Introduction
Different rearing factors (including breed, sex, age, diet) can modify meat and carcass quality in cattle. Among these rearing factors, dietary conditions are known to affect carcass characteristics by modifying the content and composition of adipose tissue (AT), especially when comparing beef produced in forage v. grain finishing systems (French et al., Reference French, Stanton, Lawless, O’Riordan, Monahan, Caffrey and Moloney2000). Consumers throughout Europe are showing a growing interest for meat produced from grass-fed ruminants, which is considered to be healthier and more natural than that obtained from grain-fed animals (Geay et al., Reference Geay, Bauchart, Hoquette and Culioli2001). Indeed, a grass-based diet compared with the maize-silage-based diet increased the polyunsaturated fatty acid (PUFA) to saturated fatty acid (SFA) ratio and decreased the n-6-to-n-3 PUFA ratio in intermuscular and subcutaneous AT (Noci et al., Reference Noci, Monahan, French and Moloney2005). However, nutritional treatment can also affect the metabolism of bovine AT, with roughage diets resulting in decreased carcass fatness and AT lipogenesis compared with concentrate diets (Schoonmaker et al., Reference Schoonmaker, Fluharty and Loerch2004). These data have been explained on the basis of differences in energy intake. However, one study in ewes fed diets with the same energy density reported that lipogenesis was lower in the subcutaneous AT of ewes fed roughage compared with those fed a concentrate diet (Okine and Arthur, Reference Okine and Arthur1997). The first objective of the present study was therefore to evaluate the influence of a grass diet compared with a maize silage diet on AT lipogenesis in steers reared at the same growth rate. Moreover, one characteristic of animals produced on pasture is a higher physical activity than those raised indoors. Thus, it is difficult to dissociate, at pasture, the respective effects of the nature of the diet and the activity of the animal, since the mobility of animals could decrease lipogenic activity and fat deposition, as has been observed in rodents (Nara et al., Reference Nara, Kanda, Tsuhui, Inukai, Umeda, Inoue and Kobayashi1999; Faulconnier et al., Reference Faulconnier, Arnal, Patureau Mirand, Chardigny and Chilliard2004). Thus, our second objective was to compare the respective effects of feeding grass either at pasture or indoors. Lipogenic enzyme activities involved in de novo lipogenesis, fatty acid (FA) esterification, triglyceride uptake and adipose cell size were assessed in different AT sites (perirenal, intermuscular and/or subcutaneous AT) since regional differences have been reported in the responses of bovine AT deposits to dietary manipulations (Chilliard and Robelin, Reference Chilliard and Robelin1985; Gilbert et al., Reference Gilbert, Lunt, Miller and Smith2003; Bonnet et al., Reference Bonnet, Faulconnier, Hocquette, Bocquier, Leroux, Martin and Chilliard2004). Other parameters linked to lipogenesis and adiposity, such as plasma glucose, non-esterified FA (NEFA), insulin and leptin, are also reported.
Material and methods
Animals, diets and experimental design
The two experiments were conducted at the INRA Le Pin-au-Haras experimental farm in full compliance with national legislation on animal care and on experimentation on living animals.
