reference Kubes2018). Neutrophils are necessarily able to respond rapidly to stimuli, and Kubes (Reference Kubes2018) argues that although neutrophils are capable of protein synthesis and may tailor cytokine production once in situ, they must have already synthesised their cargo of effector molecules before recruitment.
The lifespan of neutrophils is still unclear, complicated by the fact simply collecting a blood sample and labelling neutrophils for study may activate them. Neutrophils are generally thought to circulate for a short time (6 to 8 h half-life in humans; less in mice), but neutrophils’ lifespan is extended once activated, although still to less than ∼1 day (Kubes, Reference Kubes2018; Liew and Kubes, Reference Liew and Kubes2019). Additionally, recent data suggest that neutrophils may be trafficked through more complex cycles and sites, and perhaps longer lifespans than in the current paradigm (Hidalgo et al., Reference Hidalgo, Chilvers, Summers and Koenderman2019).
It is now recognised that there are important links of systemic metabolism (especially adipose metabolism and insulin resistance) with immune function and inflammation in humans and laboratory animals (Hotamisligil and Erbay, Reference Hotamisligil and Erbay2008; Osborn and Olefsky, Reference Osborn and Olefsky2012) as well as in dairy cows (Bradford et al., Reference Bradford, Yuan, Farney, Mamedova and Carpenter2015). Both obese people and periparturient dairy cows are characterised by elevated circulating NEFA, insulin resistance, hepatic lipid accumulation and systemic inflammation, and there is great interest in the phenomenon of metabolic or sterile inflammation in dairy cows (LeBlanc, Reference LeBlanc2014). Non-esterified fatty acids, particularly saturated fatty acids which predominate in transition cows, may also impair neutrophil function (Ingvartsen and Moyes, Reference Ingvartsen and Moyes2013). Briefly, certain NEFA activate toll-like receptor 4 (TLR4), a main receptor for lipopolysaccharide (LPS), which activates nuclear factor κβ (NF-κβ) and leads to secretion of tumour necrosis factor α (TNFα), interleukin (IL)-1 and IL-8. Tumour necrosis factor α and IL-1 act on intracellular messengers to upregulate inflammation and increase insulin resistance. Based on work in mice and humans, local, classical neutrophil actions may feed into chronic, systemic, sterile inflammation (Buck et al., Reference Buck, Sowell, Kaech and Pearce2017) under conditions that may include lipolysis, ketosis and lack of supply of substrates for immune cells – all of which are common in dairy cows in the transition period. Host microbiome(s) are starting to be recognised as having important interactions with regulation of inflammation well beyond the gut (Kubes, Reference Kubes2018). However, there are also important differences between cows in early lactation and people with ‘metabolic syndrome’ or non-alcoholic fatty liver disease.
Neutrophil function in dairy cows
Neutrophils are activated in cattle as in other species by chemokine (C-X-C motif) ligand 8 (CXCL8) (also called IL-8), platelet activating factor, TNFα and complement component C5a (Bassel and Caswell, Reference Bassel and Caswell2018). Neutrophils can also directly detect and be activated by pathogen associated molecular patterns (PAMP) through toll-like receptors, of which TLR1, TLR2, TLR4, TLR6, TLR7 and TLR10 are known to be present on bovine neutrophils (Bassel and Caswell, Reference Bassel and Caswell2018). Toll-like receptor 4, which responds to LPS, is especially relevant in the postpartum cow. Once stimulated by CXCL8 or TNFα released from macrophages resident in damaged tissue, or by epithelial cells reacting with PAMP or damage associated molecular patterns (Sheldon et al., Reference Sheldon, Cronin and Bromfield2019), neutrophils begin migration.
In cattle, the circulating half-life was estimated to be 9 h (Paape et al., Reference Paape, Bannerman, Zhao and Lee2003). Neutrophils in dairy cows in the transition period may have a shorter-than-normal lifespan due to early activation of apoptosis, but administration of recombinant bovine G-CSF stimulates production and release of neutrophils (Van Schyndel et al., Reference Van Schyndel, Carrier, Bogado Pascottini and LeBlanc2018). In humans, G-CSF delayed apoptosis by inhibiting caspase-9 and -3 (van Raam et al., Reference van Raam, Drewniak, Groenewold, van den Berg and Kuijpers2008). Based on the magnitude and duration of increased circulating neutrophil counts following treatment of dairy cows with a long-acting formulation of G-CSF (Van Schyndel et al., Reference Van Schyndel, Carrier, Bogado Pascottini and LeBlanc2018), there may be some extension of neutrophil lifespan in addition to ongoing stimulus of production and release. That hypothesis is supported by inhibition of caspase-9 expression in neutrophils in cows receiving the same G-CSF product (Heiser et al., Reference Heiser, LeBlanc and McDougall2018).
The two best-studied elements of neutrophil function in dairy cows are phagocytosis and oxidative burst. These are traditionally measured in isolated, live neutrophils collected from blood, which are stimulated in vitro by bacteria or chemokines, with functional assays that employ flow cytometry to assess the per-cell capacity to engulf fluorescent micro-spheres or to generate a reactive oxygen molecule tagged with a fluorescent marker. These techniques have provided a lot of useful insights into innate immune function. This approach is constrained by the requirement to perform the assays within hours (<24 h) of blood collection, being labour-intensive, and the need for expensive equipment. Additionally, it is challenging to perform the assays on cells that represent the sites of greatest interest (neutrophils in the uterus or the mammary gland). Neutrophils in the uterine lumen or in the milk have by definition already been activated and migrated, and so may be near the end of their life and less responsive to another stimulation in vitro, possibly leading to false-negative results. Additionally, functional assays performed on neutrophils from circulation are necessarily normalised to standard numbers of cells (e.g., 1 × 105 cells), which removes the possible effect of the number of cells in circulation. Therefore, immune functional capacity should perhaps be expressed as the product of per-cell measures of function and circulating numbers (concentration) of cells, as in the ‘phagocytic overall performance’ index used by Sander et al. (Reference Sander, Piechotta, Schlamberger, Bollwein, Kaske, Sipka and Schuberth2011). Conversely, there do not appear to be important differences in the circulating concentrations of neutrophils between healthy and diseased cows, preceding the onset of disease (Cai et al., Reference Cai, Weston, Lund, Brodie, McKenna and Wagner1994).
