Implications
Water and soil salinization is an increasing worldwide phenomenon, thus creating new threats for farm animal production. We investigated a stepwise adaptation to saline drinking water in Boar goats. The method was effective to habituate the animals to saline water intake without health impairment. However, after the adaptation period the goats reacted more sensitively when offered the choice between fresh water and different concentrations of saline water. Apparently, the acceptance thresholds for saline water change with the total sodium balance of the body.
Introduction
Domestic farm animals have demonstrated remarkable adaptation capacities to a broad range of environments. Apart from climatic challenges due to global changes, salinization of groundwater and soil is an increasing threat for livestock, in particular in coastal areas (Hallegatte et al., Reference Hallegatte, Green, Nicholls and Corfee-Morlot2013). Compared to other mammals, ruminants are able to tolerate more salt in drinking water (Goatcher and Church, Reference Goatcher and Church1970b), depending on the duration of salt ingestion (Peirce, Reference Peirce1957), age (Wilson and Dudzinski, Reference Wilson and Dudzinski1973), physiological status and environmental factors. Several studies show that among ruminants, goats are well adapted to dry environments and water scarcity (Silanikove, Reference Silanikove2000), and can even survive on seawater (Dunson, Reference Dunson1974).
Ruminants are particularly sensitive to sudden and drastic changes in feed composition due to their rumen physiology (Grubb and Dehority, Reference Grubb and Dehobity1975; Mackie et al., Reference Mackie, Gilchrist, Robberts, Hannah and Schwartz1978) and a stepwise adaptation is recommended for changes in diets (Mackie et al., Reference Mackie, Gilchrist, Robberts, Hannah and Schwartz1978). Similarly, a stepwise habituation to saline water might help exposed animals to cope with higher concentrations of saline water without health impairment. So far, some studies have been conducted on the sensory perception and perception thresholds of salt in drinking water in goats (Bell, Reference Bell1959; Runa et al., Reference Runa, Brinkmann, Riek, Hummel and Gerken2019) and sheep (Goatcher and Church, Reference Goatcher and Church1970a and Reference Goatcher and Church1970b). However, there is a lack of experimental studies on how to adapt ruminants to saline water ingestion. There are only practical recommendations available (Department of Agriculture and Food, 2014) to mix fresh and saline water for a few days as an adaptation period. Therefore, the aim of our study was to investigate the individual adaptation capacity of Boer goats towards sodium chloride (NaCl) in drinking water. The focus was on the possibility to adapt the animals to increased saline water concentrations via a stepwise habituation. We hypothesized that Boer goats would tolerate saline water after an adaptation period.
Material and methods
Animals, management and experimental treatments
The study was carried out at the Department of Animal Sciences, University of Göttingen, Germany between November 2015 and January 2016. Twelve non-pregnant Boer goats aged between 1.5 and 7.5 years with an average body weight of 50.5 ± 9.0 kg were involved. Animals originated from the breeding herd of the Department. Animals were transferred to the experimental pens 2 weeks before the start of the trial for acclimatization. Animals were kept individually. As described in a previous experiment (Runa et al., Reference Runa, Brinkmann, Riek, Hummel and Gerken2019), three identical rooms, subdivided into four individual straw pens each (each pen 3.0 m2) were used. Each pen was equipped with an individual feed trough (diameter 53 cm, approximate capacity: 3 kg hay) and two water buckets (diameter 28 cm, 10 l capacity per bucket), which were placed at each side of the feed trough to allow free choice between contents. Animals had ad libitum access to chopped hay, salt lick and water (fresh and saline water) throughout the experimental period. The rooms were equipped with windows to provide natural light. The lighting schedule was kept constant at 14 h light : 10 h dark, with additional artificial lighting from 0630 h until 2030 h.
Throughout the study, a two-choice preference test (Goatcher and Church, Reference Goatcher and Church1970a) was used. The study was conducted in four phases (Figure 1). In the control phase (phase 1), only fresh tap water was supplied in two identical buckets for 1 week to record individual water intake from each bucket. In phase 2 (sensitivity test, 2 weeks), water with ascending salt concentrations (0.25%, 0.5%, 0.75%, 1.0%, 1.25% and 1.5% NaCl) was offered in one container and unsalted tap water in the other. Each salt concentration was tested for two consecutive days. This procedure allowed to determine the individual salt sensitivity. During the third phase (adaptation, 4 weeks), goats were stepwise habituated to saline water by offering only saline water in both buckets. The saline water of the lower concentration was offered in one container and the salt water with higher concentration in the other (Figure 1). The concentrations were gradually increased between 0% and 1.5% NaCl. The highest concentration was close to the rejection threshold (RET) described by Bell (Reference Bell1959) and Goatcher and Church (Reference Goatcher and Church1970a). Each combination of salt concentrations was tested for four consecutive days during phase 3 (adaptation). Subsequently, in phase 4 (sensitivity re-test, 2 weeks), the same treatment as in phase 2 was repeated to test for differences in discrimination thresholds after the habituation period.

