Introduction
Two underlying processes are responsible for increased adiposity of adipose tissue depots in beef cattle, hypertrophy, or larger adipocyte size, and hyperplasia, or larger numbers of adipocytes (Hood, Reference Hood1982; Novakofski, Reference Novakofski2004). These two processes are influenced by such factors as genotype, sex, age, feeding regimen, food supply, and the individual adipose tissue depot concerned (Hood and Allen, Reference Hood and Allen1975; Vernon and Houseknecht, Reference Vernon, Houseknecht and Cronjé2000).
Few studies have dealt with the effect of genotype on the cellularity of adipose tissue in cattle. Some that have, include papers by Hood and Allen (Reference Hood and Allen1973), who compared young Holstein and Hereford × Angus bulls the same age (14 months) and weight (275 kg carcass weight); Robelin (Reference Robelin1981), who examined young Friesian and Charolais bulls at different maturity stages (15 to 65 percent of mature weight); Truscott et al. (Reference Truscott, Wood and Denny1983), who compared young Friesian and Hereford bulls at different ages (10, 13, 17, and 20 months), and May et al. (Reference May, Savell, Lunt, Wilson, Laurenz and Smith1994) who compared Angus and Wagyu crossbed steers at 2 years of age. A review of the findings of these papers brings home the important influence of genotype on lipid accumulation, because the effect of genotype in all cases turns out to be significant at any given weight, age, or maturity stage.
With a view to deepening our understanding of the lipid accumulation process in young bulls of the main Spanish beef cattle breeds, the present study examined the size and number of adipocytes in different adipose tissues in bulls slaughtered at two body weights (BW), a low BW of approximately 320 kg and a high BW of approximately 540 kg. The Avileña, Morucha, and Retinta breeds, which are raised in western Spain's extensive-grazing Dehesa ecosystem, and the Rubia Gallega, Asturiana, Parda Alpina, and Pirenaica breeds, which are raised in northern Spain, were studied. The object of studying the size and number of adipocytes was to elucidate the differing contributions of hypertrophy and hyperplasia of adipose cells to lipid accumulation in various depots during animal growth in each breed. The results are intended to complement the results obtained for bulls of these same breeds slaughtered at an intermediate BW of approximately 470 kg (Mendizabal et al., Reference Mendizabal, Albertí, Eguinoa, Arana, Soret and Purroy1999).
Material and methods
Animals and feeding
A total of 168 young bulls of the Asturiana, Avileña, Morucha, Pirenaica, Parda Alpina, Retinta, and Rubia Gallega breeds were used, 24 animals per breed. Half of the bulls of each breed were slaughtered at a BW of around 320 kg (low weight) and half at a BW of around 540 kg (high weight). The animals were weaned at around 6 to 7 months of age at a BW of around 270 kg and were fed at a facility operated by the Agrifood Research and Technology Centre of the Government of Aragon (Saragossa, Spain). During the feeding stage the animals were fed a commercial concentrate (grain barley, soya-bean cake, dehydrated lucerne, calcium biphosphate, calcium carbonate and oligo-mineral-vitamin balancing concentrate) and cereal straw, both ad libitum. A weaning feed was used for all animals up to a BW of 320 kg (per kg dry matter (DM): 12.5 MJ metabolisable energy (ME); 180 g crude protein (CP); 30 g ether extract). After that weight, a finishing feed was used for the high-weight animals (per kg DM: 13.0 MJ ME; 155 g CP; 30 g ether extract). The animals were weighed every 2 weeks, and the animals in the low-weight group were slaughtered when the mean weight for the group reached 320 kg BW. The high-weight animals were slaughtered when the mean weight for the group reached 540 kg BW. Care and use of the animals followed the European guidelines (European Community (1986) and the Official Gazette of Spain (1988).