Another approach to assessing neutrophil status is through quantification of expression of genes for important aspects of neutrophil function. Especially with highly efficient techniques such as Nanostring which allows for simultaneous measurement of many genes with minimal extraction, this has the advantage of providing a more comprehensive profile of the suite of functional steps in neutrophil function (Heiser et al., Reference Heiser, LeBlanc and McDougall2018). Arguably, RNA-Seq (transcriptome sequencing) provides more comprehensive information than targeted hybridisation methods including Nanostring, subject to the completeness of the reference genome. By whatever method, the caveat is that expression of RNA may not equate with function in vivo. For example, in contrast to the temporal differences in function discussed below, Heiser et al. (Reference Heiser, LeBlanc and McDougall2018) found that many genes related to key neutrophil functions had increased expression in the first week postpartum.
Classic work by Kehrli et al. (Reference Kehrli, Nonnecke and Roth1989) demonstrated impairment of elements of innate immune function around calving, although the temporal changes may have been confounded to some extent by clinical or subclinical mastitis in five of the eight primiparous animals studied. Most measures of function in neutrophils (except random migration the week before) were increased in the weeks preceding calving, followed by a nadir in week 1 after calving, particularly for their three measures of oxidative burst function. Each of retained placenta, metritis, purulent vaginal discharge, endometritis and mastitis (which collectively affect at least 25% of dairy cows in early lactation) is strongly associated with impairments of one or more aspects of neutrophil function (Gunnink, Reference Gunnink1984; Cai et al., Reference Cai, Weston, Lund, Brodie, McKenna and Wagner1994; Kimura et al., Reference Kimura, Goff, Kehrli and Reinhardt2002; Hammon et al., Reference Hammon, Evjen, Dhiman, Goff and Walters2006; Ballou et al., Reference Ballou2012). While some reports document differences in neutrophil migration capacity between cows that subsequently have disease or remain healthy, the bulk of evidence points to impairment of neutrophil killing function (i.e., reduction of measures of oxidative burst such as iodination (myeloperoxidase activity), or cytochrome C reduction (generation of superoxide anion)). There is less indication of impairment of ingestion (phagocytic capacity) by neutrophils. The changes and differences in neutrophil function precede detection of clinical disease (Hammon et al., Reference Hammon, Evjen, Dhiman, Goff and Walters2006) and may precede calving (Gunnink, Reference Gunnink1984; Cai et al., Reference Cai, Weston, Lund, Brodie, McKenna and Wagner1994; Kimura et al., Reference Kimura, Goff, Kehrli and Reinhardt2002).
It is important to be specific when discussing neutrophil function, because not all aspects are typically impaired in transition cows, and different variables affect particular elements of neutrophil function.
In the transition period, there are inevitable substantial changes in circulating hormone concentrations in late pregnancy and the postpartum period and numerous profound endocrine adaptations to support lactation. These are compounded by imposed changes applied with good intentions but not always good effect (such as diet and pen or social group changes), as well as variable changes that may result from the foregoing or be somewhat independent of it, such as the degree of reduction in feed intake. Finally, other variables such as heat stress, competition for feeding or lying space, the quality of resting places, feed and water quality and availability, and the extent of social turmoil may combine to abate or exacerbate the inevitable challenges of the periparturient period.
In a bold experiment, researchers at the US National Animal Disease Center attempted to separate the effects of late pregnancy and calving from those of lactation by mastectomising 10 cows in early to mid-pregnancy and comparing three markers of innate immune function to eight intact cows that calved and lactated (Kimura et al., Reference Kimura, Goff and Kehrli1999). A caveat is that the effects of lactation may be confounded by the fact that all intact cows had milk fever and three of the eight had ketosis and displaced abomasum. Expression of L-selectin on the surface of neutrophils (necessary for initial (rolling) endothelial adhesion) decreased in both groups at calving but recovered within 1 to 3 days. The decrease was likely caused by inhibition of L-selectin (CD62) by glucocorticoids (Burton et al., Reference Burton, Kehrli, Kapil and Horst1995), that is, cortisol released as part of parturition. Neutrophil surface expression of β2-integrins (needed for final adhesion for diapedesis) was actually greater before calving and to 3 days postpartum in the cows that lactated. However, myeloperoxidase activity (a measure of oxidative burst capacity) declined in both groups from 3 weeks before to 3 days after calving, but then quickly and fully recovered in the mastectomised cows while remaining at the reduced level until the end of the study at 21 days postpartum in the lactating cows. On the available evidence, oxidative burst is the most consistently impaired element of neutrophil function in dairy cows after calving, and these unique data support the inference that factors related to the demands of lactation sustain but do not initiate this impairment.
Factors affecting neutrophil function
There are numerous factors that are likely to affect neutrophil functional capacity in dairy cows, the headlines of which are illustrated in Figure 2. Elaboration is provided below.