Experiment 1: comparison of grazed grass-based and maize-silage-based diets on adipose tissue lipogenesis and plasma metabolites and hormones
Twenty-four Charolais steers weaned at 8 months of age were allotted into two treatment groups, i.e. either maize silage or grazed grass groups, with 12 animals in each group, based on birth weight, weaning weight and body condition score (scale of 0 (very thin) to 5 (very fat)). The animals were castrated at 9 months of age. The whole study lasted 23 months from weaning and covered two winter seasons and two summer seasons. The feeding value of the food ingredients and diets and the nutritional requirements of the animals were calculated according to INRA (1978) feeding standards. Steers of the maize-fed group were housed in open sheds with 12 animals per pen of 80 m2 and were fed maize silage (0.9 UFL, 50 g PDIN and 70 g PDIE per kg dry matter (DM)) with a small amount of wheat straw (0.4 UFL, 22 g PDIN and 44 g PDIE per kg DM) and rapeseed meal (1.1 UFL, 260 g PDIN and 165 g PDIE per kg DM) throughout the duration of the experiment. Over the last summer season, ingredient composition of the maize diet calculated on a dry matter basis averaged 72% maize silage, 17% wheat straw and 11% rapeseed meal, respectively. During the winter seasons, steers on the grass treatment were fed ad libitum grass silage (from a permanent pasture of perennial rye-grass (0.7 UFL, 49 g PDIN and 52 g PDIE per kg DM)) supplemented with a commercial concentrate (1.1 UFL, 111 g PDIN and 128 g PDIE per kg DM) and/or rapeseed meal (1.1 UFL, 260 g PDIN and 165 g PDIE per kg DM). Over the last winter season, grass silage, concentrate and rapeseed meal accounted for 85%, 11% and 4% of the total dry matter intake, respectively. During the two summer seasons, animals rotationally grazed on a perennial rye-grass pasture (estimated to have a mean value of 0.9 UFL, 94 g PDIN and 89 g PDIE per kg DM) without supplementation.
Experiment 2: Comparison of the effects of grazed grass-based, cut grass-based or maize-silage-based diets on adipose tissue lipogenesis and plasma metabolites and hormones
Twenty-four Charolais steers weaned at 8 months of age were allotted into one of the two treatment groups, with 8 and 16 animals to the maize silage and grass treatment groups, respectively, based on birth weight, weaning weight and body condition score. The animals were castrated at 9 months of age. The whole study lasted 23 months from weaning, and covered two winter seasons and two summer seasons. The animals were housed in open sheds, with eight animals per 80 m2 pen, except at pasture. The steers of the maize silage group were fed maize silage (0.9 UFL, 50 g PDIN and 70 g PDIE per kg DM) with a small amount of wheat straw (0.4 UFL, 22 g PDIN and 44 g PDIE per kg DM) and rapeseed meal (1.1 UFL, 260 g PDIN and 165 g PDIE per kg DM) throughout the duration of the experiment. Over the last summer season, ingredient composition of the maize diet calculated on a DM basis averaged 73% maize silage, 15% wheat straw and 12% rapeseed meal, respectively. The animals on the two grass treatments were fed during the winter seasons ad libitum grass silage (from a permanent pasture of perennial rye-grass (0.83 UFL, 61 g PDIN and 61 g PDIE per kg DM)) with a small amount of wheat straw (0.4 UFL, 22 g PDIN and 44 g PDIE per kg DM) and supplemented with a commercial concentrate (1.1 PDIE, 111 g PDIN and 128 g PDIE per kg DM) and/or rapeseed meal (1.1 UFL, 260 g PDIN and 165 g PDIE per kg DM). Over the last winter season, grass silage, straw and concentrate accounted for 85%, 12% and 3%, of the total DM intake, respectively. During the first summer season, the animals of the grass treatment were grazed rotationally on a perennial rye-grass pasture. For the second summer season, the animals were split into two homogenous groups of eight animals each, on the basis of their age and live weight. The first group (grazed grass) was finished by rotational grazing of a perennial rye-grass pasture (0.85 UFL, 88 g PDIN and 86 g PDIE per kg DM), without any supplementation. The second group (cut grass) was offered ad libitum fresh cut grass daily, coming from the same plot as that used by the grazing group.
In these two experiments, feed intake of the grazing animals was estimated by determining the average grass height at entry and exit for each plot as well as the average weight of grass cut over a 30 m2 area from each plot. Daily feed intake of steers fed indoors was determined by the daily weighing of feed allowances and refusals. Live weights were measured every 2 weeks. Feeding allowance of the maize-silage-fed group was adjusted every 2 weeks so that animals would show the same average daily gain as those of the grazed grass group. The animals were then slaughtered at the end of the second grazing season, at the same final age (31 to 32 months of age) and at the same final average live weight (Table 1).