Slaughter and sample collection
The young bulls were slaughtered at the General Refrigerated Slaughterhouse in Zaragoza. At slaughter, samples consisting of 2 g adipose tissue each were collected from the omental depot (OM; middle portion of the greater omentum), perirenal depot (PR; surrounding the cranial portion of the left kidney), subcutaneous adipose tissue (SC; at the sternum), and intermuscular adipose tissue (IM; from between the sternum and pectoral muscles) to determine adipocyte size. The samples were stored in glass vials with 10 ml of Tirode's solution (0.15 mol/l NaCl; 6 mmol/l KCl; 2 mmol/l CaCl2; 6 mmol/l glucose; 2 mmol/l NaHCO3; pH 7.62) at a temperature of 39°C and were taken to the Animal Production Laboratory at the Agricultural Production Department at the Public University of Navarre in thermos containers filled with water warmed to that same temperature. In addition, 10 g adipose tissue were taken from each depot to determine the lipid content and stored in hermetically sealed bags at − 40°C in the laboratory.
The entire OM tissue was removed and weighed out on the processing line at the slaughterhouse. The entire PR tissue was removed and weighed out 24 h after slaughter. At the same time the 10th rib was removed, weighed, and stored at 4°C for later dissection to yield the corresponding whole SC and IM tissues. The tissue composition around this rib was deemed representative of the tissue composition of the carcass as a whole (Oliván et al., Reference Oliván, Martínez, García, Noval and Osoro2001).
Adipocyte size and number
The samples used to determine adipocyte size underwent digestion with collagenase in the laboratory to dissolve the matrix of connective tissue surrounding the adipocytes. The microscope images obtained were digitised and saved on a computer storage medium for measurement of adipose cell diameter (200 adipocytes per depot per animal) (Mendizabal et al., Reference Mendizabal, Albertí, Eguinoa, Arana, Soret and Purroy1999) using image analysis software (Optimas, 1996).
The number of adipocytes in the OM and PR depots and in the SC and IM tissue from the 10th rib was then calculated based on the amount of adipose tissue present, the lipid content of the adipose tissue (as determined by the Soxhlet method in accordance with the International Standards Organisation (1973), a lipid density value of 0.915 g/ml, and the mean adipocyte volume, assuming cells to be spherical in shape (lipid weight × chemical lipid content / 0.915 × mean adipocyte volume).
Statistical analysis
Statistical processing of the data was carried out by analysis of variance (anova) using the program of the Statistical Packages for the Social Sciences (1999). Because the data fit an exponential rather than a normal distribution, before processing the data were log transformed so that the variance would be normally distributed and homogeneous as required by anova. The mean values were subsequently antilog transformed back to linear scale to facilitate interpretation of the data.
BW at slaughter was included in the anova equation as a covariable for both the low-weight and high-weight bulls, to adjust for differences in BW at slaughter. Means were reported as the least-squares means.
After correcting for differences in the BW at slaughter in each body weight category, the breeds (no. = 7) were compared applying Tukey's test using the following equation:
where y ijk = amount of adipose tissue, adipocyte number, or adipocyte size; μ = least squares mean value, R i = fixed effect of breed (i = 1: Asturiana; i = 2: Avileña; i = 3: Morucha; i = 4: Parda Alpina; i = 5: Pirenaica; i = 6: Retinta; i = 7: Rubia Gallega); P j = fixed effect of BW at slaughter (j = 1: low weight; j = 2: high weight); R i × P j = fixed effect of the BW × breed interaction; ![P _{ j }[ b _{ j }( x _{ ij }( k ) - x _{ j })] = BW](https://static.cambridge.org/binary/version/id/?pub-status=live)
![j ( x _{ 1 } = 320.9\hairsp kg, x _{ 2 } = 544.6\hairsp kg)];](https://static.cambridge.org/binary/version/id/?pub-status=live)
Multivariate analysis, namely, factor analysis, was used to examine the relationships between all the adipose tissue variables considered here, which not only enabled plots of the relationships between the variables but also attempted to explain those relationships. Factors were extracted using the principal component analysis (PCA) method. The purpose of PCA is usually to determine a few linear combinations of the original variables that can be used to summarise the data with minimal loss of information. The PCA transforms the original variables into new axes, or principal components (PC), the said axes being orthogonal so that the data presented on the axes are uncorrelated with each other. Each successively derived PC expresses decreasing amounts of variation (Destefanis et al., Reference Destefanis, Barge, Brugiaplagia and Tassone2000). The Varimax rotation method was applied to the factors to facilitate interpretation and to maximise the explained variance (Morrison, Reference Morrison1990).