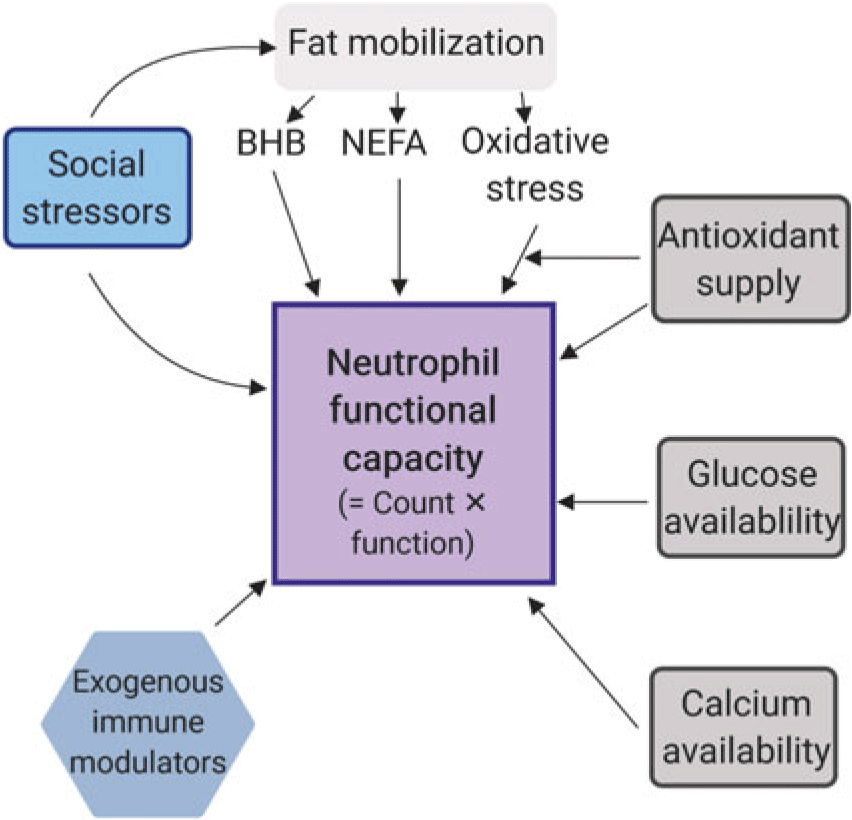
Figure 2 Factors that influence the functional response capacity of neutrophils in dairy cows. The scheme is simplified because there are likely interactions among these known factors, and others including genetics. Feed intake lags milk production in early lactation, so essentially all dairy cows mobilise fat stores to some degree. Nutritional formulation and feeding management determine the potential supply of immune system inputs, with additional variability imposed by social group and competitive pressures, as well as heat stress, and the comfort of the lying space. BHB = β-hydroxybutyrate; NEFA = Non-esterified fatty acids.
Management and social stress effects
Lesser feed intake up to 2 weeks before calving is a risk factor for metritis (Hammon et al., Reference Hammon, Evjen, Dhiman, Goff and Walters2006; Huzzey et al., Reference Huzzey, Veira, Weary and von Keyserlingk2007). That finding makes sense because lower nutrient intake would plausibly reduce the availability of fuel and substrates for neutrophil function and so diminish immune response, which in turn is a risk factor for metritis (Cai et al., Reference Cai, Weston, Lund, Brodie, McKenna and Wagner1994; Hammon et al, Reference Hammon, Evjen, Dhiman, Goff and Walters2006). The reasons for which some cows consume less than others before calving and before the onset of visible disease are not well understood. In the study by Huzzey et al. (Reference Huzzey, Veira, Weary and von Keyserlingk2007), competitive behaviour at the feed bin was studied, and cows that were more socially submissive (had fewer interactions in which they displaced another cow for feed access) were more likely to have lower intake and metritis. That study showed that cows apparently have a strong drive to eat at the same time, most acutely after fresh feed delivery, and that more submissive animals do not compensate their intake by eating at off-peak times. That is consistent with empirical observations that crowding or competition for space at the feed bunk (i.e., <75 cm of feed bunk per cow or >80% cows to headlocks) during the transition period is a risk factor for postpartum disease (Nordlund et al., Reference Nordlund, Cook and Oetzel2006). Observations of the adverse effects on feed intake and lying time of pen moves and social group changes led to the logical suggestion that stable social groups and fewer movements of cows to new pens during the transition period (Cook and Nordlund, Reference Cook and Nordlund2004), along with adequate feeding space, should reduce the incidence of postpartum disease, plausibly by reducing stressors and/or improving feed intake for cows at the submissive end of the social spectrum, thus improving metabolic status and thereby immune function. Endogenous cortisol has a transient but substantial increase at calving, which is associated with decreased expression of glucocorticoid receptor on neutrophils and increased circulating neutrophil counts (Preisler et al., Reference Preisler, Weber, Tempelman, Erskine, Hunt and Burton2000). Both elevated endogenous cortisol concentrations and administration of dexamethasone decreased expression of L-selectin on neutrophils, leading to neutrophilia but likely contributing to decreased functional capacity of the neutrophil system for 1 to 2 days (Weber et al., Reference Weber, Toelboell, Chang, Durrett Tirrell, Saama, Smith and Burton2004).
However, this hypothesis of social stress (mediated by acute or chronic elevations of cortisol or catecholamines and/or effects on feed intake or behaviour) leading to impaired immune function or health has generally not been supported in controlled studies. Huzzey et al. (Reference Huzzey, Nydam, Grant and Overton2012) provided 4 groups of 10 cows in late gestation with 67 cm of feed rail space and 1 freestall per cow or 34 cm of feeding space and 2 cows per stall for 14 days per treatment in a crossover design. On average, feed intake was greater in the overstocked group, particularly in the second week of the treatment. There were significantly but modestly greater plasma NEFA and faecal cortisol metabolite concentrations, and lower plasma glucose concentrations in overcrowded primiparous animals, and a lesser insulin response to a glucose tolerance test in overcrowded cows. However, it is unclear that the magnitude of the effects observed would contribute to disease risk.
In an experiment, 756 Jersey cows were assigned 26 days before expected calving to separate pens for heifers or parous cows, stocked at either 80% cows to headlocks and 80% cows to freestalls, or approximately 100% cows to headlocks and stalls. There were more competitive displacements at the feed bunk in the crowded groups, but only small differences in mean feeding and lying times (Lobeck-Luchterhand et al., Reference Lobeck-Luchterhand, Silva, Chebel and Endres2015). There were no differences between treatments in the incidence of retained placenta, metritis, purulent vaginal discharge or culling, nor in plasma concentrations of NEFA or BHB, energy-corrected milk yield to 155 days of lactation, or probability of pregnancy at the first two inseminations (Silva et al., Reference Silva, Dresch, Machado, Moraes, Lobeck-Luchterhand, Nishimura, Ferreira, Endres and Chebel2014). In a subset of 48 cows per treatment per parity, measures of innate and adaptive immune function and chronic stress were assessed from 2 to 3 weeks before, to 2 to 3 weeks after calving (Silva et al., Reference Silva, Lobeck-Luchterhand, Cerri, Haines, Ballou, Endres and Chebel2016). There were no differences between treatments in neutrophil phagocytosis or oxidative burst function, expression of L-selection or cluster of differentiation (CD)18, production of immunoglobulin (Ig)G against ovalbumin, serum haptoglobin concentrations or concentrations of cortisol in blood or hair.
