Introduction
Rationale
Porcine respiratory disease complex (PRDC) is a major cause of morbidity and mortality in swine production. PRDC is a multifactorial disease frequently involving the interplay of environmental, host, and infectious factors (Opriessnig et al., Reference Opriessnig, Giménez-Lirola and Halbur2011). Bacterial pathogens involved in PRDC may be sufficient to cause disease themselves, or may present as co-infections with other bacteria or viruses (Opriessnig et al., Reference Opriessnig, Giménez-Lirola and Halbur2011). Some of the major bacterial pathogens frequently involved in PRDC include Actinobacillus pleuropneumoniae, Actinobacillus suis, Bordetella bronchiseptica, and Mycoplasma hyopneumoniae (Opriessnig et al., Reference Opriessnig, Giménez-Lirola and Halbur2011; VanAlstine, Reference VanAlstine, Zimmerman, Karriker, Ramirez, Schwartz and Stevenson2012).
Management of respiratory disease is a major source of antibiotic use in swine production (Karriker et al., Reference Karriker, Coeteze, Friendship, Prescott, Zimmerman, Karriker, Ramirez, Schwartz and Stevenson2012). Antimicrobials may be used for several purposes; animals showing signs of clinical illness suggestive of bacterial infection may be treated individually, groups of animals may be treated prophylactically to prevent infection and clinical illness from occurring (although this use is not global and likely will become historical), or groups of animals with some animals already infected may be treated metaphylactically to prevent the spread of infection and clinical disease within-group (McEwen and Fedorka-Cray, Reference McEwen and Fedorka-Cray2002). Regulations regarding the use of antibiotics for these purposes vary by country and over time. It has been estimated that 60% of swine nursery sites use injectable antibiotics to treat respiratory disease (USDA, 2016). Similarly, 70% of swine grower-finisher sites reported using injectable antibiotics to treat respiratory disease (USDA, 2016). In Canada in 2015, approximately 67% of grower-finisher pigs in the provinces of Ontario and Quebec received antimicrobials in feed for preventive purposes, with 44% of herds surveyed in the western provinces reporting the use of preventive antimicrobials in grower-finisher pigs (Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS), 2017).
Swine veterinarians and producers are encouraged to minimize antimicrobial use, make appropriate antibiotic selections, and use antimicrobials only when they provide measurable benefits (Canadian Association of Swine Veterinarians, 2016; Pork Checkoff, 2016; Canadian Pork Council, 2018; American Association of Swine Veterinarians, 2019). This requires decision-making informed by accurate comparative estimates of antimicrobial efficacy. Such comparisons require interpreting data from published research, which may sometimes report inconsistent or even opposing findings across studies.
Systematic reviews are widely recognized as a transparent and credible methodology for synthesizing evidence regarding intervention efficacy (European Food Safety Authority, 2010; Higgins and Green, Reference Higgins and Green2011; Codex Alimentarius Commission, 2014). When combined with meta-analysis (the statistical pooling of effect estimates across studies), a systematic review can summarize the publicly available research on an intervention, and produce a summary estimate of effect with greater precision than those reported in the individual studies (Higgins and Green, Reference Higgins and Green2011). Network meta-analysis (NMA), an extension of pairwise meta-analysis, allows the assessment of the comparative, or relative, efficacy of multiple interventions across a network of trials, even though not every intervention-comparison may have been directly assessed in an individual trial in the dataset (Cipriani et al., Reference Cipriani, Higgins, Geddes and Salanti2013). For instance, suppose that there were three antibiotic treatment options (tetracycline, tiamulin, and tilmicosin) for preventing a specific outcome such as mortality, but studies were only available evaluating the relationship between tetracycline and tiamulin and between tiamulin and tilmicosin (the ‘direct’ comparisons). It is possible within an NMA to indirectly estimate the relationship between tetracycline and tilmicosin (the ‘indirect’ comparisons) using the information from studies reporting on the direct comparisons.
A systematic review and NMA of antibiotics for the treatment of swine respiratory disease has been published (O'Connor et al., Reference O'Connor, Totton and Shane2019); however, the scientific literature on preventive uses of antibiotics has not been formally synthesized. A systematic review and NMA of the efficacy of currently used antimicrobials for the prevention of swine respiratory disease could provide credible evidence regarding the comparative efficacy of antimicrobials for prevention of swine respiratory disease, to aid veterinarians and swine producers in decision-making for the prudent use of antibiotics.
Objective
The objective of this study was to conduct a systematic review and, if supported by data, an NMA to address the question, ‘What is the comparative efficacy of antimicrobials for the prevention of swine respiratory disease?’
Methods
Protocol
A protocol, reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocol extension (PRISMA-P) guidelines (Moher et al., Reference Moher, Shamseer, Clarke, Ghersi, Liberati, Petticrew, Shekelle and Stewart2015), was prepared a priori and published to the University of Guelph's institutional repository (https://atrium.lib.uoguelph.ca/xmlui/handle/10214/10046) and is also available on the Systematic Reviews for Animals and Food (SYREAF) website (http://www.syreaf.org/contact/). This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (Liberati et al., Reference Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche, Ioannidis, Clarke, Devereaux, Kleijnen and Moher2009; Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009).
Eligibility criteria
Primary research studies, both published and unpublished (gray literature), available in English were eligible. In addition, eligibility criteria specific to the PICOS components of the review question were as follows:
Population (P): Healthy swine at any stage of production.
Intervention (I): Antibiotics administered parenterally, in the feed, or in the water at a labeled therapeutic dose to prevent respiratory disease. Eligible antibiotics include any antibiotic licensed for use for respiratory disease in swine in any country and included in the OIE list of antimicrobial agents of veterinary importance (OIE, 2015). Because there are differences in regulations on use of antibiotics over time and by country, we did not attempt to determine, or restrict, the intervention to on-label or legal usage.
Comparator (C): Placebo, untreated control group, another antibiotic, or an alternative treatment.