Figure 1 Experimental design with Boer goats based on a two-choice preference test: provision of different concentrations of salt in drinking water during the different phases of the experiment. Two water containers were placed at each side of the feed trough for ad libitum intake. The positions of the concentrations were changed daily at random in order to avoid a bias due to position effects. The same combination of concentrations was offered for two (phases 2 and 4) or four (phase 3) consecutive days, respectively.
The water buckets were replenished with test solutions daily and the positions of the concentrations were changed daily at random in order to avoid a bias due to position effects. The individual water intake from each container was determined daily. The average Na+ content of tap water was 7.55 mg/l. Saline water was prepared by adding a defined amount of salt (EscoSiede-Speisesalz, Hannover, Germany) with 99.8% NaCl purity to tap water. The accurate salt concentration provided was measured by using a refractometer (HI96821 refractometer, Hanna Instruments Inc., Woonsocket, RI, USA) (Runa et al., Reference Runa, Brinkmann, Riek, Hummel and Gerken2019).
Body weight, body condition score, water and feed intakes
As described in Runa et al. (Reference Runa, Brinkmann, Riek, Hummel and Gerken2019), individual body weight was recorded every 2nd week with a mobile scale (Salter Brecknell LS300, capacities: 300 kg, resolution: 0.2 kg, Salter Brecknell, Smethwick, West Midlands, UK). The body condition score (BCS scale: 1 = emaciated, 5 = obese with 0.5 increments), a palpable and visual assessment of the degree of fatness and muscle over and around the lumbar vertebrae, sternum, ribs and intercostal (between ribs) spaces was assessed every 2nd week according to Villaquiran et al. (Reference Villaquiran, Gipson, Merkel, Goetsch and Sahlu2007).
As described in a previous experiment (Runa et al., Reference Runa, Brinkmann, Riek, Hummel and Gerken2019), individual water intake from each bucket was recorded daily by weighing and re-weighing water buckets before and after water administration with an electronic scale (Sartorius model CP 34000, Sartorius AG, Göttingen, Germany). Water refusals were discarded and the buckets refilled after cleaning. The daily amount of test solution consumed was expressed as a percentage of the total fluid taken from both containers. A separate bucket (10 l) containing water was placed in an adjacent area and reweighed daily to estimate the amount of water lost by evaporation and the total daily drinking water intake (TDWI) was corrected accordingly. The total water intake (TWI) was determined as the sum of TDWI from both containers and the water content in consumed hay. Water intake was also expressed as intake per kg BW0.82 since water use by animals is related to the live weight of animals to the power of 0.82, as water is used in the body for intermediary metabolism and also for evaporative cooling (Wilson, Reference Wilson and Cloudsley-Thompson1989).
Hay was chopped to avoid spillage. The hay with an average dry matter content of 90.3% ± 0.2% and metabolizable energy concentration of 8.8 ± 0.1 MJ/kg DM was offered for ad libitum intake daily and individual feed consumption was measured by weighing the remaining feed on the next day. As described in a previous experiment (Runa et al., Reference Runa, Brinkmann, Riek, Hummel and Gerken2019), hay samples of approximately 150 g were collected weekly and analyzed for dry matter content. Collected hay samples were ground through a 1-mm screen and analysed using standard methods (Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten (VDLUFA), 2012) for dry matter (VDLUFA method 3.1), ash (method 8.1), crude protein (method 4.1.2 – Dumas; ElementarVarioMAX CN, Langenselbold, Germany), ether extracts (method 5.1.1), neutral detergent fibre and acid detergent fibre (expressed without residual ash – NDFom and ADFom, methods 6.5.1 and 6.5.2; Fiber Analyzer 220, Ankom Technology, Macedon, NY, USA). The metabolizable energy was estimated using prediction equations for ruminants, including values from Hohenheim gas test (Menke et al., Reference Menke, Raab, Salewski, Steingass, Fritz and Schneider1979; VDLUFA method 25.1). For the feed and water, Na and Cl were determined from aqueous extract using ion chromatography with conductivity detection (VDLUFA 10.5.2) (Dionex DX-100, Dionex, Sunnyvale, CA, USA). For Ca, Mg, P and K analyses, samples were ashed and dissolved in HCl. Ca, Mg and K were measured via atomic absorption spectroscopy (Varian SpectrAA-300, Varian, Palo Alto, CA, USA), and P was measured photometrically. A mineral licking block (Solsel®, Esco-European Salt Company, Hannover, Germany) containing 37% sodium, 1.1% calcium, 0.6% magnesium, 0% phosphorous, manganese oxide (1000 mg/kg), zinc oxide (1000 mg/kg), calcium iodate (100 mg/kg), cobalt carbonate (20 mg/kg) and sodium selenite (20 mg/kg) was supplied for each animal throughout the experimental period. To avoid spillage, the mineral block was placed into the feeding trough. Consumption of the mineral lick was determined by weekly re-weighing the salt block with a scale (Sartorius model CP 34000, Sartorius AG, Göttingen, Germany) to the nearest 1 g. Total sodium (Na+) intake was determined as the sum of the amount of sodium consumed from hay, licking mineral block, fresh and saline drinking water. To correct for differences in animals’ body weights, feed and sodium intakes were also expressed on the basis of their metabolic body weight (kg BW0.75.)