To assess the effect of stable v. dynamic social group (pen populations) in the 4 weeks before calving, Silva et al. (Reference Silva, Moraes, Mendonça, Scanavez, Nakagawa, Fetrow, Endres and Chebel2013b) assigned a total of 567 Jersey cows to groups of 44 cows per freestall pen in 6 replicates of stable (‘all-in-all-out’; no additions to the pen) or dynamic (weekly additions of new animals to replace cows that left for calving) social groups, resulting in differences in social stability as well as average stocking density (72% and 87%, respectively, but with much greater variance in the stable groups). There were no differences between treatments in plasma concentrations of NEFA or BHB, the incidence of retained placenta, metritis, endometritis, lameness or early culling, the prevalence of persistent anovulation, pregnancy at first or second insemination or energy-corrected milk yield. In a subset of 34 to 40 cows per treatment, there were no differences between treatments in neutrophil phagocytosis or oxidative burst function or expression of L-selection or CD18, production of IgG against ovalbumin, serum haptoglobin concentrations or concentrations of cortisol in blood (Silva et al., Reference Silva, Moraes, Mendonça, Scanavez, Nakagawa, Ballou, Walcheck, Haines, Endres and Chebel2013a).
Miltenburg et al. (Reference Miltenburg, Duffield, Bienzle, Scholtz and LeBlanc2018a) assigned a total of 48 cows in groups of 6 to 10 to either of two space allowances for the 3 weeks before calving: 90 cm of feeding rail space per cow and 80% cows to freestalls, or 45 cm of feeding space and 120% cows to stalls. Lying time was reduced by 2 h/day in the overcrowded group but was still >12 h/day on average. As seen by Silva et al. (Reference Silva, Dresch, Machado, Moraes, Lobeck-Luchterhand, Nishimura, Ferreira, Endres and Chebel2014), there were more competitive displacements at the feed bunk in overcrowded groups, although that was confounded with a greater number of such interactions among multiparous cows generally. Overcrowded cows tended to have greater liver fat content at week 3 postpartum. However, during the treatment period and to 5 weeks postpartum, there were no differences between treatments in serum BHB, NEFA, glucose, insulin, insulin-like growth factor 1, aspartate aminotransferase, bilirubin or haptoglobin concentrations, and no differences in neutrophil phagocytosis or oxidative burst function. One interesting finding was that when cows were ranked by successful displacements of other cows at the feed bunk, in under-crowded groups, medium and high success index animals had greater neutrophil oxidative burst function than low success cows or any cows in overcrowded groups. This suggests that greater space allowance may have an effect on innate immune function, but it is not to improve function for the most submissive cows. Similarly, Chebel et al. (Reference Chebel, Silva, Endres, Ballou and Luchterhand2016) found that the cows in the 90th percentile of social rank (most dominant) had modestly but significantly greater neutrophil phagocytosis and oxidative burst capacity, particularly at calving. Also interestingly, and in contrast to Huzzey et al. (Reference Huzzey, Veira, Weary and von Keyserlingk2007) compilation of data from several large experiments on space and social group stability indicated that, counter-intuitively, more dominant cows (based on displacements at the feed bunk) were at greater risk of retained placenta or metritis (Chebel et al., Reference Chebel, Silva, Endres, Ballou and Luchterhand2016). These authors hypothesise that such cows may spend more time in aggressive interactions but fail to use their apparent advantage to consume more feed or achieve greater health (at least with respect to uterine disease). We concluded from our study (Miltenburg et al., Reference Miltenburg, Duffield, Bienzle, Scholtz and LeBlanc2018a) and the published data on the effects of crowding on metabolic health and innate immune function that the evidence does not refute the potential importance of space allowances under field conditions, but feeding and lying space alone are not the critical determinants of immune function in transition dairy cows. The minimal and optimal amounts of feeding and lying space likely depend on other variables, including whether primiparous and multiparous animals are commingled in the prepartum period (Chebel et al., Reference Chebel, Silva, Endres, Ballou and Luchterhand2016).
Nutrient supply for neutrophil function
Mounting an immune or inflammatory response consumes meaningful quantities of nutrients, and is notably energetically costly. In an elegant experiment, Kvidera et al. (Reference Kvidera, Horst, Abuajamieh, Mayorga, Sanz Fernandez and Baumgard2017) employed an IV LPS challenge with a euglycemic clamp technique to estimate the minimal requirement of glucose to mount an acute inflammatory response, which was approximately a net of 1 kg of glucose in 12 h. The glucose requirement for immune response appears to be consistent among dairy and beef cattle at 0.7 to 1.0 g/kg body weight0.75 per hour.
Homeorrhetic adaptions in support of lactation are oriented around partitioning nutrients to the mammary gland, notably increasing the supply of glucose for milk synthesis by sparing its use by other tissues, primarily by inducing peripheral insulin resistance. This situation likely contributes to reduction of immune function in early lactation. Activated immune cells appear to be obligate users of glucose and to increase their consumption of glucose (Kvidera et al., Reference Kvidera, Horst, Abuajamieh, Mayorga, Sanz Fernandez and Baumgard2017). The fuels used by bovine neutrophils are not well characterised but glucose appears to be crucial (Ingvartsen and Moyes, Reference Ingvartsen and Moyes2013). Conversely, in vitro supplementation of glucose in neutrophils from early- or mid-lactation dairy cows modestly increased phagocytic capacity but mostly did not meaningfully affect neutrophil function (Garcia et al., Reference Garcia, Elsasser, Qu, Zhu and Moyes2015). However, the basal concentration of glucose in the cell media (7.2 mmol/l) used by Garcia et al. was considerably greater than in circulation in cows of either stage of lactation (∼3.2 mmol/l), or even in dry cows. Once migrated, neutrophils likely must rely on stores of glycogen to function. Galvão et al. (Reference Galvão, Flaminio, Brittin, Sper, Fraga, Caixeta, Ricci, Guard, Butler and Gilbert2010) showed that there were lesser glycogen stores in neutrophils in circulation at calving in cows that 3 to 7 days later had metritis. That is consistent with Hammon et al. (Reference Hammon, Evjen, Dhiman, Goff and Walters2006), who showed that circulating neutrophils had lower oxidative burst capacity in cows that 1 week later had metritis, or 3 to 4 weeks later had endometritis. In that study, one explanatory variable was that neutrophils from cows in the lowest quartile of feed intake through the 3 weeks before calving (despite ad libitum availability) had (in relative terms) 50% lesser oxidative capacity from 1 week before to 3 weeks after calving than cows in the top quartile of feed intake. In summary, it seems that the availability of glucose to fuel neutrophil function may be a contributing factor to the impaired capacity observed in the transition period.