During each treatment period (i.e. at 400, 480, 780 and 840 days of age) and just prior to slaughter (at 964 days of age), jugular blood samples were collected after a night fast into EDTA-containing tubes and centrifuged. Plasma was collected and frozen at −20°C until analysis of plasma leptin, insulin, glucose and NEFA. Just after slaughter, samples of subcutaneous, perirenal and/or intermuscular AT were taken and either immediately placed at 37°C for adipocyte volume determination or stored at −80°C until analysis of lipogenic enzyme and lipoprotein lipase (LPL) activities. Subcutaneous AT was dissected on the rump in front of the tail and intermuscular AT from the longissimus thoracis muscle. Total fat (i.e. carcass fat depots plus internal fat depots) was also determined. Internal fat depots (i.e. perirenal, peritoneal, mesenteric and pericardiac AT) were weighed and carcass fat depots (i.e. essentially intermuscular, subcutaneous and intramuscular AT) were estimated from the composition of the sixth ribcut by regression equations, as described by Robelin and Geay (Reference Robelin and Geay1977).
Plasma metabolites and hormones
Plasma glucose and NEFA concentrations were determined enzymatically by the glucose dehydrogenase method (Glucose RTU kit; BioMérieux, Lyon, France) and the acyl-CoA Synthetase method (Wako-Unipath NEFA-C kit; Oxoid, Dardilly, France), respectively, as described by Ferlay and Chilliard (Reference Ferlay and Chilliard1999).
The leptin content of blood plasma was analysed in duplicate with an ovine-specific RIA that has been validated for the bovine species (Delavaud et al., Reference Delavaud, Ferlay, Faulconnier, Bocquier, Kann and Chilliard2002). Within- and between-assay variations were 6.5% and 7.6%, respectively.
Plasma insulin concentrations were assayed in duplicate using a commercially available porcine RIA kit (Cis Bio International, Gif sur Yvette, France) validated for bovine plasma. Within- and between-assay variations were 5.4% and 6.4%, respectively.
Tissue measurements
Adipose tissue cellularity was measured on tissue fixed with osmium tetroxide and digested in urea solution (Robelin, Reference Robelin1981). Cell diameters greater than 12.5 μm were measured with the Optimas software, and the mean volume was calculated from the individual cell volumes.
LPL activity was measured in AT using an artificial emulsion containing 3H-triolein after a detergent (Deoxycholate-Nonidet P40; Sigma Chemical, Saint-Quentin-Fallavier, France) extraction procedure (Faulconnier et al., Reference Faulconnier, Thévenet, Fléchet and Chilliard1994). The activities of glucose-6-phosphate dehydrogenase (G6PDH), malic enzyme (ME), fatty acid synthase (FAS) and glycerol-3-phosphate dehydrogensase (G3PDH) were assayed spectrophotometrically in AT as described previously (Faulconnier et al., Reference Faulconnier, Arnal, Patureau Mirand, Chardigny and Chilliard2004). Enzyme activities were expressed either as nmol of released fatty acids (LPL) or as nmol of reduced (G6PDH, ME) or oxidized (FAS, G3PDH) nucleotides per min and per 106 adipocytes.
Statistical analyses
The data presented in Tables 1 and 2 and Figure 2 were analysed using the anova procedure of the statistical package (Statistical Analysis Systems Institute (SAS), 1985). Differences between the two treatments were tested using the PLSD Fisher test. Values were considered to be significantly different if P values were less than 0.05, and tendencies with P < 0.10 are also indicated.
Table 1 Body weight, average daily gain and fat weight before slaughter in Charolais steers in Experiments 1 and 2Footnote †

† There was no significant between-treatment effect (P > 0.10).
‡ Measured between 26 and 32 months of age (last summer season).
§ Perirenal, peritoneal, mesenteric and pericardiac adipose tissues.