Statistical analysis
The results from earlier studies showed a higher salt sensitivity in young compared to adult sheep (Wilson and Dudzinski, Reference Wilson and Dudzinski1973). In order to take into account a possible age effect, goats were classified as young (n = 4) or adult (n = 8) when younger or older than 2 years, respectively. Data recorded on a daily basis were averaged for each goat per phase. Analyses of variance were performed using the PROC MIXED procedure of the software package Statistical Analysis Systems version 9.3 (SAS, Inst. Inc., Cary, NC). Animal was included as a random factor to account for repeated measurements of the same individuals. For all traits, the model included the fixed effect of the room, the experimental phase, age of the animal, their interactions, and the random effect of the goats. The model (1) was
 $${Y_{ijklm}} = \mu + {R_i} + {P_j} + {A_k} + {\left( {R*P} \right)_{ij + }}{\left( {P*A} \right)_{jk}} + {\left( {R*A} \right)_{ik}} + {\left( {R*P*A} \right)_{ijk}} + {G_l} + {e_{ijklm}}$$
$${Y_{ijklm}} = \mu + {R_i} + {P_j} + {A_k} + {\left( {R*P} \right)_{ij + }}{\left( {P*A} \right)_{jk}} + {\left( {R*A} \right)_{ik}} + {\left( {R*P*A} \right)_{ijk}} + {G_l} + {e_{ijklm}}$$
where Yijklm : observation value; μ: overall mean; Ri : room (i = rooms 1 to 3); Pj : phase (j = control, phases 2, 3 and 4); Ak : age (k = young, old); interactions: (R*P), (P*A), (R*A), (R*P*A); Gl : random effect of the goat; and eijklm : random error.
Data related to different salt concentrations during experimental phases 2, 3 and 4 were analysed by using the following mixed model (2):
 $${Y_{ijklmno}} = \mu + {R_i} + {P_j} + {A_k} + {C_l} + {B_m} + {\left( {P*A} \right)_{jk}} + {\left( {P*C} \right)_{jl}} + {\left( {P*B} \right)_{jm}} + {\left( {A*C} \right)_{kl}} + {\left( {C*B} \right)_{lm}} + {\left( {P*A*C} \right)_{jkl}} + {G_n} + {e_{ijklmno}}$$
$${Y_{ijklmno}} = \mu + {R_i} + {P_j} + {A_k} + {C_l} + {B_m} + {\left( {P*A} \right)_{jk}} + {\left( {P*C} \right)_{jl}} + {\left( {P*B} \right)_{jm}} + {\left( {A*C} \right)_{kl}} + {\left( {C*B} \right)_{lm}} + {\left( {P*A*C} \right)_{jkl}} + {G_n} + {e_{ijklmno}}$$
where Yijklmno : observation value; μ: overall mean; Ri : room (i = rooms 1 to 3); Pj : phase (j = phases 2, 3 and 4); Ak : age (k = young, old); Cl : concentration (l = 1 to 7); Bm : bucket position (m = right, left); interactions: (P*A), (P*C), (P*B), (A*C), (C*B), (P*A*C); Gn : random effect of the goat; and eijklmno : random error.
An integrated Tukey test was used to detect the differences between means at a 5% significance level. All values are presented as least squares means ± SEM. Theoretically, 50% of the TWI was expected to be consumed from each bucket. A 95% confidence interval was then placed on the theoretical mean. The 40% level of intake referred to as lower discrimination threshold (LDT) and the 60% intake as upper discrimination threshold (UDT). An RET was set at 20% intake (Bell, Reference Bell1959; Goatcher and Church, Reference Goatcher and Church1970a).
Results
Ambient temperature, body weight and body condition score
Ambient temperature (Ta ) varied during the different phases of the experiment and ranged from 17.9°C to 26.2°C. Daily mean Ta was 21.1°C ± 1.2°C in the control phase and phase 2, and 19.7°C ± 0.7°C during phases 3 and 4 of the study. Body condition score significantly (P = 0.002) decreased during the experiment, while there was no effect of treatment on body weight (Table 1). However, older goats had a significantly (P<0.001) higher body weight and lower body condition score (54.56 ±7.52 kg and 2.89 ± 0.45 points) compared to young goats (41.60 ± 3.57 kg and 3.41 ± 0.35 points).
Table 1 Average body weight, body condition score, daily feed, water and sodium intakes for control (phase 1 : 1 week) and treatment phases (phase 2 : 2 weeks; phase 3 : 4 weeks and phase 4 : 2 weeks) in Boer goats (n=12), corrected for body weight

BW = body weight; BCS = body condition score; DMI = dry matter intake; TDWI = total drinking water intake; TWI = total water intake; TNaI = total sodium intake; NaIF = NaIW; NaISL = sodium intake from feed, water and salt lick, respectively.
Phase 1 (C) = control, only fresh (tap) water was supplied in two buckets; Phase 2 (ST) = sensitivity test, water with ascending salt concentrations (0.25%, 0.5%, 0.75%, 1.0%, 1.25% and 1.5% NaCl) was offered in one container and unsalted tap water in the other; Phase 3 (A) = adaptation to saline water, only saline water (concentration between 0% and 1.5%) was provided in both buckets; Phase 4 (SRT) = sensitivity re-test, the same treatment as in phase 2 was repeated.
abcdMeans within the same row with different superscripts differ significantly by P<0.05.