Outcomes (O): The outcomes of interest were respiratory-related incidence of clinical morbidity (as defined by the authors), mortality, or total antibiotic use over any period of time within the same production stage. If antibiotics were administered to groups where some pigs were ill, outcome data needed to be presented separately for the pigs that were healthy at the time the antibiotic treatment was initiated for the study to be eligible.
Study design (S): Controlled trials with natural disease exposure.
Search
Sources
Databases searched were: Agricola (via ProQuest, 1970 to search date), CAB Abstracts and Global Health (via the University of Guelph CAB interface, 1900 to search date), MEDLINE® (via PubMed; 1946 to search date), Conference Proceedings Citation Index – Science (via Web of Science, 1990 to date of search), and Science Citation Index (via Web of Science, 1900 to date of search). No date restrictions were placed on the search beyond inception dates of the databases. Likewise, no restrictions were placed on the study designs or language of publication in the search. In addition, a single reviewer hand-searched the table of contents of the Proceedings from the American Association of Swine Veterinarians Annual Meeting (1999–2018), the Proceedings of the International Pig Veterinary Society Congress (2000–2018), and the Food and Drug Administration website for registered animal drugs (FDA FOI).
Search strategy
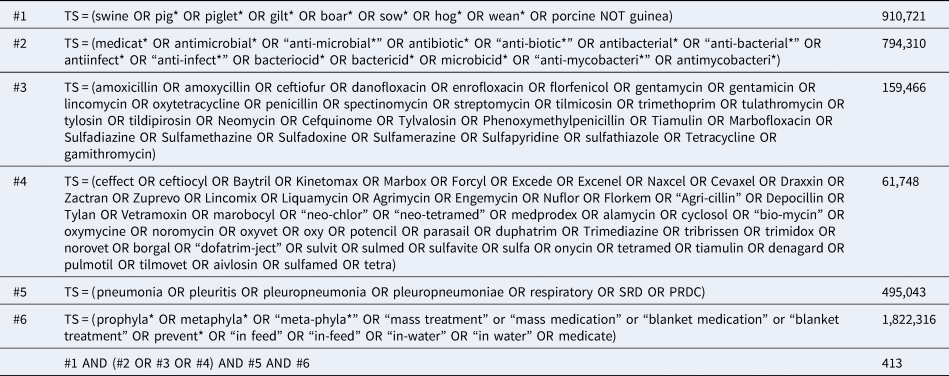
The search strategy was developed for the Science Citation Index (Web of Science) and comprised of search terms related to four concepts: swine at any stage of production, antibiotics, respiratory disease outcomes, and disease prevention (as opposed to treatment). The full Science Citation Index search strategy is listed in Table 1. Database searches were conducted on 17–20 September 2018 and accessed through the library at the University of Guelph, Canada. Search results were uploaded to EndNoteX7 (Clarivate Analytics, Philadelphia, PA) and duplicate results documented and removed. Records were then uploaded to DistillerSR® (Evidence Partners Inc., Ottawa, ON) and additionally de-duplicated.
Table 1. Full electronic search strategy used to identify studies on the efficacy of antibiotics to prevent respiratory disease in swine as formatted for Science Citation Index (Web of Science) conducted on 17 September 2018
Study selection
DistillerSR® was used for managing eligibility screening, data extraction, and risk-of-bias assessments. Titles and abstracts were initially screened for eligibility. All reviewers were trained on a pre-test of the first 250 titles and abstracts. Thereafter, two reviewers independently evaluated each citation. The following questions were used to assess eligibility:
(1) Is this a primary study which evaluates the use of one of more antibiotics to prevent respiratory disease in swine? YES, NO, UNCLEAR
(2) Is there a concurrent comparison group (i.e. controlled trial with natural or deliberate disease exposure, or analytical observational study)? YES, NO, UNCLEAR
(3) Is the full text available in English? YES, NO, UNCLEAR
Citations were excluded if both reviewers responded ‘NO’ to any of the questions; agreement was at the level of the decision to exclude the citation versus advancing to full-text screening. Disagreements were resolved by consensus, or by JMS or CBW if agreement could not be reached. Full-text eligibility was then conducted on remaining studies independently by two reviewers, using the first 10 articles as a pre-test by all reviewers. The full-text eligibility screening used the initial three questions with only YES or NO options, and the following additional questions:
(4) Is the full text available with >500 words? YES, NO
(5) Does the study evaluate swine? YES, NO
(6) Does the study assess the use of one or more of the antibiotics for the PREVENTION of respiratory disease(s) (i.e. animals were healthy at the time of enrolment)? YES, NO
(7) Are at least one of the following outcomes described: respiratory disease-related clinical morbidity, mortality, or antibiotic use? YES, NO
(8) Is the study a controlled trial with natural disease exposure?
YES (moves to data extraction)
NO, the study is a controlled trial with deliberate disease induction (indicate the antibiotic(s) evaluated, but exclude from data extraction)
NO, the study is an observational study (indicate the antibiotics(s) evaluated but exclude, from data extraction)
Agreement was at the question level, with conflicts resolved by consensus or with input from JMS or CBW if agreement could not be reached.
Data collection
Data from eligible trials were independently extracted by two reviewers using a standardized form, which was piloted on the first 10 articles by all reviewers. Any discrepancies between reviewers in data extraction were resolved by consensus, with mediation by JMS or CBW if agreement could not be reached.
Data items
Study characteristics
Study-level characteristics extracted included country of conduct, setting (research or commercial herd), number of farms enrolled, inclusion criteria at the animal- and farm-levels, year the study was conducted, and the months of data collection.
Interventions and comparators
Data extracted for each trial on the interventions comprised of antibiotic name, dose, route, and frequency of administration, additional concurrent therapy, and description of the comparison group. Data were also collected on the stage of production when the intervention was allocated and when the outcomes were evaluated.