Table 2 Effect of the diet and grazing on plasma metabolite and hormone concentrations in Charolais steers before slaughter in Experiments 1 and 2

A,BIndicate that values with different superscript letters within the same row are significantly different (P < 0.05).
a,bIndicate that values with different superscript letters within the same row tend to be significantly different (P < 0.10).
The data presented in Tables 3 and 4 were analysed using the MIXED procedure of the SAS statistical package. The model accounted for diet treatment (D), AT sites (S) and their interaction (D × S) as fixed effects, and animal within-diet treatment was the random effect. A comparison of means was performed using the LSMEANS statement of the MIXED procedure. Differences between diet treatments and/or AT sites were considered significant if P values were less than 0.05.
Table 3 Effect of the diet and adipose tissue site on adipose tissue lipogenic enzyme activities and adipose cell volume in Charolais steers (n= 24) in experiment 1 (activities are expressed as mmol/min per 106 adipocytes)

Abbreviations are: IMAT = intermuscular adipose tissue; PRAT = perirenal adipose tissue.
A,BIndicate that values with different superscript letters within the same row are significantly different (P < 0.05).
‡There was no significant interaction between diet and adipose tissue site (P > 0.10).
Table 4 Effect of the diet and grazing on adipose tissue lipogenic enzyme activities and adipose cell volume in Charolais steers (n = 24) in Experiment 2 (activities are expressed as mmol/min per 106 adipocytes)

Abbreviations are: SCAT = subcutaneous adipose tissue; IMAT = intermuscular adipose tissue; PRAT = perirenal adipose tissue.
A,BIndicate that values with different superscript letters within the same row are significantly different (P < 0.05).
‡There was no interaction between diet and adipose tissue site (P > 0.10) except for G6PDH and ME (P < 0.05).
The data illustrated in Figure 1 were analysed using the MIXED procedure of the SAS statistical package. The model accounted for the diet treatment (D), age of the steers (A) and their interaction (D × A) as fixed effects, and animal within diet treatment was the random effect. A comparison of means was performed using the LSMEANS statement of the MIXED procedure. Differences between diet treatment and/or age of the steers were considered significant if P values were less than 0.05.
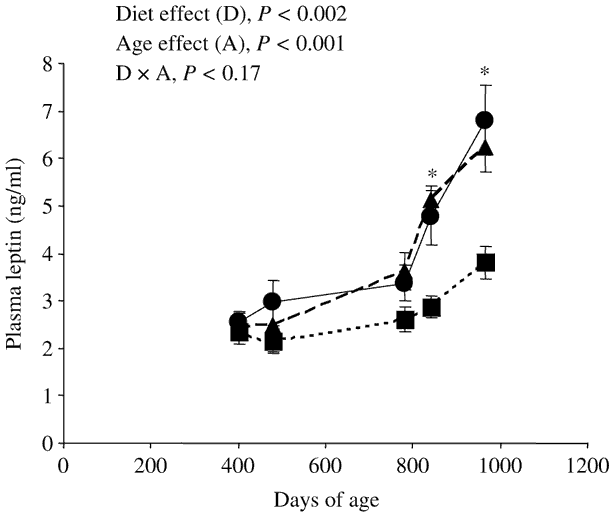
Figure 1 Effect of nature of forage [maize silage (●); grazed grass (▪); cut grass (▴)] and of age of the steers on plasma leptin concentrations (Experiment 2, mean ± s.e., eight steers per group). *Plasma leptin concentrations were significantly (P < 0.05) lower in steers grazing grass than in steers of the same age fed maize silage or cut grass.
Results
Effect of the diet and grazing on body weight, average daily gain and body fat weight
Growth performance remained similar across treatment groups. Final body weight (BW) and average daily gain before slaughtering did not differ between the two (Experiment 1) or the three (Experiment 2) treatment groups (Table 1). Total fat, internal AT and perirenal AT were not significantly affected by nutritional treatments (Table 1).