Feed and sodium (Na+) intakes
Dry matter intake (DMI) decreased significantly during the experiment (Table 1). Total sodium intake was higher in phase 2 (sensitivity test) and phase 3 (adaptation), while it approached control phase values in the sensitivity re-test (phase 4; Table 1). In phases 2 and 3 most sodium intake originated from the drinking water. Due to the experimental design, sodium intake was highest in the adaptation period (phase 3) when only saline water was offered. Sodium ingestion from the salt lick was strongly reduced during phases 2, 3 and 4 compared to the control phase (Table 1). Significant age effects were found for all traits recorded with higher values in older than younger goats. The age × phase interactions was significant for total sodium intake, sodium intake from water and sodium intake from salt lick. However, when corrected for body weight, these interactions became non-significant, with the exception of sodium intake from salt lick in phase 1, where old goats had a higher consumption (Table 2).
Table 2 Average total sodium intake, sodium intake from water and salt lick for young (n=4) and old (n=8) Boer goats during the control (phase 1 : 1 week) and treatment phases (phase 2 : 2 weeks; phase 3 : 4 weeks and phase 4 : 2 weeks), corrected for body weight

TNaI = total sodium intake; NaIW and NaISL = sodium intake from water and salt lick, respectively.
Phase 1(C) = control; Phase 2 (ST) = sensitivity test; Phase 3 (A) = adaptation to saline water; Phase 4 (SRT) = sensitivity re-test.
abMeans within the same row and phase with different superscripts differ significantly by P<0.05.
Water intake and sensitivity responses