Results
Table 1 gives the results for the amount of adipose tissue in the different depots in the low-weight and high-weight young bulls of the seven breeds considered. The high weight at slaughter bulls had a greater amount of adipose tissue than did the lowweight at slaughter bulls in all four adipose tissue depots analysed in all seven breeds. The rate of lipid accumulation was higher in the Morucha, Avileña, and Retinta breeds than in the Asturiana and Rubia Gallega breeds, with the Pirenaica and Parda Alpina breeds falling in between. Differences (P < 0.001) in the rate of lipid accumulation were observed between the different breeds at both BWs at slaughter considered, with the low-weight bulls of the Morucha, Retinta, and Avileña breeds depositing the largest quantities of lipids and the low-weight bulls of the Asturiana and Rubia Gallega breeds the least. This pattern was more distinct in the high-weight bulls, which explains why the body weight × breed (BW × B) interaction was significant for all four adipose tissue depots considered.
Table 1 Least-squares means for amount of adipose tissue (g) in different depots in bulls of seven local Spanish breeds slaughtered at 320 kg (low) and 540 kg (high) body weight (12 animals per group)†
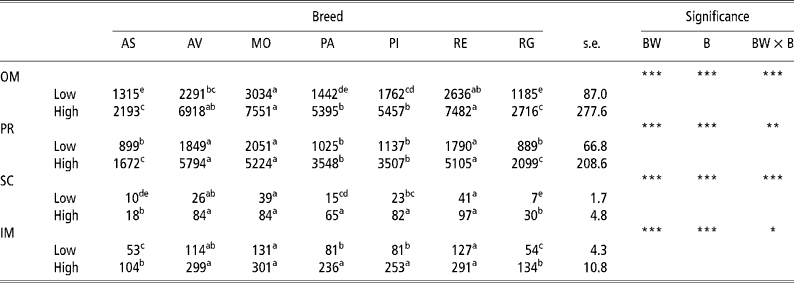
a,b,c,d Different superscripts in the same row denote significant differences (P < 0.05).
† Abbreviations are as follows. Depots: omental (OM), perirenal (PR), subcutaneous (SC), intermuscular (IM). Breeds: Asturiana (AS), Avileña (AV), Morucha (MO), Parda Alpina (PA), Pirenaica (PI), Retinta (RE), Rubia Gallega (RG). Significance: BW = body weight effect; B = breed effect; BW × B = body weight × breed interaction.
Table 2 presents the results for adipocyte size. There was an increase (P < 0.001) in adipocyte size in the OM and PR depots with body weight at slaughter in all the breeds examined in this study, though the amount of increase differed according to the breed, thus explaining the interactions observed (P = 0.05 for OM and P < 0.001 for PR). There was also an increase in cell size in the SC depot in the Rubia Gallega (P = 0.047), Avileña (P = 0.022), Parda Alpina (P = 0.006), and Pirenaica (P < 0.001) breeds and in the IM depot in the Avileña, Parda Alpina, Pirenaica, and Retinta (P < 0.001) breeds. On examining the effect of breed, in the low-weight bulls adipocyte size was generally largest in the OM, PR, and SC depots in the Morucha, Retinta, and Avileña breeds, in that order, and smallest in the Parda Alpina, Rubia Gallega, and Asturiana breeds. In the IM depot there were differences (P < 0.05) between the Morucha bulls, which had the largest adipocytes, and the Parda Alpina bulls, which had the smallest. In the OM and PR depots in the high-weight bulls, the Morucha and Avileña breeds had the largest adipocytes and the Asturiana and Rubia Gallega breeds the smallest. The largest adipocytes in the SC depot were observed in the Avileña bulls, the smallest in the Asturiana bulls. Finally, in the IM depot the Morucha breed had the largest adipocytes and the Parda Alpina and Rubia Gallega breeds the smallest. The differing behaviours of the different breeds at the two BWs studied here account for the BW × B interactions found for the SC (P = 0.005) and IM (P = 0.003) depots.