Effect of the diet and grazing on plasma metabolites and hormones
During the first experiment, plasma NEFA concentrations were higher (+137%) in steers grazing grass compared with those fed maize silage (Table 2), while plasma glucose and insulin concentrations remained similar between the two groups.
During the second experiment, plasma NEFA concentrations were also higher (+103%) in steers grazing grass compared with those fed maize silage, whereas plasma insulin concentrations were lower (−30%) in the grazing steers (Table 2). Plasma glucose concentrations did not differ between steers grazing grass and those fed maize silage. Steers fed cut grass showed lower plasma glucose and insulin concentrations (−17% and −27% for glucose and insulin, respectively) than steers fed maize silage (Table 2), whereas plasma NEFA concentrations were similar. Grazing per se (i.e. grazed grass v. cut grass diets) led to an increase in plasma glucose (+20%) and NEFA (+87%) concentrations but had no significant effect on plasma insulin concentrations.
At slaughter, plasma leptin concentrations were lower (−27% and −44% in the first and second experiments, respectively) in steers grazing grass than in steers fed maize silage (Table 2). In the second experiment, plasma leptin concentrations were similar between steers fed cut grass and steers fed maize silage, whatever the age of the steers (Table 2 and Figure 1). Grazing per se decreased (−44% and −39% at 840 and 964 days of age, respectively) plasma leptin concentrations. Moreover, whatever the diet, leptinaemia was significantly higher in older than in younger steers (Figure 1).
Effect of the diet, grazing and adipose tissue site on adipose cell size
During the first and the second experiments, the volume of adipocytes in perirenal and intermuscular AT was not significantly affected by the nature of the diet (Tables 3 and 4). Adipocyte number (×109) expressed per total perirenal AT was not affected by the nature of the diet (experiment 1: 7.1 ± 0.8 and 6.9 ± 1.4 in maize silage and grazed grass groups, respectively, P = 0.93; experiment 2: 7.1 ± 1.2, 5.4 ± 0.6 and 5.4 ± 0.2 in maize silage, grazed grass and cut grass-fed groups, respectively, P = 0.24). Grazing per se had no significant effect on adipocyte volume whatever the AT site studied (Table 4), nor on adipocyte number expressed per total perirenal AT (P = 0.98). Adipocyte volume was significantly affected by AT site (Tables 3 and 4) and was globally greater in perirenal than in intermuscular and subcutaneous AT (Figure 2).

Figure 2 Lipogenic enzyme activities (nmol/min per 106 adipocytes) and adipose cell size (pl) in perirenal (PR), intermuscular (IM) and subcutaneous (SC) adipose tissues (AT) in steers (Experiment 2, mean ± s.e., 24 steers). A,BMeans with different superscript letters are significantly different (P < 0.05).
Effect of the diet, grazing and adipose tissue site on lipogenic enzyme and lipoprotein lipase activities
The lipogenic enzyme activities measured in Experiment 1 (Table 3) were similar in the perirenal and intermuscular AT (except for ME activity, which was lower in perirenal AT), whereas there was a significant effect of AT site in Experiment 2 with greater activities in subcutaneous and/or perirenal AT than in intermuscular AT, whatever the nature of the diet (Figure 2) (except for ME activity which was again lower in perirenal AT).
During the first experiment, LPL, G6PDH, ME, FAS and G3PDH activities were lower in grazing steers compared with maize silage-fed animals, both in perirenal (−56%, 70%, 58%, 61% and 57%, respectively) and intermuscular (−53%, 67%, 50%, 39% and 34%, respectively) AT (Table 3).