Outcomes
Data were collected for the following outcomes: respiratory-related morbidity (clinical illness or illness requiring treatment, or cough index within a group), mortality, or total antibiotic use. For morbidity outcomes, results were collected for incident cases of illness. If antibiotics were administered to a group that contained some animals that were ill at the time of treatment initiation, data were only extracted if results were reported separately for the incident cases. Because results were presented in different ways among trials, and because some trials presented the results in more than one way, the type of outcome data extracted was prioritized. If presented, the adjusted summary effect (adjusted odds ratio (OR) or risk ratio (RR)) and corresponding measures of variability were extracted, including information on which factors were included in the adjustment. If an adjusted measure was not reported, unadjusted summary effect sizes and corresponding measures of variability were extracted. If no summary measures were reported, then arm-level data (number of events and total number of study subjects within each intervention group) were extracted. If summary measures were reported without variance estimates, then arm-level data were extracted, if reported. For each intervention, the number lost to follow-up was extracted, if reported.
Some publications included multiple trials, or trials in multiple farms where the results were reported separately by herd. In these instances, the results of each trial or farm were extracted separately and are reported as separate trials.
Risk of bias in individual studies
Risk of bias was assessed by outcome for clinical morbidity and for mortality, with the nursery and grower-finisher stages of production where the intervention was administered combined. The Cochrane Risk-of-Bias tool for Randomized Trials (RoB 2.0) (Higgins et al., Reference Higgins, Sterne, Savović, Page, Hróbjartsson, Boutran, Reeves, Eldridge, Chandler, McKenzie, Boutron and Welch2016) was used. Signaling questions were modified to address common practices in swine where animals are grouped in pens. All outcomes were evaluated using this tool, regardless of whether interventions were allocated at the individual or pen level, or whether animals within intervention groups were comingled or grouped by intervention group within pens. For assessing risk of bias due to allocation, the Cochrane Risk-of-Bias tool includes a question on whether the authors described the method for generating the random sequence. This question was modified to allow a response for studies where allocation to intervention group was reported as ‘random,’ but no information was provided on the actual method for generating the random sequence. In the risk-of-bias domain related to deviation from intended interventions, there is a question on whether participants were aware of their intervention allocation; this was answered as ‘no’ for all trials, because the participants in these trials were animals. An additional question in this domain asks about blinding of study personnel; for the purposes of this review, the wording of this question was clarified to mean blinding of animal caregivers.
The overall risk of bias within each domain was calculated based on the algorithms suggested by Higgins et al. (Reference Higgins, Sterne, Savović, Page, Hróbjartsson, Boutran, Reeves, Eldridge, Chandler, McKenzie, Boutron and Welch2016), with the exception of the domain related to randomization. For this domain, the allocation concealment question was not included in the algorithm, as all eligible pens and animals are included in swine trials, and it is unlikely that a producer or investigator would have any treatment preference for a given pen, as the differential economic value of animals or pens would not be known at the time that interventions were allocated. This approach is consistent with risk of bias assessments of other livestock studies (Moura et al., Reference Moura, Totton, Sargeant, O'Sullivan, Linhares and O'Connor2019). Trials where the authors stated that allocation to treatment group was random, but did not describe how the random allocation was generated, were categorized as ‘no information’ for the question related to random allocation.
Summary measures
The RR was used as the summary measure for clinical morbidity and mortality outcomes. For comparisons where both intervention arms had no events (zero cells), a relative risk was not calculated. For comparisons where one of the two treatment arms had a zero cell, a value of 0.5 was assigned to that cell for illustrative purposes only in the forest plots used to show the trial results.
Synthesis of results
As described in the protocol, the intention of this review was to conduct an NMA to assess the comparative efficacy of antibiotic intervention options. However, due to the lack of replication in the intervention and outcome comparisons that were identified in the literature, no quantitative synthesis was performed. Trial results were presented in forest plots for the purpose of visualization, but no summary measure was calculated and heterogeneity in the results among trials was not formally assessed. Forest plots were produced for each outcome and separately based on the stage of production where the intervention was administered (nursery or grower stage).
Risk of bias across studies
Risk of bias across studies (publication bias) is usually evaluated by examining a funnel plot for small-study effects using pairwise comparisons. This is generally a two-step process: a visual evaluation of the symmetry in the funnel plots, and a formal statistical test for symmetry, if sufficient data are available (>10 studies) (Higgins and Green, Reference Higgins and Green2011). In this review, too few observations were available for each intervention, so any statistical assessments of symmetry would not be reliable. Therefore, an evaluation of risk of bias across studies was not conducted.
Additional analyses
No additional analyses were conducted.
Results
Study selection
There were 1249 unique citations identified by the search. After full-text screening, 58 trials from 50 publications were eligible for inclusion (Fig. 1). Of these, 44 trials from 36 publications had extractable data for one or more of the eligible outcomes. Respiratory-related clinical morbidity was evaluated in eight trials where interventions were given to nursery pigs, and in 10 trials where interventions were given to grower pigs. Mortality was evaluated in one trial where the antibiotics were given to pre-weaned pigs, 22 trials where the antibiotics were given to nursery pigs, and 12 trials where the antibiotics were given to grower pigs (Fig. 1 and Table 2).