Both TWI and TDWI were significantly (P<0.001) higher in phase 2 (sensitivity test) and phase 3 (adaptation), compared to control phase and sensitivity re-test (phase 4; Table 1). When goats had the choice between fresh water and saline water for the first time (phase 2), they preferred higher salt concentrations and consumed higher amounts of saline water than in the re-test (phase 4) after prolonged exposure to saline water (Figure 2). Thus, daily saline water intake was significantly (P<0.001) higher (75.4 ± 53.2 g/kg BW0.82 per day) in phase 2 (sensitivity test) than in phase 4 (sensitivity re-test) (40.4 ± 34.0 g/kg BW0.82 per day). Thus, salt RETs were lowered to 1.25% in phase 4 compared to 1.5% in phase 2 (Figure 3). In both phases (2 and 4), saline water intake was reduced with increasing further salt concentrations and the lowest (10% to 20% of TWI) salt water intake was recorded at a concentration of 1.5% (Figure 3).
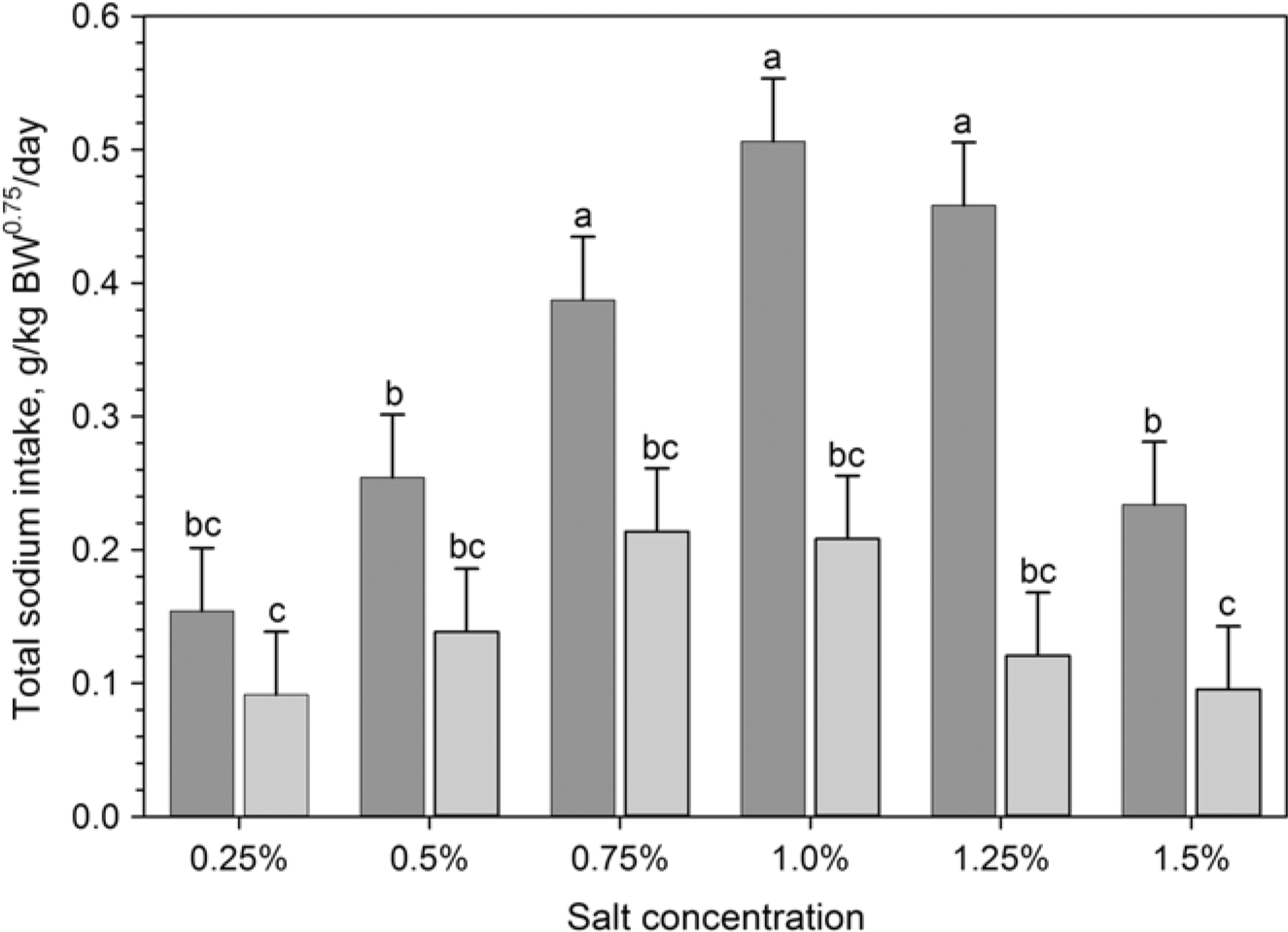
Figure 2 Daily average total sodium intake (g/kg BW0.75 per day) for the sensitivity test in phase 2 (dark grey bar) and the sensitivity re-test in phase 4 (grey bar) of Boer goats (n = 12) exposed to a two-choice preference test (see Figure 1 for details). One bucket contained fresh water while the second was filled with different salt concentrations. Values are presented as means ± SE. abcMeans with different superscripts differ significantly by P<0.05.
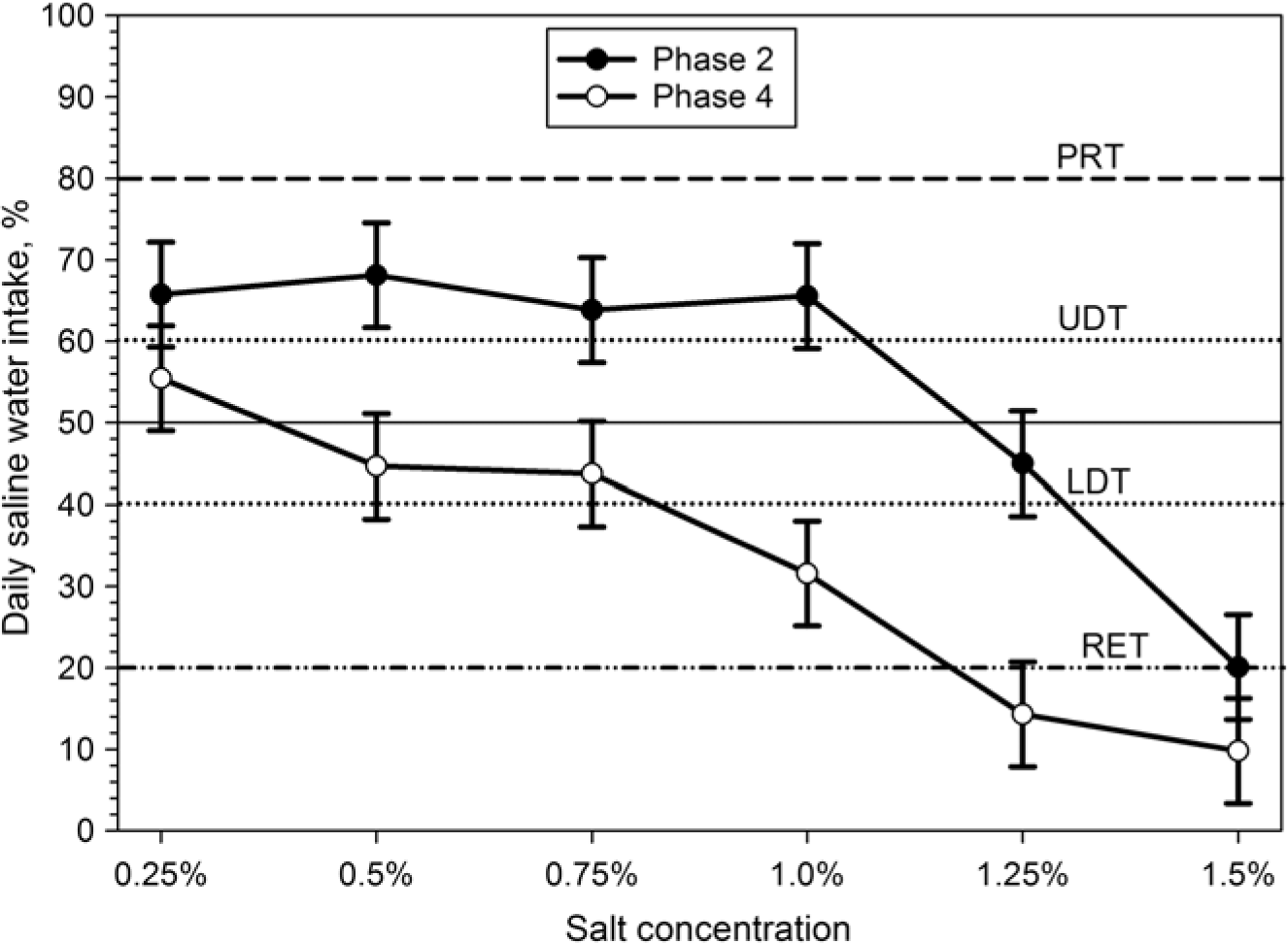
Figure 3 Responses of Boer goats (n = 12) to saline drinking water in a two-choice preference test during the sensitivity test (phase 2) and the sensitivity re-test (phase 4): daily amount of test solution consumed expressed as a percentage of the total fluid taken from both containers. The horizontal lines indicate threshold limits; dash-dotted line = RET, dashed line = preference threshold (PRT), dotted lines indicate LDT and UDT (see text for details).