The supply of antioxidants (e.g., selenium and vitamin E) is important to contain the potent oxygen free radicals generated within neutrophils as part of their killing function. If there is insufficient selenium or sulphur-containing amino acids, glutathione peroxidase may not be able to detoxify hydrogen peroxide in the cytosol. Similarly, insufficient vitamin E may allow hydroxyl radicals to initiate a chain reaction of cell membrane peroxidation. Either could result in neutrophils conducting a short-lived suicide mission rather than a more sustained response with numerous iterations of ingestion and degradation of bacteria.
There is an abundance of large-scale observational studies that demonstrate associations of elevated serum concentrations of NEFA and/or BHB (hyperketonemia or ketosis) with increased risk of infectious and metabolic disease (summarised in McArt et al., Reference McArt, Nydam, Oetzel, Overton and Ospina2013). Briefly, serum NEFA > 0.3 mmol/l in the 1 to 2 weeks before expected calving is associated with increased risk of retained placenta, metritis or displaced abomasum, decreased milk production and worse reproductive performance. Serum or blood BHB ≥ 1.2 mmol/l in the first 2 weeks after calving is associated with increased risk of displaced abomasum, endometritis, prolonged anovulation and early culling, decreased milk yield in early lactation (conditional on the concentration of BHB and the timing of onset of ketosis) and decreased reproductive performance (McArt et al., Reference McArt, Nydam, Oetzel, Overton and Ospina2013). Elevated serum NEFA concentrations and hyperketonemia are indicators of some degree of maladaptive response to the demands of lactation, probably in some measure reflect the availability of glucose to fuel neutrophils, and more generally are correlated with a greater degree of negative energy balance and perhaps heightened systemic inflammation, all of which are intertwined. In the whole animal, it is difficult to distinguish between these markers as only being indictors of other complex processes, or whether NEFA or BHB might have direct effects on innate immune function. Several studies provide insight into this question, with inconsistent results.
Non-esterified fatty acids
Neutrophils were collected from eight Holstein cows in mid-lactation and incubated with mixtures of NEFA to resemble concentrations from low to typical in the days after calving (2-fold increments from 0.06 to 1 mmol/l) to extremely high (2 mmol/l) (Scalia et al., Reference Scalia, Lacetera and Bernabucci2006). Phagocytic function was not affected at any concentration of NEFA, and oxidative burst function was only affected at 2 mmol/l, where it was substantially increased, but in conjunction with massive neutrophil necrosis (49% v. <1% at lower concentrations), though not increased apoptosis. It is unclear if brief in vitro exposure of circulating neutrophils to moderately elevated concentrations of NEFA replicates the direct or indirect effects of maturation and circulation in the milieu of a peripartum cow with elevated blood NEFA concentrations, which likely also includes increased concentrations of pro-inflammatory cytokines and other modulators of neutrophil function. Ster et al. (Reference Ster, Loiselle and Lacasse2012) also mixed neutrophils from mid-lactation cows with a mixture of NEFA at 0, 0.1, 0.25, 0.5 or 0.75 mmol/l to mimic concentrations in early postpartum cows. They observed a dose-dependent reduction in oxidative burst function, with significant and substantial impairment at the two higher concentrations. They did not measure other aspects of neutrophil function with the spiked NEFA approach, but they did demonstrate inhibition of proliferation of peripheral blood mononuclear cells (PMBC) with NEFA as low as 0.13 mmol/l. Hammon et al. (Reference Hammon, Evjen, Dhiman, Goff and Walters2006) found a significant but modest correlation between in vivo plasma NEFA concentration and neutrophil oxidative burst activity in vitro (R 2 = 0.2). Taken together, these studies support a possible direct and rapid effect of elevated concentrations of NEFA on the functional capacity of circulating neutrophils, but encourage more investigation into the mechanisms by which perhaps specific fatty acids modulate neutrophil functions and whether such effects are more relevant during maturation or in circulation.
Ketosis
Greater severity of clinical mastitis has been observed in cows with ketosis (Kremer et al., Reference Kremer, Burvenich, Noordhuizen-Stassen, Grommers, Schukken, Heeringa and Brand1993). Hoeben et al. (Reference Hoeben, Heyneman and Burvenich1997) isolated neutrophils from seven high-producing (presumably non-ketotic) cows and employed several assays to assess oxidative burst function in samples with BHB added at 0.01, 0.05, 0.1, 1.0 or 2.5 mmol/l. That study demonstrated impairment of generation of hydrogen peroxide specifically, through not of superoxide anion or myeloperoxidase activity, with BHB ≥ 1.0 mmol/l. In an experiment with only three to six cows per group with naturally occurring differences in blood BHB concentration, neutrophil chemotaxis was reduced in cows with BHB > 1.6 mmol/l (Suriyasathaporn et al., Reference Suriyasathaporn, Daemen, Noordhuizen-Stassen, Dieleman, Nielen and Schukken1999). Neutrophils from these same cows were then incubated with different combinations of ketone bodies (BHB alone at 1.0 or 4.8. mmol/l; acetoacetate or acetone alone; or a combination of all three at high or low concentrations). β-hydroxybutyrate alone did not consistently impair chemotaxis, but the other ketones and the combination of all did in all cases. This experiment has the advantage of using neutrophils that had natural exposure to ketotic cows (presumably not only elevated BHB, although the cows were at 5 to 10 weeks in lactation, so not the complex metabolic and endocrine milieu of the transition period). Ster et al. (Reference Ster, Loiselle and Lacasse2012; details above) demonstrated that adding BHB up to 1 mmol/l had no effect on blood mononuclear cell proliferation or interferon-γ production, and up to 10 mmol/l (i.e., extremely high) had no effect on neutrophil oxidative burst function. Similarly, Hammon et al. (Reference Hammon, Evjen, Dhiman, Goff and Walters2006) found no association of blood BHB concentrations with neutrophil killing ability. As with NEFA, the effects of ketone bodies are inconsistent, but there is sufficient evidence to suggest a potential effect on migration and killing functions of neutrophils. It is unclear if BHB alone is a sufficient cause of impaired neutrophil function.