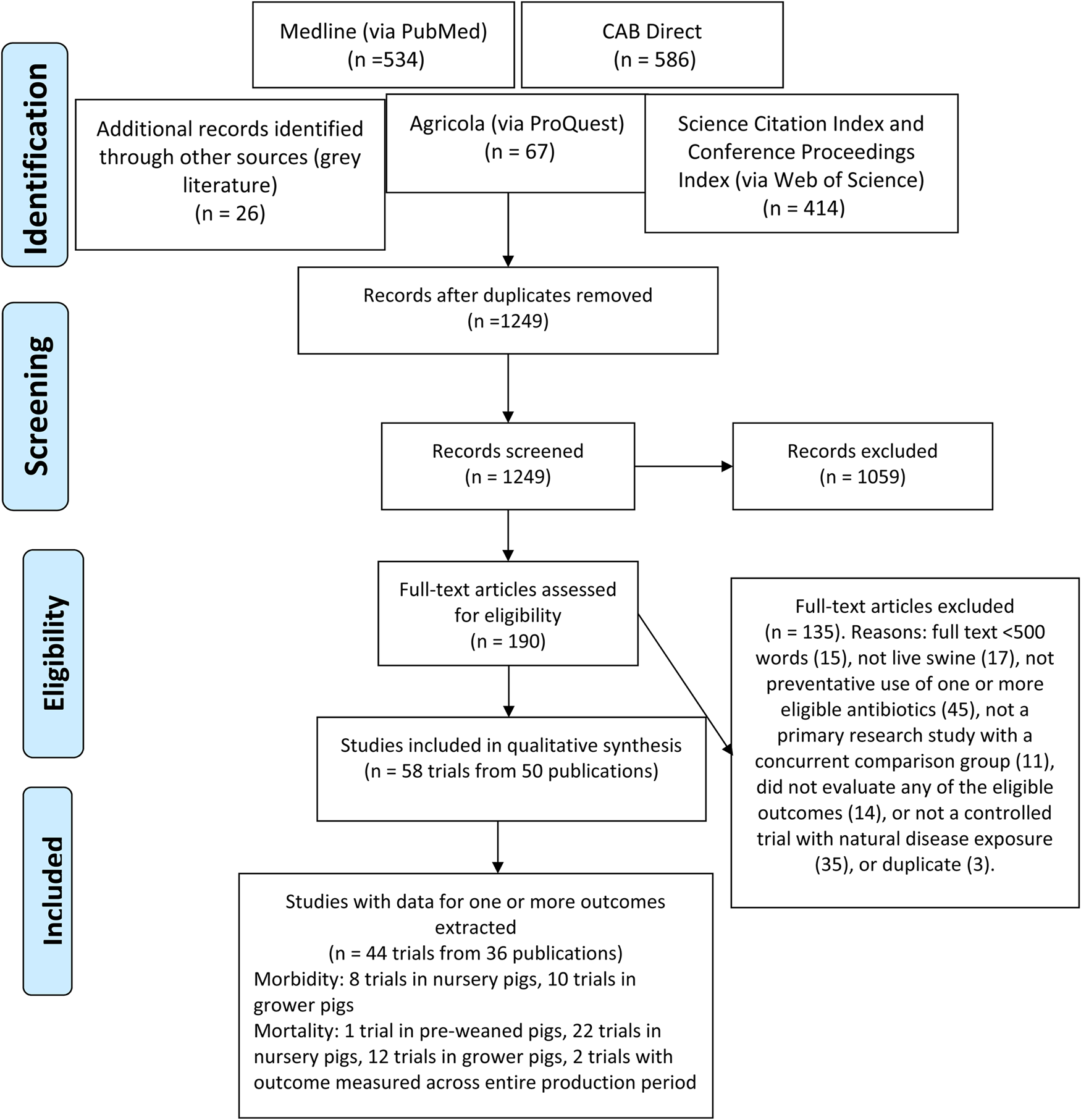
Fig. 1. Flow of literature through the review process for a systematic review of the efficacy of antibiotics to prevent respiratory disease in swine.
Table 2. Characteristics of trials evaluating the efficacy of antibiotics for preventing respiratory disease in swine
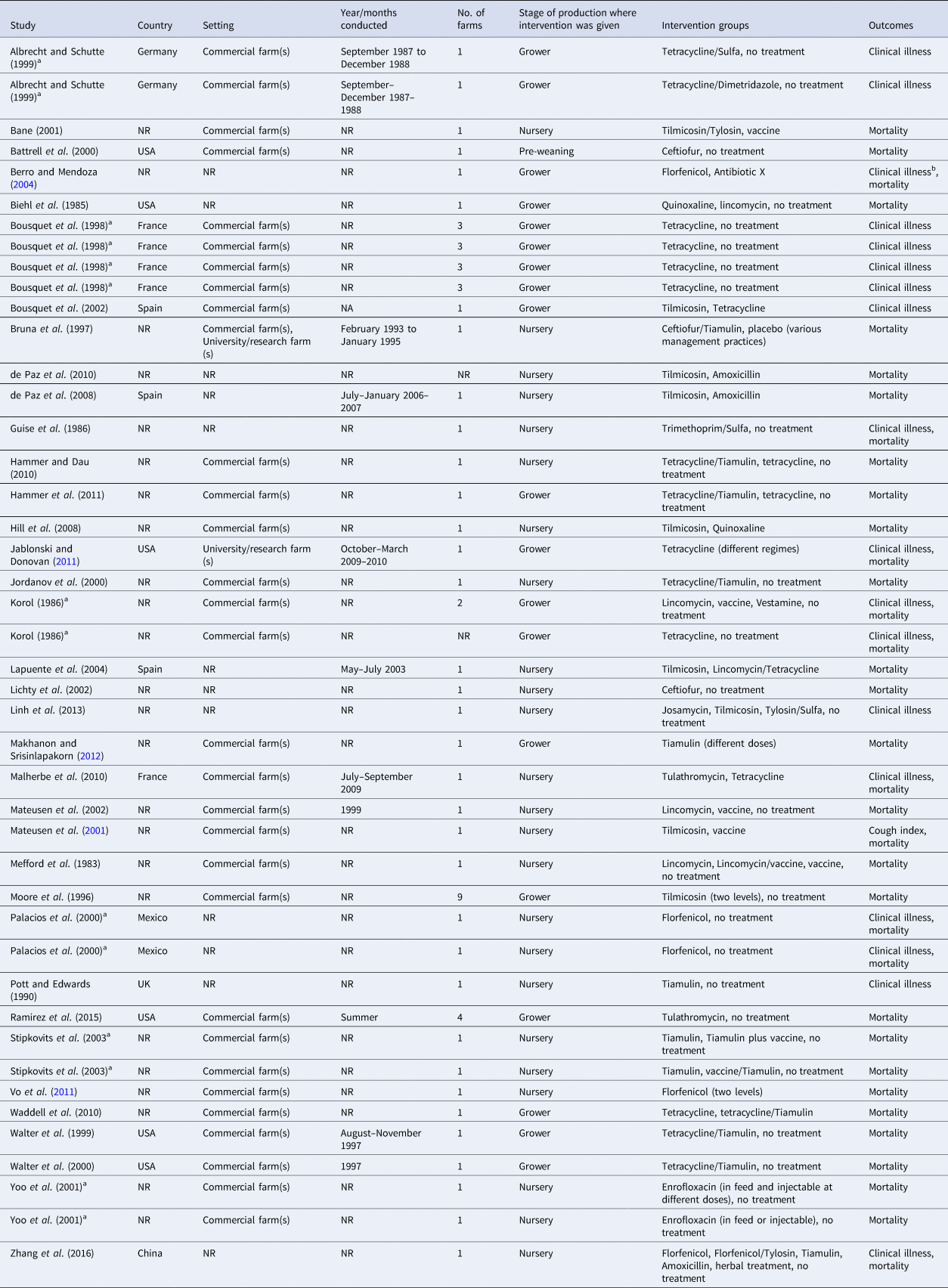
Label of tetracycline included tetracycline, chlortetracycline, and doxycycline.