In the second experiment, LPL, G6PDH, ME, FAS and G3PDH activities were significantly affected by the nature of the diet (Table 4). They were lower (from −22% to −74%) in perirenal and subcutaneous AT with the two grass diets compared with the maize silage diet, except for FAS activity in perirenal AT (Table 4). Similar trends were observed in intermuscular AT, although the effects on ME, FAS and G3PDH activities were less marked, especially when comparing grazed grass to maize silage diets (Table 4). Grazing per se had no significant effect on lipogenic enzymes in the three AT sites, except for G6PDH in subcutaneous AT, ME in intermuscular AT and G3PDH in subcutaneous and intermuscular AT, which all showed increased activity (from 50% to 99%) under the grazing treatment (Table 4).
Discussion
This study shows that the activity of lipogenic enzymes involved in NADPH generation for de novo lipogenesis (G6PDH, ME), FA synthesis and uptake (FAS and LPL, respectively) and esterification (G3PDH) were all lower in Charolais steers fed a cut grass diet compared with a maize silage diet in the three AT sites studied, although the effects were less marked in intermuscular AT. Depot-specific differences in lipogenic enzymes were observed, with greater activities (except for ME activity) in subcutaneous and/or perirenal AT than in intermuscular AT, which is consistent with previous reports (Whitehurst et al., Reference Whitehurst, Beitz, Cianzio and Topel1981; Mendizabal et al., Reference Mendizabal, Alberti, Eguinoa, Arana, Soret and Purroy1999) in different breeds of steers. However, the absence of interaction between adipose depot and dietary treatments for most enzymes indicates that diet-induced changes in lipogenic enzyme activities are consistent across adipose depots. The absence of any difference across treatment groups in final BW, average daily gains before slaughtering, total fat weight and AT weight, cell size and adipocyte number suggests that the effects observed on lipogenic activities were associated with the nature of the diet (cut grass v. maize diets) and not to differences in available net energy. To our knowledge, there is no published work comparing the effects of grass-based v. maize silage diets on AT lipogenesis in isoenergetically fed cattle. Our results are, however, in agreement with Okine and Arthur (Reference Okine and Arthur1997) who showed that acetyl CoA carboxylase (ACC) and FAS activities and the rate of FA esterification were 34%, 28% and 47% lower, respectively, in the subcutaneous AT of ewes isoenergetically fed roughage v. concentrate diets having the same energy density. Thus, the present study shows in cattle that lipogenic activities may be associated not only with the energy available for growth but also with the type of feed.
Among different putative mechanisms, the decrease in AT lipogenesis observed in the present study is probably at least partly due to differences in FA composition between the experimental diets. Indeed, grass-based diets generally present a lower proportion of oleic acid 18:1 (n-9) and linoleic acid 18:2 (n-6) and higher α-linolenic acid 18:3 (n-3) than the concentrate-based or maize-silage-based diets (Ferlay et al., Reference Ferlay, Martin, Pradel, Coulon and Chilliard2006). Literature data on ruminant AT have shown that diets supplemented with different unsaturated FA inhibited FA synthesis or lipogenic activities, the effects being more or less marked according to experiment duration, to the amount of the dietary lipid supplements, and to the degree of protection against ruminal biohydrogenation (see Chilliard, Reference Chilliard1993 for a review). Furthermore, Fickova et al. (Reference Fickova, Hubert, Crémel and Leray1998) showed, in rat epididymal adipocytes, that higher dietary levels of (n-3) PUFA increased the lipolytic response relative to (n-6) PUFA diets while diminishing insulin-stimulated glucose transport and lipogenesis. In pigs, Mourot et al. (Reference Mourot, Peiniau and Mounier1994) observed that the elevation of dietary linoleic supply 18:2 (n-6) increased ACC, ME and G6PDH activities in subcutaneous and perirenal AT. Thus, it is likely that the lower lipogenic enzyme activities observed in the present study in AT from cut-grass-fed steers was partly related to the more-abundant 18:3 (n-3) and less-abundant 18:2 (n-6) in grass diet compared with maize silage diet. Indeed, several studies have shown that the inclusion of pasture compared with concentrates in bovine diets led to an increase in proportion of PUFA, PUFA/SFA ratio and 18:3 (n-3) percentage and a decrease in 18:2 (n-6) percentage in subcutaneous and/or intermuscular AT (Realini et al., Reference Realini, Duckett, Brito, Dalla Rizza and De Mattos2004; Noci et al., Reference Noci, Monahan, French and Moloney2005). Pasture feeding (as compared with concentrate) also increased bovine subcutaneous AT concentrations of several trans isomers of 18:1 and 18:2 (Noci et al., Reference Noci, Monahan, French and Moloney2005; Dannenberger et al., Reference Dannenberger, Nuernberg, Nuernberg, Scollan, Steinhart and Ender2005), which could have antilipogenic effects (Faulconnier et al., Reference Faulconnier, Roy, Ferlay, Chardigny, Durand, Lorenz, Gruffat and Chilliard2006).