Table 2 Least-squares means for adipocyte size (pl) in different depots in bulls of seven local Spanish breeds slaughtered at 320 kg (low) and 540 kg (high) body weight (12 animals per group)†
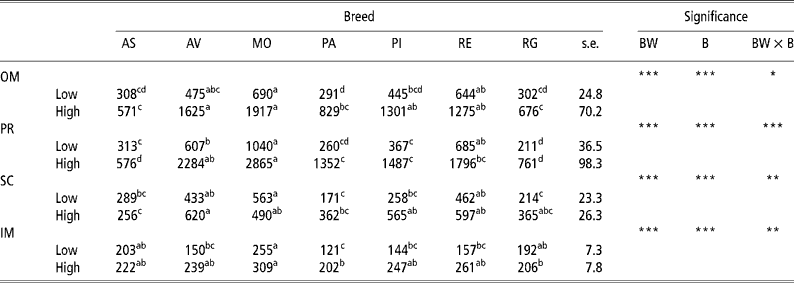
a,b,c,d Different superscripts in the same row denote significant differences (P < 0.05).
† Abbreviations are as follows. Depots: omental (OM), perirenal (PR), subcutaneous (SC), intermuscular (IM). Breeds: Asturiana (AS), Avileña (AV), Morucha (MO), Parda Alpina (PA), Pirenaica (PI), Retinta (RE), Rubia Gallega (RG). Significance: BW = body weight effect; B = breed effect; BW × B = body weight × breed interaction.
Table 3 summarises the results for the number of adipocytes in the different depots and shows that the differences observed between the low-weight and high-weight bulls varied according to the depot considered. In the OM depot there was no increase in the number of adipocytes between the two body weight at slaughter categories except in the Parda Alpina (P = 0.001) and Retinta (P = 0.006) breeds (BW × B interaction: P = 0.017). In contrast, the number of adipocytes in the SC and IM depots increased (P < 0.05) in the higher body weight at slaughter bulls in all the breeds considered. Comparing the individual breeds at each body weight at slaughter, the results again depended on the adipose tissue depot concerned. The OM depot did not exhibit any significant differences between breeds in the low-weight bulls, whereas in the high-weight bulls the Parda Alpina breed had the most adipocytes and the Asturiana breed the fewest. For the PR depot in the low-weight bulls, the Rubia Gallega breed had the most adipocytes and the Morucha breed the fewest (P < 0.05). In the case of the SC and IM depots, the Asturiana and Rubia Gallega breeds had fewer adipocytes than did the other breeds in both the low-weight and high-weight bulls (P < 0.05).
Table 3 Least-squares means for adipocyte number (cells per depot, 106) in different depots in bulls of seven local Spanish breeds slaughtered at 320 kg (low) and 540 kg (high) body weight (12 animals per group)†

a,b,c,d Different superscripts in the same row denote significant differences (P < 0.05).
† Abbreviations are as follows. Depots: omental (OM), perirenal (PR), subcutaneous (SC), intermuscular (IM). Breeds: Asturiana (AS), Avileña (AV), Morucha (MO), Parda Alpina (PA), Pirenaica (PI), Retinta (RE), Rubia Gallega (RG). Significance: BW = body weight effect; B = breed effect; BW × B = body weight × breed interaction.
‡Approaching significance (P < 0.1).
Table 4 and Figures 1 and 2 present the results of the factor analysis carried out to interrelate all the variables considered. The factor analysis performed for all the variables studied together yielded two factors that together explained 75.07% of the total variance for the group of test animals studied (Table 4). Factor 1 explained 61.78% of the variance and displayed a high positive relation between the amount of adipose tissue and the number of adipocytes in the SC and IM depots and adipocyte size in the OM and PR depots. Factor 2 explained 13.29% of the variance and related the amounts of OM and PR adipose tissue with adipocyte size in the four depots considered. The plots of the animals by body weight at slaughter on the factor space defined by factors 1 and 2 (Figure 1) grouped the bulls into two clusters, one for each body weight at slaughter. The plots of the animals by breed and body weight at slaughter yielded three clusters (Figure 2), one (cluster A) grouping the low-weight Asturiana, Parda Alpina, Pirenaica, and Rubia Gallega bulls, another intermediate cluster (cluster B) grouping the low-weight Avileña, Morucha, and Retinta bulls and the high-weight Asturiana and Rubia Gallega bulls, and a third (cluster C) grouping the high-weight bulls of the other five breeds (Pirenaica, Parda Alpina, Morucha, Avileña, and Retinta).