NR = not reported.
a Studies where results were presented by farm/flock.
b Studies with outcomes that could not be used due to no variance, results presented in graphs, etc.
There were 15 unique antibiotics evaluated in the relevant trials. Based on the World Health Organization (WHO) 2018 categorization of the importance of antibiotics for human medicine, the antibiotics evaluated in this review ranged from those of critical importance to human medicine to antibiotics that currently are not used in humans (Table 3) (WHO, 2019).
Table 3. Antibiotics evaluated for prevention of swine respiratory disease, categorized based on medical importance to humans

Study characteristics
The characteristics of the included trials are presented in Table 2 and a reference list is provided in Supplementary material. The trials were conducted in seven countries, although the country where the trial was conducted was not reported for over half of the trials (25/44). For the trials where country was reported, six trials were conducted in the United States, five were conducted in France, three were conducted in Spain, two were conducted in each of Germany and Mexico, and a single trial was conducted in each of the United Kingdom and China. The majority of the trials were conducted in commercial herds (31/44) and the month(s) and year when the trial was conducted was not reported for most trials (33/44). The number of farms per trial ranged from 1 to 9; there were 34 trials conducted on a single farm or where the results were presented separately by farm.
Risk of bias within studies
Summaries of the risk of bias by domain for trials that measured clinical morbidity and for trials that measured mortality are shown in Figs. 2 and 3, respectively. All except for two of the trials were rated as ‘some concerns’ or ‘high’ for the risk of bias related to the randomization process. This was because the authors of these trials reported that randomization was not used, or reported that randomization was used but did not provide information on how the random sequence was generated, or did not provide any information to describe how study subjects were allocated to treatment group. The differentiation between ‘some concerns’ and ‘high’ was based on whether or not the authors presented information on baseline imbalances that would suggest a potential for bias.
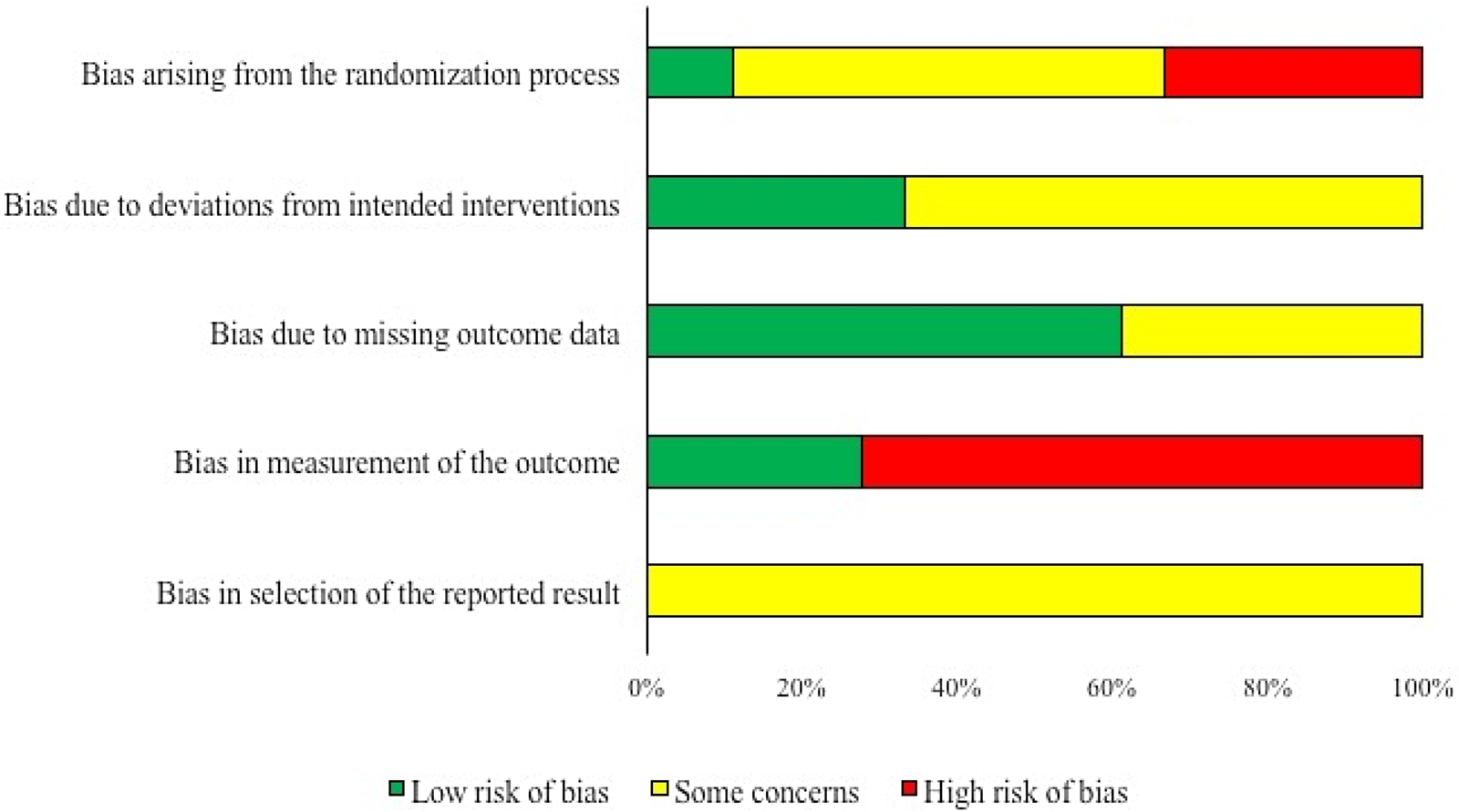
Fig. 2. Summary of the risk of bias by domain for 18 trials evaluating the efficacy of preventive antibiotics to reduce the incidence of clinical morbidity in swine.