Calcium
Intracellular calcium signalling is a key element in the activation of neutrophils. Calcium acts as a second messenger for intracellular signal transduction for a variety of cell-surface receptors (Vig and Kinet, Reference Vig and Kinet2009). Neutrophils treated in vitro with ethylene diamine tetraacetic acid, an extracellular calcium ion chelator, had severely reduced phagocytosis capacity (Ducusin et al., Reference Ducusin, Sarashina, Uzuka, Tanabe and Ohtani2001). Therefore, hypocalcemia around calving may contribute to immune cell dysfunction. Neutrophils collected from cows with clinical milk fever had lower intracellular calcium concentrations and impaired phagocytosis compared to cows without parturient paresis (Ducusin et al., Reference Ducusin, Uzuka, Satoh, Otani, Nishimura, Tanabe and Sarashina2003). Kimura et al. (Reference Kimura, Reinhardt and Goff2006) isolated PMBC from 27 cows through the transition period, 8 of which had developed clinical milk fever, and assessed the quantity and release of intracellular calcium stores. They showed that the stimulated flux of intracellular ionised calcium (iCa) was reduced at calving, and lower in cows that developed milk fever days from 12 days before the onset of clinical signs. Intracellular iCa response of PMBC was low at the time of milk fever, but doubled following treatment with intravenous calcium, indicating prompt response of PMBC intracellular iCa to increased plasma concentrations of total calcium. The releasable intracellular iCa store decreased before calving and was correlated with blood calcium concentration and with intracellular iCa flux in response to stimulation. The authors suggested that intracellular stores of iCa may be diminished before calving as there is next efflux of iCa in an attempt to maintain blood calcium concentration, likely contributing to impaired PMBC function by decreasing the magnitude of intracellular iCa flux available to activate cell function.
Recent work demonstrates the calcium ‘cost’ of mounting an inflammatory response to an acute LPS challenge. Using a model analogous to Kvidera et al. (Reference Kvidera, Horst, Abuajamieh, Mayorga, Sanz Fernandez and Baumgard2017) for glucose, Horst et al., (Reference Horst, Mayorga, Al-Qaisi, Abeyta, Portner, McCarthy, Goetz, Ramirez-Ramirez and Baumgard2018) showed that in the 12 h after challenge with LPS, blood calcium concentration was reduced by 32%, and maintenance of eucalcemia during that time required infusion of 12 g of Ca, or somewhat more than the typical deficit (8 to 10 g) in a cow recumbent with milk fever.
Subclinical hypocalcemia is highly prevalent among periparturient cows and is associated with increased risk of displaced abomasum (Chapinal et al., Reference Chapinal, Carson, Duffield, Capel, Godden, Overton, Santos and LeBlanc2011) and milk production losses (Chapinal et al., Reference Chapinal, Carson, LeBlanc, Leslie, Godden, Capel, Santos, Overton and Duffield2012) and increased culling risk in early lactation (Roberts et al., Reference Roberts, Chapinal, LeBlanc, Kelton, Dubuc and Duffield2012). Cows classified at high risk for metritis (having one or more of dystocia, twins, stillbirth or retained placenta) that were able to maintain serum calcium concentrations above 2.15 mmol/l had one-half and one-third the incidence of metritis and puerperal metritis, respectively, when compared to low metritis risk cows that were below this cut-point at least once in the first 3 days postpartum (Martinez et al., Reference Martinez, Risco, Lima, Bisinotto, Greco, Ribeiro, Maunsell, Galvão and Santos2012). That study showed reduced total circulating neutrophil number, neutrophil phagocytosis and neutrophil oxidative burst capacity in cows with blood calcium <2.15 mmol/l through the first 3 days postpartum. Based on the variables measured in the study, at least two-thirds of the cases of metritis were estimated to be attributable to having blood calcium below 2.15 mmol/l in the first 3 days postpartum. Even if that is an overestimate if more variables in more herds were considered, it points to suboptimal calcemia contributing meaningfully to the occurrence of metritis, mediated at least in part by impairment of neutrophil function.
The same research group explored this association through experimental induction of hypocalcemia with a 24-h infusion of a selective iCa chelator (Martinez et al., Reference Martinez, Sinedino, Bisinotto, Ribeiro, Gomes, Lima, Greco, Risco, Galvão, Taylor-Rodriguez, Driver, Thatcher and Santos2014). They used 10 mature, non-pregnant, non-lactating cows in a crossover design. By about 4 h after the start of treatment, steady-state plasma concentrations of ∼0.75 mmol/l iCa and ∼1.75 mmol/l total calcium were maintained for 20 h; therefore, the model replicated blood calcium levels in a hypocalemic (but not milk fever) cow in the day after calving. Feed intake (∼5 v. 10 kg DM/day), blood glucose (∼4.2 v. 4.4 mmol/l) and insulin concentrations decreased and NEFA increased during treatment, so the effects of treatment may not all be directly attributable to calcium. Neutrophil phagocytosis and oxidative burst function decreased at the end of the infusion and continued to diverge negatively from the controls until 3 days after the end of the infusion. The data patterns for both measures of neutrophil function were similar to those in their field study (Martinez et al., Reference Martinez, Risco, Lima, Bisinotto, Greco, Ribeiro, Maunsell, Galvão and Santos2012). Similar to Kimura et al. (Reference Kimura, Reinhardt and Goff2006), experimentally induced hypocalcemia decreased the stimulated intracellular iCa flux (Martinez et al., Reference Martinez, Sinedino, Bisinotto, Ribeiro, Gomes, Lima, Greco, Risco, Galvão, Taylor-Rodriguez, Driver, Thatcher and Santos2014). The data from the latter study support earlier experimental and observational data that transient (≤1 day) hypocalcemia contributes to impaired neutrophil function and consequently to disease risk.