Another explanation for the lower lipogenic activities in steers fed cut grass compared with maize silage could be the absence of starch as compared with the maize diet. Maize is known to increase whole-body glucose turnover (Ortigues-Marty et al., Reference Ortigues-Marty, Vernet and Majdoub2003) through an enhanced supply of either gluconeogenic precursor or absorbed glucose (Chilliard et al., Reference Chilliard, Bocquier and Doreau1998), both stimulating insulin secretion (Gross et al., Reference Gross, Harmon, Minton and Avery1990). These known effects are coherent with the increase in plasma glucose and insulin concentrations observed in the present study in steers fed maize silage compared with cut grass and could result in higher lipogenic enzyme and LPL activities in AT. Indeed, although glucose is of minor importance as a carbon source for FA synthesis, the oxidation of glucose via the pentose phosphate pathway is an important source of reduced NADP, whereas its oxidation via glycolysis contributes to 3-glycerol phosphate for FA esterification in ruminant AT (Ballard et al., Reference Ballard, Filsell and Jarret1972; Janes et al., Reference Janes, Weekes and Armstrong1985). It could thus be hypothesized that lipogenic potential (as measured in vitro) was increased by glucose and insulin in the maize silage group and/or decreased by PUFA in the grass-fed groups, but that this potential was not expressed in vivo because of the management of animals at similar growth rates.
Leptin is a hormone primarily secreted by white AT, which is implicated in the regulation of food intake, energy expenditure and body fat stores and is modulated by dietary macronutrient composition (see Havel, Reference Havel2004 for a review). In cattle, plasma leptin levels are closely linked to AT cellularity, body condition score and nutritional state (see Chilliard et al., Reference Chilliard, Delavaud and Bonnet2005 for a review). To our knowledge, there is no published work on the effects of the grass diet v. maize silage diet on plasma leptin concentrations in ruminants. In the present study, the nature of the forage (cut grass v. maize silage diets) did not affect significantly plasma leptin concentration, regardless of the age of the steers. This is in agreement with the lack of variation in AT weight and cell size (and total adipocyte number in perirenal AT) between steers fed cut grass and maize silage, which had similar growth rates. Furthermore, no effect of feeding sunflower seeds or corn oil was observed on leptinaemia in growing or beef cattle (see Chilliard et al., Reference Chilliard, Delavaud and Bonnet2005 for a review). The apparent contradiction between the decrease in AT lipogenic enzymes and LPL activities and the absence of variation in leptinaemia in steers fed cut grass (compared with maize silage) could be explained by the fact that lipogenic activities reflect the short-term effect of daily nutritional status while leptinaemia reflects long-term effects of feeding level and is strongly related to body fatness, which did not differ between the different groups of steers, in the present study.