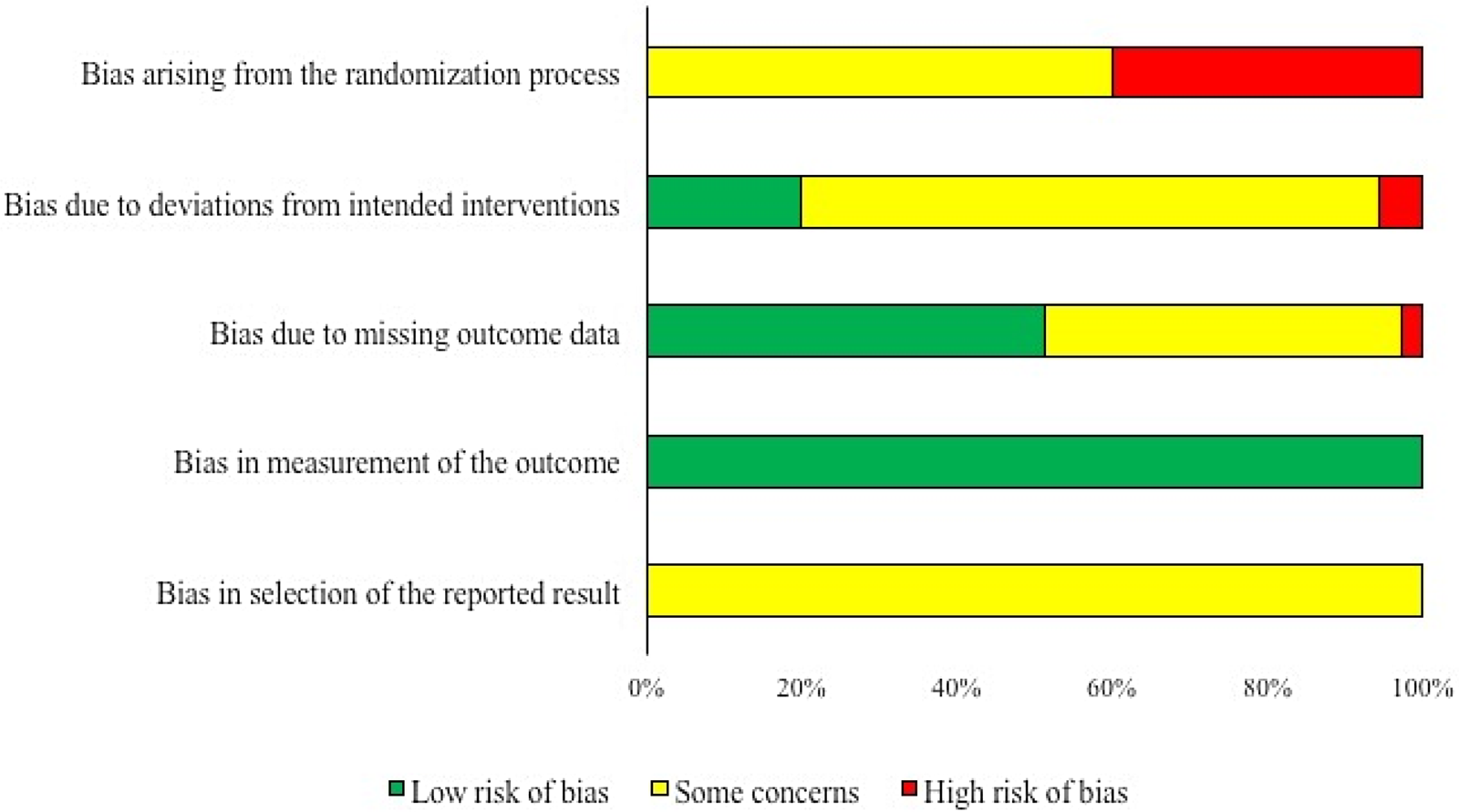
Fig. 3. Summary of the risk of bias by domain for 35 trials evaluating the efficacy of preventive antibiotics to reduce mortality in swine.
For both outcomes, bias due to deviations from intended interventions was either rated as ‘low’ risk of bias or as ‘some concerns,’ with the exception of two studies which were rated as a ‘high’ risk of bias in this domain. A ‘low’ rating was assigned to trials where outcome assessors were blinded to the intervention group and all study subjects were analyzed in the group to which they were assigned or where it was not clear whether or not outcome assessors were blinded, but there were no deviations from intended interventions that could likely affect the outcome and all study subjects were analyzed in the group to which they were assigned. ‘Some concerns’ resulted from the lack of information to assess the questions in this domain.
Bias due to missing outcome data was rated as low or some concerns except in one trial measuring mortality. The ‘low’ rating resulted when there was no loss to follow-up or when any losses to follow-up were equal between treatment groups. Ratings of ‘some concerns’ resulted from the lack of information to assess the questions in this domain.
For mortality, all studies were assigned a ‘low’ risk for bias from outcome measurement, because outcome assessors were not likely to be biased in their recordings of mortality regardless of whether they were blinded. In contrast, the risk of bias related to outcome measurement in trials reporting clinical morbidity was rated as ‘high’ in 13/18 trials, because outcome assessors were not blinded, or no information on blinding of outcome assessors was provided, and there was the potential for knowledge of intervention status to influence assessors’ measurement due to the subjective nature of measuring clinical morbidity.