Figure 4A and B depicts the stepwise adaptation to saline drinking water during the third (adaptation) phase. Across the entire adaptation phase, the total water (g/kg BW0.82 per day) consumption increased with higher salt concentrations in drinking water (Figure 4A). Initially, goats preferred saline water of 0.25% over fresh water (Figure 4B). Goats did not differentiate between concentrations of 0.25% and 0.5% when offered simultaneously. However, with increasing concentrations, the preference for the lower concentration of saline water became more pronounced.
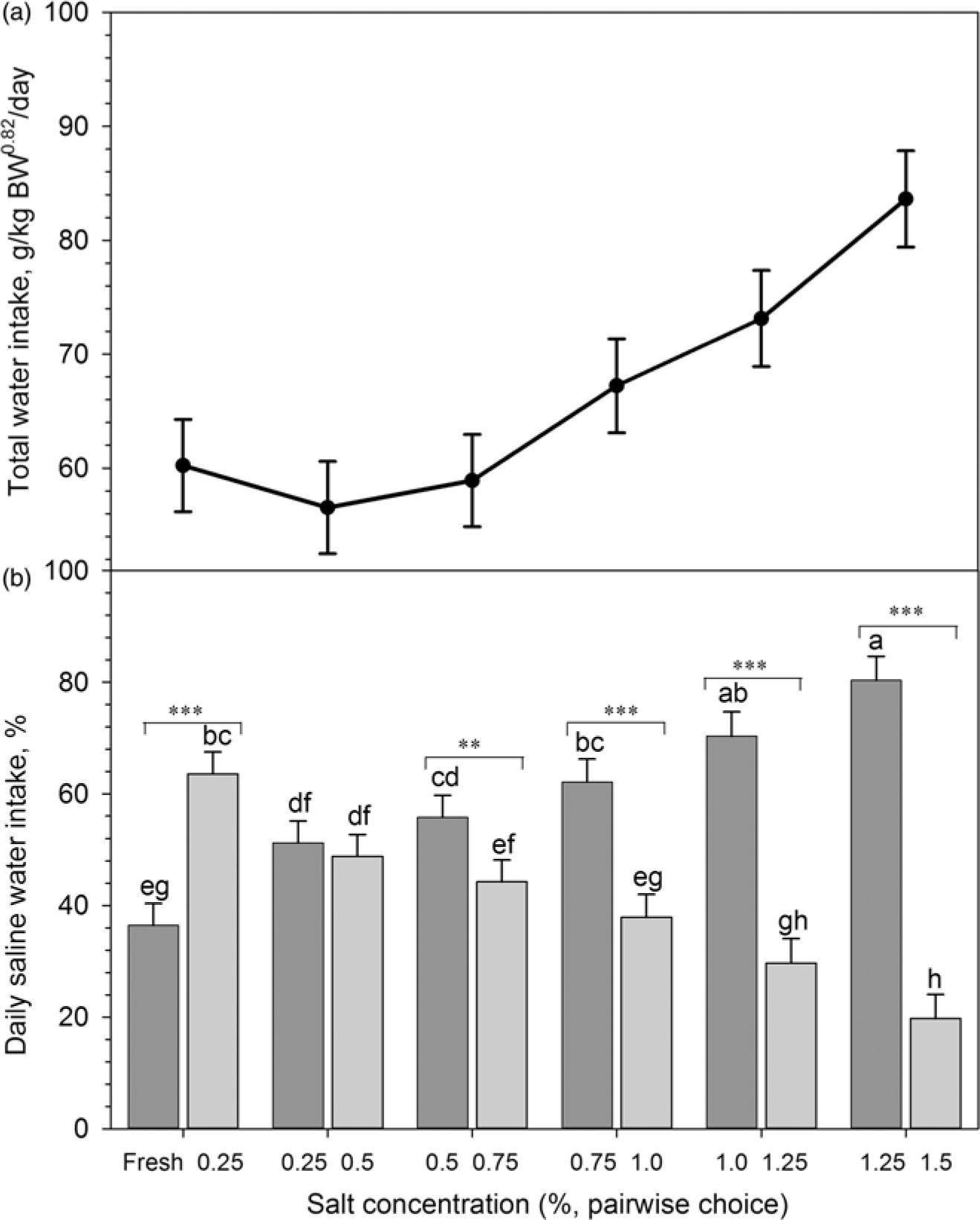
Figure 4 Stepwise adaptation of Boer goats (n = 12) to saline drinking water during the adaptation to saline water (phase 3; see Figure 1 for details). (a) Total daily saline water intake (g/kg BW0.82 per day); (b) Daily saline water intake of different salt concentrations (percentage of TWI). Dark grey and light grey colour bars indicate lower and higher concentrations, respectively; abcdefghmeans ± SE; significant differences (P<0.05) between salt concentrations (***P<0.001; **P<0.05).