Table 4 Principal component (PC) factor loadings in principal component analysis taking as the variables the amount of adipose tissue, adipocyte number, and adipocyte size in different adipose tissue depots† in bulls of seven local Spanish breeds†
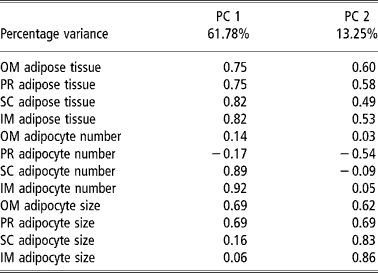
† Abbreviations are as follows. Depots: omental (OM), perirenal (PR), subcutaneous (SC), intermuscular (IM).

Figure 1 Plot of principal component (PC) factor scores (PC1 and PC2) for the principal component analysis of amount of adipose tissue, adipocyte number, and adipocyte size in the different adipose tissues of bulls of seven local Spanish breeds slaughtered at 320 kg (low) and 540 kg (high) body weight.

Figure 2 Plot of groupings of Spanish breeds slaughtered at 320 kg (low) and 540 kg (high) body weight (12 animals per group) in the two-dimensional space defined by principal component factors PC1 and PC2 for the principal component analysis taking as the variables amount of adipose tissue, adipocyte number, and adipocyte size in the different adipose tissues. Breeds are: Asturiana (AS), Avileña (AV), Morucha (MO), Parda Alpina (PA), Pirenaica (PI), Retinta (RE), Rubia Gallega (RG).
Discussion
The amount of adipose tissue in the OM and PR depots and in the SC and IM depots as sampled by dissecting the tenth rib underwent a significant increase from the low-weight bulls to the high-weight bulls in all seven breeds considered as a result of the older age needed to attain the higher body weight and higher degree of lipid accumulation (Table 1). The differences between the breeds observed at 320 kg BW (low-weight bulls) were likewise observed at 540 kg BW (high-weight bulls) and were even more pronounced, because of the older age and higher degree of maturity attained by the bulls, in keeping with the growth and maturation rate inherent to each breed.
The highest levels of lipid accumulation were attained by the young bulls of the Morucha, Retinta, and Avileña breeds, perhaps because they are rustic, early-maturing breeds raised in Spain's Dehesa ecosystem that are well adapted to their natural environment of natural pasture in holm oak woodland (Sánchez, Reference Sánchez2002). In contrast, the bulls of the Asturiana and Rubia Gallega breeds were the animals that had the lowest amounts of accumulated lipids. Over the past few decades these breeds have been bred specially for meat production, which ordinarily requires animals to mature more slowly, and as a consequence these breeds do indeed mature later (Gutiérrez et al., Reference Gutiérrez, Fernández, Alvarez, Royo and Goyache2006). The degree of lipid accumulation was intermediate in the Pirenaica and Parda Alpina breeds, which, together with the Asturiana and Rubia Gallega breeds, are raised principally in northern Spain (Casasús et al., Reference Casasús, Sanz, Villalba, Ferrer and Revilla2002). A comparison of Pirenaica bulls and Holstein bulls, deemed to be an early-maturing breed, showed that the latter exhibited a higher degree of lipid accumulation than the former (Eguinoa et al., Reference Eguinoa, Brocklehurst, Arana, Mendizabal, Vernon and Purroy2003).
Mendizabal et al. (Reference Mendizabal, Albertí, Eguinoa, Arana, Soret and Purroy1999) reported results similar to the findings reported here in bulls of these same seven breeds at an intermediate weight at slaughter of 470 kg BW. Similarly, the finding that animals that belonged to the more rustic breeds (Morucha, Retinta, and Avileña) stored larger amounts of lipids in the internal OM and PR adipose tissue depots than did animals of the other breeds was consistent with the notion that rustic breeds store more lipids in their internal adipose tissue depots than do improved meat breeds (Kempster, Reference Kempster1980–81).
Adipocyte size and number
Increases in the amount of adipose tissue in animals during growth is related both to an increase in the number of adipocytes (hyperplasia) and to an increase in adipocyte size (hypertrophy) (Novakofski, Reference Novakofski2004), though growth of the different adipose tissues in cattle after birth is attributable more to adipocyte hypertrophy (Cianzio et al., Reference Cianzio, Topel, Whitehurst and Beitz1985; Robelin, Reference Robelin1986). Robelin (Reference Robelin1986), for instance, reported that between birth and mature BW, adipocyte numbers increase approximately by a factor of 7 and adipocyte volume by a factor of 30.