Finally, for bias due to selective reporting of outcomes, all trials for both outcomes were rated as ‘some concerns’ because an a priori trial protocol is needed to judge whether a trial is at a ‘high’ or ‘low’ risk for bias in this domain, and a priori protocols were not available for any of the trials included in this review.
Results of individual studies
Incidence of clinical morbidity for trials evaluating antibiotics given to nursery pigs
There were 25 comparisons in seven trials evaluating clinical morbidity where antibiotics were given to nursery pigs (Fig. 4). A single trial measured morbidity using a cough index (Mateusen et al., Reference Mateusen, Maes, Hoflack, Verdonck and De Kruif2001); this trial was not included in the forest plot. For the 11 comparisons of an antibiotic to a non-active treatment group, all but one comparison qualitatively favored the antibiotic. However, there was insufficient replication of specific intervention/outcome comparisons to allow for quantitative synthesis. None of the trials measuring morbidity for antibiotics given to nursery pigs controlled for the pen effect. Thus, the confidence intervals as reported were likely narrower than they would have been, had the authors adjusted for the non-independence of observations within pen.
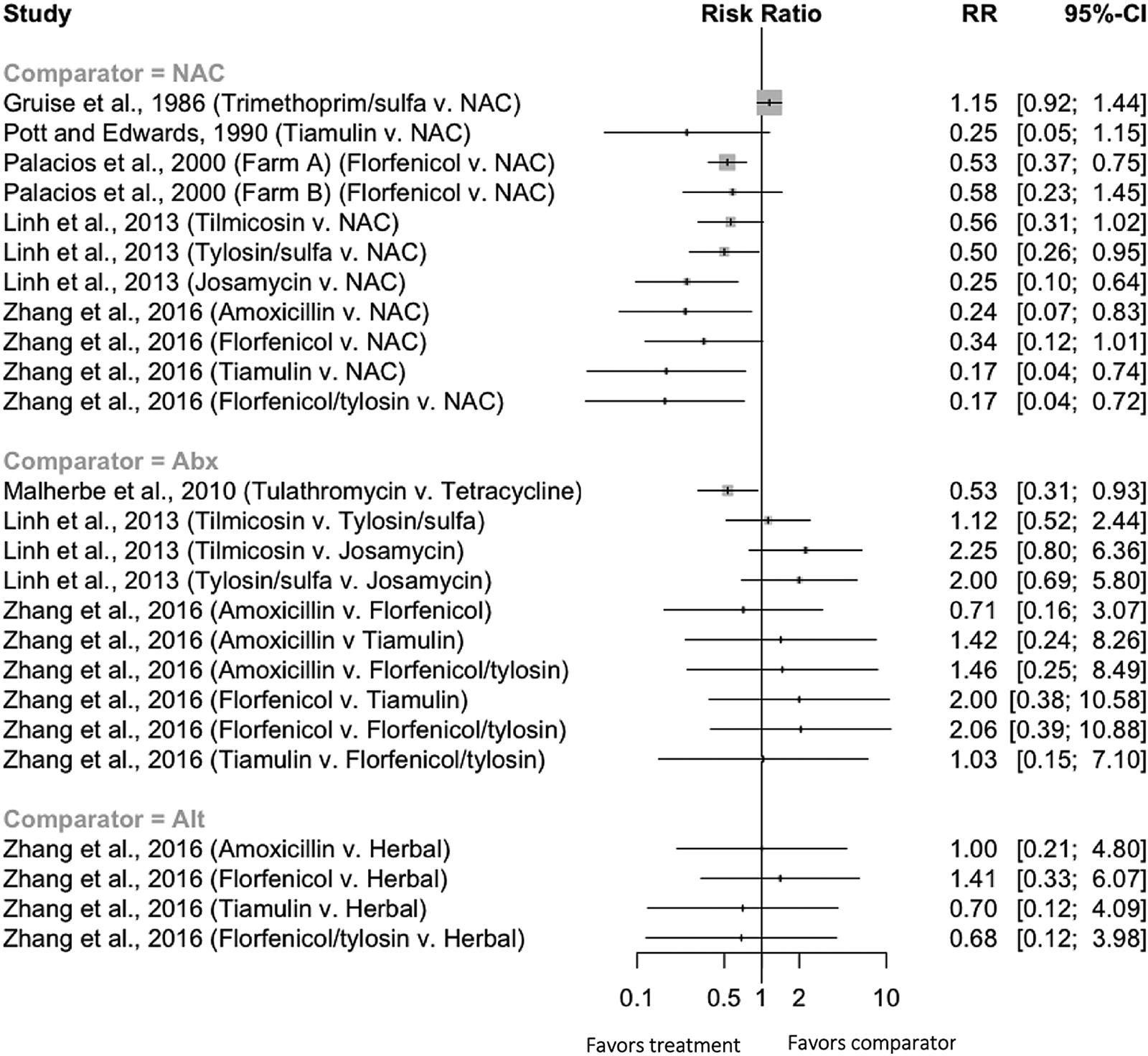
Fig. 4. Forest plot illustrating the efficacy of preventive antibiotics to reduce the incidence of clinical morbidity when given to nursery pigs. NAC = ‘non-active comparison,’ i.e. not treated.
Incidence of clinical morbidity for trials evaluating antibiotics given to grower pigs
There were 10 trials with extractable data measuring respiratory-related clinical illness where antibiotics were given during the grower phase. Of these, different treatment regimens of the same antibiotic (tetracycline) were compared in one trial (Jablonski and Donovan, Reference Jablonski and Donovan2011); this trial was not included in the forest plot. The results of the remaining nine trials are shown in Fig. 5. There were eight comparisons between an antibiotic intervention and a non-active treatment group, two comparisons to a non-antibiotic intervention, and one comparison between two different antibiotics. Qualitatively, the comparisons to a non-active control group showed a reduction in clinical morbidity associated with the antibiotic treatment. However, there was insufficient replication of specific interventions to allow a quantitative synthesis. None of the trials measuring morbidity where interventions were given to grower pigs controlled for the pen effect; controlling for non-independence would be expected to result in wider confidence intervals.