Discussion
To our knowledge, this study is the first experimental adaptation trial for saline drinking water in goats. While body weight remained unchanged during the study, the changes in body condition scores of our experimental goats were directly associated with feed and water consumption. Continuous intake of high saline water depressed appetite, reduced feed intake and digestibility, resulting in body weight loss in sheep (Masters et al., Reference Masters, Rintoul, Dynes, Pearce and Norman2005). The lower body condition score found in our treatment phases could indicate that water retention may have contributed to a constant body weight (Masters et al., Reference Masters, Rintoul, Dynes, Pearce and Norman2005). Similarly, sheep increased their body water content in the intracellular space to excrete excess sodium from the body fluids (Assad and El-Sherif, Reference Assad and El-Sherif2002).
Feed and water intakes
Saline water intake induced only small changes in DMI in our study. Animals initially ingested more feed when saline water was first introduced together with fresh water, most likely due to increasing feed palatability. Similar results have been reported by Ru et al. (Reference Ru, Fischer, Glatz and Bao2004) where the feed intake of red and fallow weaner deer was increased at the beginning of drinking saline water and then decreased when drinking water salinities increased from 1.2% to 2.4%. Studies in rusa deer stags (Kii and Dryden, Reference Kii and Dryden2005) reported similar results. Furthermore in a study on sheep, feed intake was not affected by drinking water containing 1.3% to 1.5% salt and a greater reduction of feed intake was only observed when animals were given 2% sodium chloride in water (Peirce, Reference Peirce1957).
In our experiment, the TDWI was increased with an increased salt concentration, which is in agreement with previous findings (Peirce, Reference Peirce1957; Kattnig et al., Reference Kattnig, Pordomingo, Schneberger, Duff and Wallace1992). Abou Hussien et al. (Reference Abou Hussien, Gihad, El-Dedawy and Abdel Gawad1994) found that the TWI of goats and sheep was increased by 59% and 99%, respectively, when drinking water salinity was increased to 1.7%. Similar results were recorded in rusa deer stags (Kii and Dryden, Reference Kii and Dryden2005) and in fallow and red deer (Ru et al., Reference Ru, Fischer, Glatz and Bao2004). However, several studies with cattle found that water intakes were not increased with water salinity up to 1.1% (Bahman et al., Reference Bahman, Rooke and Topps1993). It has been postulated that increased water consumption is a physiological response to an excess in salt in the body (Kattnig et al., Reference Kattnig, Pordomingo, Schneberger, Duff and Wallace1992) in order to maintain systemic osmotic balance. This strategy, in turn, allows animals to adapt to increased quantities of ingested salt through renal adjustment (Potter, Reference Potter1961). Macfarlane (Reference Macfarlane1982) observed an induction of Na+/K+ATPase in the kidney, liver and ileum in goats exposed to saline water. As this enzyme plays a central role in the active transfer of sodium out of the cell and other ion-transporting mechanisms (Suttle, Reference Suttle and Suttle2010), the higher tolerance to saline water found in goats compared to sheep may be attributed to their slightly more effective sodium pumps (Macfarlane, Reference Macfarlane1982).
Sensitivity responses
The comparison between salt acceptance thresholds before and after the habituation period revealed very interesting results. Contrary to our expectation, prolonged higher intake of sodium in phase 3 did not lead to a higher salt acceptance as there was a considerable shift towards lower thresholds in the sensitivity re-test (phase 4), when offered a choice between fresh and saline water. Accordingly, discrimination and RETs are not constant but depend on the total sodium balance of the animal indicating flexible regulation mechanisms.
Sodium is the only mineral that mammals recognize sensorial by taste. Variations in salt taste sensitivity have been associated with individual differences such as genetic variations, age, sex, and feed or water containing high sodium contents (Noh et al., Reference Noh, Paik, Kim and Chung2013). In the adaptation phase of this study (phase 3), goats reacted more sensitively to higher concentrations of saline water which may be related to aversion behavioural responses (Chandrashekar et al., Reference Chandrashekar, Kuhn, Oka, Yarmolinsky, Hummler, Ryba and Zuker2010). Thus, different salt taste pathways are involved in response to salt stimulation via drinking water, including prominent taste receptor cells in the salt sensing system and transfer mechanisms of the taste information to the brain (Yoshida et al., Reference Yoshida, Yasumatsu, Shigemura and Ninomiya2006; Chandrashekar et al., Reference Chandrashekar, Kuhn, Oka, Yarmolinsky, Hummler, Ryba and Zuker2010). It is open to question, whether the prolonged exposure to saline water modified the sensitivity of the taste buds or the epithelial sodium ion channel of the salt receptor cells.