Emerging data (McArt and Neves, Reference McArt and Neves2020) indicate that the pattern and duration of reduced blood calcium concentrations in the 4 days after calving are more predictive of disease risk and milk yield than the nadir concentration or single point measurements in the first 24 h after calving. This new approach to classifying hypocalcemia should be applied to study its effects on neutrophil function.
The effect of calcium supplementation or prepartum diet on neutrophil function
We evaluated (Miltenburg et al., Reference Miltenburg, Duffield, Bienzle, Scholtz and LeBlanc2018b) whether administration of an injectable calcium supplement product at time of calving increased neutrophil oxidative burst or phagocytosis capacity. Cows (n = 27) from four farms were blocked by parity and randomly assigned to receive either a commercial injectable calcium supplement or a placebo within 12 h after calving and again 24 h later. In a separate study with the same protocol (Miltenburg et al., Reference Miltenburg, Duffield, Bienzle, Scholtz and LeBlanc2016), treatment increased serum total calcium at 24 h postpartum, conditional on calcium concentration before treatment. Total serum calcium concentration (tCa), neutrophil oxidative burst and neutrophil phagocytosis capacity were measured from coccygeal blood samples before and 72 h after the first treatment. The study animals were 23 first parity heifers and 6 multiparous cows. There was no effect of treatment on oxidative burst or phagocytosis. Therefore, despite plasma calcium concentration being associated with neutrophil function as described above, this study does not support the ability of supplemental calcium, as given to low-parity parturient cows soon after calving, to improve oxidative burst or phagocytosis capacity of neutrophils.
Martinez et al. (Reference Martinez, Rodney, Block, Hernandez, Nelson, Lean and Santos2018) used 80 cows in a 2 × 2 factorial experiment of positive or negative (−130 mEq/kg DM) dietary cation–anion difference (DCAD) with different dietary sources of vitamin D fed for 4 weeks before calving to assess a variety of health outcomes. The negative DCAD treatment increased plasma iCa and tCa at calving and 1 day later. Regarding neutrophil function, there were no effects of treatments on phagocytosis, and no interactions of the effects of DCAD and source of vitamin D on neutrophil function. Overall, cows fed calcidiol had better oxidative burst function postpartum than those fed cholecalciferol. Among multiparous cows, there was a modest effect of the negative DCAD diet to improve phagocytosis function before calving and oxidative burst function after calving. Therefore, improving maintenance of calcium homeostasis through dietary prevention approaches holds some promise for support of neutrophil function.
Immune modulation treatment
Almost since reduction of aspects of peripartum neutrophil function was documented (Kehrli et al., Reference Kehrli, Nonnecke and Roth1989), there has been interest in interventions that might mitigate the problem, initially with reference to mastitis (reviewed by Kehrli et al., Reference Kehrli, Cullor and Nickerson1991a). The focus has been on stimulation of the circulating numbers, and possibly function of neutrophils using G-CSF. Daily injections of recombinant bovine G-CSF increased circulating neutrophil count and some but not all elements of function (Kehrli et al., Reference Kehrli, Goff, Stevens and Boone1991b). Another early attempt (Cai et al., Reference Cai, Weston, Lund, Brodie, McKenna and Wagner1994) incubated isolated bovine neutrophils from healthy cows or those with retained placenta, metritis or mastitis with recombinant human G-CSF or granulocyte-macrophage CSF. No effect was found on six measures of migration, chemotaxis, ingestion or killing capacity. Incubation of bovine neutrophils with G-CSF did not induce oxidative burst, but enhanced it when the cells were later activated by exposure to bacteria; this effect was augmented by prior exposure to both IL-8 and G-CSF (Mitchell et al., Reference Mitchell, Albright and Caswell2003). Interest was revived when a pegylated recombinant bovine G-CSF (PEG-rbG-CSF) product that only needed to be injected approximately weekly became commercially available. A small study with that product (Imrestor; Elanco Animal Health Greenfield, IL, USA) demonstrated substantial increases in circulating neutrophil count and an increase in myeloperoxidase exocytosis, but not phagocytosis or oxidative burst (Kimura et al., Reference Kimura, Goff, Canning, Wang and Roth2014). Those effects have been confirmed in subsequent studies (McDougall et al., Reference McDougall, LeBlanc and Heiser2017; Van Schyndel et al., Reference Van Schyndel, Carrier, Bogado Pascottini and LeBlanc2018). Blood neutrophils from cows treated with PEG-rbG-CSF had greater expression of 3 (ICAM1, TLR2 and PTGS2) of a panel of 20 genes selected to represent the chain of functional steps of neutrophils (Heiser et al., Reference Heiser, LeBlanc and McDougall2018). These differences in gene expression suggested possible enhancement of cell adhesion for migration, recognition of pathogens (Gram-positive bacteria in particular) and generation of inflammation through the cyclooxygenase pathway. However, functional assays from the same study (McDougall et al., Reference McDougall, LeBlanc and Heiser2017) showed increased myeloperoxidase release but no difference in phagocytosis or oxidative burst. Heiser et al. (Reference Heiser, LeBlanc and McDougall2018) also isolated neutrophils from the uterus at 4 and 7 days postpartum and showed 11 differentially expressed genes in cows treated with PEG-rbG-CSF which collectively suggested enhanced antimicrobial capacity. Several randomised controlled trials demonstrated an approximately one-third relative reduction in the incidence of clinical mastitis in early lactation following treatment with PEG-rbG-CSF. Hassfurther et al. (Reference Hassfurther, Terhune and Canning2015) assigned just over 50 cows per group to control, 5, 10 or 20 μg/kg PEG-rbG-CSF (2 doses: at 7 days before expected calving and within 24 h after). Treatment produced the expected ∼4-fold increase in circulating neutrophil counts the day after the first administration (to ∼20 × 109 cells/l), with lesser, dose–responsive increases present 7 days after the second administration. The study was conducted on one commercial farm, where the dirt resting area was kept wet to increase the incidence of mastitis for the study. The incidence of clinical mastitis to 28 days postpartum was 34%, 20%, 17% and 9% for control, 5, 10 or 