Whatever the diet, plasma leptin concentrations were significantly higher in the older than in younger steers (Figure 1), probably due to the increase in body fatness during the ageing process. These data corroborate previous studies (Vega et al., Reference Vega, Lee, Kuwayama, Matsunaga and Hidari2002; Higashiyama et al., Reference Higashiyama, Abe, Hayashi and Hodate2003; Hersom et al., Reference Hersom, Wettemann, Krehbiel, Horn and Keisler2004) showing increased circulating leptin levels during fattening in heifers and steers. The late increase of leptinaemia with age in Charolais steers (from 28–32 months of age in the present study, Figure 1) compared with Holstein or Japanese Black steers (from 16 to 18 months of age, Vega et al., Reference Vega, Lee, Kuwayama, Matsunaga and Hidari2002; Higashiyama et al., Reference Higashiyama, Abe, Hayashi and Hodate2003) could be a breed-related difference due to an earlier lipid deposition in the early-maturing Holstein and Japanese Black breeds than in the late-maturing Charolais breed.
Grazing per se (grazing v. cut grass diets) either did not affect lipogenic enzyme activities in the three AT sites or even increased some of them (G3PDH and G6PDH), whereas it decreased plasma leptin concentrations from the older steers and increased plasma glucose and NEFA concentrations without affecting AT weight and adipose cell size. These data on lipogenesis were unexpected, since previous studies in rat AT (Applegate et al., Reference Applegate, Upton and Stern1984; Ladu et al., Reference Ladu, Kapsas and Palmer1991) showed that physical exercise lowered fat gain and lipogenic enzyme activities. However, the decrease in plasma leptin and the increase in plasma NEFA levels in grazing steers are in agreement with previous studies in physical exercise-trained rats (Nara et al., Reference Nara, Kanda, Tsuhui, Inukai, Umeda, Inoue and Kobayashi1999, Faulconnier et al., Reference Faulconnier, Arnal, Patureau Mirand, Chardigny and Chilliard2004). An increase in β-adrenergic stimulation occurring during physical activity (Van Aggel-Leijssen et al., Reference Van Aggel-Leijssen, Saris, Homan and Van Baak2001) could explain the increase in plasma NEFA and the decrease in plasma leptin (Chilliard et al., Reference Chilliard, Bonnet, Delavaud, Leroux, Djiane and Bocquier2001) observed in the present study. Furthermore, the decrease in leptinaemia with grazing could have increased the energetic efficiency of these steers (see Chilliard et al. (Reference Chilliard, Delavaud and Bonnet2005) for review), which would compensate the increase in energy expenditure generally observed during physical activity (Lobley, Reference Lobley1990), and thus explain, at least in part, why grazing had no effect on AT weight and lipogenic activities in the present study.
In conclusion, this is the first study to report that steers reared at similar growth rates and slaughtered at the same age show significantly lower lipogenic enzyme activities in perirenal, intermuscular and subcutaneous AT when fed cut grass compared with a maize silage diet. Plasma insulin and glucose concentrations were also decreased whereas plasma leptin concentrations and adipose cell size were similar. Grazing per se did not affect lipogenic enzyme activities in the three AT sites studied, whereas it decreased plasma leptin concentrations in the older steers (from 840 days) and increased plasma NEFA and glucose concentrations without affecting plasma insulin concentrations. Future studies using specific nutrient infusions and/or measurement of energy metabolism and expenditure are needed to unravel the mechanisms responsible for the respective effects of forages (maize, grass) and the physical activity of grazing on the regulation of bovine AT lipogenesis and to investigate the molecular events that could be involved in these effects. Moreover, to confirm our results on the effect of grazing on AT lipogenesis and leptinaemia, it would be useful to test the influence of more intensive and longer physical activity in grazing steers.
Acknowledgements
The authors are grateful for the expert contributions from the staff of the two INRA experimental stations (Le Pin-au-Haras and Intrabois, Theix) for running the experiments, to the INRA Theix Slaughterhouse for slaughtering the animals, to C. Labonne, D. Bany, M. Tourret and A. Thomas for technical laboratory assistance and to M. Bonnet for helpful discussions. This study received financial support from the French Fonds National d’Aménagement et du Développement du Territoire.