Adaptation to saline water
In ruminants, gradual changes in diets are recommended to enable the animals to adjust with the microbiological and chemical changes occurring in the rumen (Grubb and Dehority, Reference Grubb and Dehobity1975; Mackie et al., Reference Mackie, Gilchrist, Robberts, Hannah and Schwartz1978). In previous studies on sheep, a stepwise adaptation was successfully achieved through diets containing different ratios of roughage and concentrates (Grubb and Dehority, Reference Grubb and Dehobity1975), or high concentrate diets containing 1% NaCl (Mackie et al., Reference Mackie, Gilchrist, Robberts, Hannah and Schwartz1978). To our knowledge, the current study is the first applying a stepwise adaptation towards saline drinking water in goats. We observed a remarkable shift of preferences during the adaptation period with indifferent choices up to 0.5%, followed by strong preferences for lower sodium concentrations, indicating the capabilities of goats to regulate their sodium intake even when they had only the choice between different concentrations of saline water.
Adaptation to saline water could be achieved in several ways. In our study, the goat breed was adapted to temperate climatic conditions and had no prior experience with saline water. Their higher sensitivity after the long-term exposure to saline water may indicate a learning process. Taste cells may have been modified in their responsiveness thus causing taste alteration in the animal (Bernays and Singer, Reference Bernays and Singer2005). Our results support the view of Ginane et al. (Reference Ginane, Baumont and Favreau-Peigné2011) that the attractiveness of sodium greatly depends on the mineral status of the animal. Thus, calves deprived of sodium exhibited a marked preference for NaCl solutions over water (Bell and Sly, Reference Bell and Sly1979). Similarly, cows without sodium supplement preferred to graze on a pasture with NaCl application (Chiy and Phillips, Reference Chiy and Phillips1991).
Examples for a long-term morphological and behavioural adaptation to saline drinking water are given by reports; the goats on arid islands and beaches voluntarily drink sea water (Dunson, Reference Dunson1974). Interestingly, Dunson (Reference Dunson1974) found that the kidneys of feral goats from arid islands had greater relative medullary thickness (RMT) than those from domestic goats. That in turn allows these goats to adapt to increased salinity of water through a greater capacity of the kidneys to reabsorb water, concentrate urine, and reduce urinary water loss during dehydration.
Conclusions
The present study shows that a stepwise adaptation to saline drinking water in goats is an effective method to habituate the animals to saline water intake. The adult goats tolerated prolonged choices between 1.25% and 1.5% saline without health impairment. Yet there was a significant decline in body condition score without a change in body weight. This would indicate negative energy balance and lipid mobilization due to the lowered feed intake (Ferreira et al., Reference Ferreira, Ferreira, Cunha, Anastácio, Lima and Correia2013) and could result in a possible health challenge during long-term exposure to saline water.
The applied stepwise adaptation for a shorter period (4 weeks) did not result in an overall higher tolerance of saline water. After the adaptation period, the animals reacted more sensitively when offered the choice between fresh water and different concentrations of saline water. Accordingly, the observed adaptation to saline water may be of short-term duration only. Thus, more detailed investigation is required to determine the goats’ ability to tolerate higher salinity for longer periods. In regions, where salinization of drinking water becomes an increasing challenge for livestock production, the controlled exposure to salt during gestation could be beneficial to the offspring because of fetal imprinting (Digby et al., Reference Digby, Masters, Blache, Hynd and Revell2010). In this context, it would be interesting to investigate whether the high tolerance of saline water described for several species such as camels (Abou Hussien, Reference Abou Hussien, Gihad, El-Dedawy and Abdel Gawad1994; Assad and El-Sherif, Reference Assad and El-Sherif2002) is also influenced by epigenetic factors.
Acknowledgements
The authors wish to thank Jürgen Dörl (Department of Animal Sciences, University of Göttingen) for his technical assistance. We thank Prof. Dr J. Hummel and U. Vehlow (Department of Animal Sciences, University of Göttingen) for feed analyses. This research was supported by the German Academic Exchange Service (DAAD), Bonn, Germany.
A. Riek, 0000-0002-1045-6904
Declaration of interest
The authors declare no conflict of interest.
Ethics statement
Procedures performed in this study were in accordance with the German animal ethics regulations and approved by the State Office of Lower Saxony, Germany for Consumer Protection and Food Safety, Germany (Ref. no.: 33.9-42502-04-15/1946).
Software and data repository resources
The data used during the current study are deposited at the Division of Livestock Ecology, Department of Animal Sciences, University of Göttingen, Germany. Anybody who is interested can apply for access rights at the University of Göttingen, Department of Animal Sciences.