The pattern of lipid deposition varies in the different adipose tissues. Truscott et al. (Reference Truscott, Wood and Denny1983) observed that the internal OM and PR adipose tissue depots have a constant number of adipocytes fixed at an early age. In the present study adipocyte hypertrophy was the principle method of lipid accumulation during the growth period from 320 to 540 kg BW in both the OM and PR depots, with adipocyte size increasing in all the breeds considered (Table 2) but there being hardly any change in the number of adipocytes in these same depots (Table 3).
Corroboration of these findings was also provided by the fact that the largest adipocytes in the OM and PR adipose tissue depots were recorded in the Morucha, Retinta, and Avileña breeds, the breeds that were the fastest maturing of the seven breeds studied and thus had the highest stored lipid levels, whereas the smallest adipocytes were recorded in the Asturiana and Rubia Gallega breeds, the breeds that had attained the lowest maturity stage and in consequence had the lowest stored lipid levels at both BWs. Additionally, this agreed with the results obtained in an earlier study using bulls of these same breeds at an intermediate weight at slaughter of 470 kg (Mendizabal et al., Reference Mendizabal, Albertí, Eguinoa, Arana, Soret and Purroy1999).
Unlike the OM and PR depots, in the SC and IM adipose tissues the number of adipocytes increased during the study period, which indicates that in these two adipose tissue depots lipid accumulation was attributable primarily to cell hyperplasia (Table 3). Schoonmaker et al. (Reference Schoonmaker, Fluharty and Loerch2004) reported this same finding in the SC adipose tissue in Holstein steers raised under various feeding regimes. In any event, where there is no evidence of an increase in mean cell size during lipid deposition in an adipose tissue depot, it still cannot be said that cell hypertrophy has not taken place, because the new cells produced by cell hyperplasia will increase in size up to the mean size of the other adipocytes in the depot.
Similarly, the lower lipid contents in the SC and IM depots at the 10th rib in the Asturiana and Rubia Gallega bulls (Table 1) can be ascribed to a smaller number of adipocytes at those locations compared with that in the other breeds (Table 3), inasmuch as the difference in adipocyte size in those two breeds compared with that in the other five breeds was quite small (Table 2). Consequently, there appears to be an association between hyperplasia in the SC and IM depots at a given point in time and the growth rate. For this reason, the animals in the slower-maturing breeds, like Asturiana and Rubia Gallega, store smaller amounts of lipids in these depots at a given weight at slaughter, primarily because of less adipocyte hyperplasia.
Lastly, the results of the factor analysis performed for all the variables considered (Table 4) corroborated the findings discussed above, with adipocyte hypertrophy being primarily responsible for lipid accumulation in the OM and PR depots (factors PC1 and PC2, Table 4) and adipocyte hyperplasia being primarily responsible for lipid accumulation in the SC and IM depots (factor PC1, Table 4). The factor analysis results further highlighted the existing differences among the genotypes with respect to adipose tissue growth, with a group of faster maturing breeds (Morucha, Retinta, Avileña), a group of slower maturing breeds (Asturiana and Rubia Gallega); and an intermediate group falling in between (Parda Alpina and Pirenaica) (Figure 2).
In conclussion, the results obtained are consistent with differing lipid deposition rates in the breeds studied here, and accordingly each breed has its own optimal BW at slaughter at which carcasses will have deposited an appropriate amount of lipids. In addition, the findings show that at between 350 and 550 kg BW, OM and PR lipid deposition is due mainly to adipocyte hypertrophy, whereas SC and IM lipid deposition is due mainly to adipocyte hyperplasia. This is important, because it means that different strategies for regulating lipid deposition will be needed for the different adipose tissue depots.
Acknowledgements
This research was supported by the Instituto Nacional de Investigaciones Agroalimentarias (National Institute of Agrifood Research) (INIA project SC9709) and carried out in collaboration with the Beef Breeders Associations of Spain.