20 μg/kg PEG-rbG-CSF, respectively. There were no differences among groups in the severity of cases of mastitis, although the study was not really powered to assess this outcome. Canning et al. (Reference Canning, Hassfurther, TerHune, Rogers, Abbott and Kolb2017) randomised a total of 640 cows across four farms to receive either 15 mg PEG-rbG-CSF (equivalent to 22 μg/kg for a 675 kg cow) or saline, twice, as above. They observed increases in circulating neutrophil counts similar to Hassfurther et al. (Reference Hassfurther, Terhune and Canning2015) for the corresponding time points and dose. The reporting of the data makes it difficult to calculate exact outcome measures, but the incidence of clinical mastitis was lower in the treated group (∼15%) than in the control group (∼23%). Reproductive outcome measures were reported, but the study was not designed to have sufficient power to assess these outcomes. The observation of a reduction in the treatment group in the proportion of cows failing to be detected in oestrus by 80 days postpartum was driven by a single farm and would require confirmation. Ruiz et al. (Reference Ruiz, Tedeschi and Sepulveda2017) used the same dose and timings of administration of PEG-rbG-CSF as in Canning et al. (Reference Canning, Hassfurther, TerHune, Rogers, Abbott and Kolb2017) in 10 238 cows across 17 farms in Mexico. Treatments were assigned based on ear-tag numbers and no placebo was employed, increasing the risk of bias due to lack of blinding and not-truly random assignment to treatment. The incidence risks of retained placenta were 5.8% and 5.6%, of metritis to 21 days 8.4% and 9.8%, and of clinical mastitis to 30 days postpartum 4.9% and 3.7% in the control and treated groups, respectively. The latter two disease risks were statistically different. Paradoxically, this study could be said to be statistically over-powered, because the biological and economic importance of these differences is questionable. Moreover, the case definition used for metritis was not well validated.
Given what is understood about the apparently important role of neutrophil dysfunction in the pathogenesis of retained placenta, metritis and endometritis (reviewed by LeBlanc, Reference LeBlanc2014), it was plausible to hypothesise that treatment with PEG-rbG-CSF would reduce the incidence of these diseases. However, in one large controlled trial (Zinicola et al., Reference Zinicola, Korzec, Teixeira, Ganda, Bringhenti, Tomazi, Gilbert and Bicalho2018), despite the substantial increase in blood neutrophil counts as expected, there were no treatment effects on the risk of retained placenta, uterine diseases or mastitis, and inexplicably increased risks of displaced abomasum and lameness. Taken together, it appears that PEG-rbG-CSF consistently causes substantial increases in the number of circulating neutrophils for several weeks, with modest increases in per-cell function, most consistently of extracellular release of myeloperoxidase, but that these effects do not consistently reduce the incidence of diseases thought to be importantly related to neutrophil function. If a central role of innate immune function in reproductive tract diseases is true, these data underline the complexity of effective neutrophil response and subsequent regulation of inflammation; even massive increases in the numbers of neutrophils available in circulation do not predictably result in less disease.
Conclusion
There is a body of evidence to support that each of glucose supply, blood concentrations of calcium, NEFA and BHB, and treatment with rbG-CSF are associated with the overall capacity of neutrophil responses in dairy cows in the 1 to 2 weeks after calving. Most commonly, the aspect of neutrophil function reported to be affected relates to oxidative burst, although that may be skewed because it is the most studied. There are fewer assessments of migration capacity, and still little in the bovine on NET function, apoptosis, cross-talk of neutrophils with the adaptive immune system or the role of neutrophils in down-regulating inflammation after the initial response. Notably, none of the effects of these factors influencing neutrophil function has been consistent among studies. Furthermore, controlled studies of socially competitive environments do not reproduce the effects on immune function or related clinical diseases that would be expected from empirical observations. These inconsistencies may be partially attributable to differences in the study populations or the methods of analysis of neutrophil function, but it seems more likely that the interactions of these known factors (and probably others) are the key determinants of effective innate immune function and inflammatory response. Future research should assess the interactions among markers of energy supply and metabolism, and of those with calcium supply, and investigate the effects of the timing and duration of these effects. Many of the recent observations and hypotheses on neutrophil function in humans and mice have not been explored in cattle, including the existence of tissue-specific recruitment mechanisms and whether tissue ‘resident’ (or at least marginated) populations exist in certain organs, notably in the uterus and udder. It would also be particularly relevant in cattle to pursue the question of whether neutrophils behave or are regulated differently in infection, injury and sterile inflammation (also known as metabolic inflammation). It would be of practical importance to understand whether glucose and calcium supply, or exposure to NEFA or BHB are critical during myelopoiesis, in circulation in blood, or both. When and how are glycogen and calcium taken onboard neutrophils? For clinical application, the present state of scientific evidence is consistent with the notion that best management practices to support adaptive metabolism and prevent excessive negative energy balance or hypocalcemia should plausibly be beneficial for innate immune function in transition dairy cows. However, there are insufficient data to make specific recommendations that would consistently enhance neutrophil function or reduce the incidence of diseases understood to be consequences of impaired or dysregulated immune function.
Acknowledgement
No funding sources supported production of this review paper.
S. J. LeBlanc 0000-0003-2027-7704
Declaration of interest
The author declares no conflicts of interest.
Ethics statement
No new animal or human studies were performed for this paper. No ethics statement was thus required.
Software and data repository resources
None of the data were deposited in an official repository.