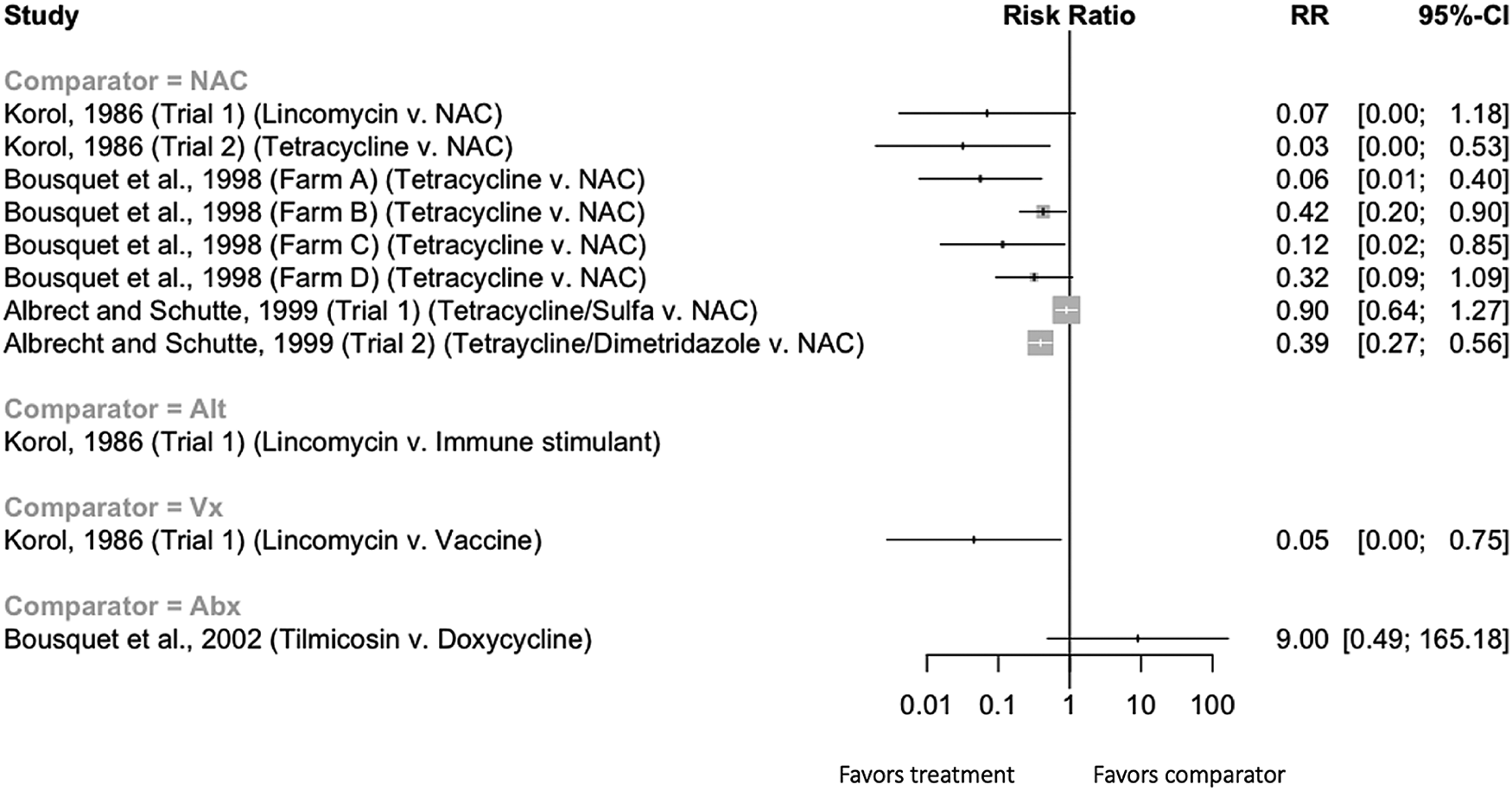
Fig. 5. Forest plot illustrating the efficacy of preventive antibiotics to reduce the incidence of clinical morbidity when given to grower-finisher pigs. Trials where no estimate of RR is provided in the figure represent trials where zero cells (no events in either intervention group) precluded estimation of an RR and confidence intervals. NAC = ‘non-active comparison,’ i.e. not treated.
Mortality for trials evaluating antibiotics given to nursery pigs
There were 38 comparisons from 19 trials measuring mortality when interventions were given to nursery pigs (Fig. 6). One trial (Vo et al., Reference Vo, Phan and Pham2011) with extractable outcome data compared two levels of the same antibiotic and therefore is not included in Fig. 6. An RR could not be calculated for eight comparisons because there were no mortality events in either intervention group. All except for two of the trials where an antibiotic was compared to a non-active treatment group found a numerical benefit to the antibiotic. However, there was insufficient replication of specific intervention/comparator comparisons to allow a quantitative synthesis. As with the clinical morbidity outcomes, none of the trials measuring mortality when interventions were given to nursery pigs controlled for the pen effect.

Fig. 6. Forest plot illustrating the efficacy of preventive antibiotics to reduce mortality when given to nursery pigs. Trials where no estimate of RR is provided in the figure represent trials where zero cells (no events in either intervention group) precluded estimation of an RR and confidence intervals. NAC = ‘non-active comparison,’ i.e. not treated.
Mortality for trials evaluating antibiotics given to grower pigs
There were 15 comparisons from nine trials measuring mortality when interventions were given to grower pigs (Fig. 7). Two comparisons had zero events in both intervention groups and therefore a relative risk was not calculated. Three trials which had extractable data were not included in the forest plot; one did not describe the antibiotic used for the comparison group (Berro and Mendoza, Reference Berro and Mendoza2004), and two trials compared different treatment regimens (e.g. dose and route) for the same antibiotic (Jablonski and Donovan, Reference Jablonski and Donovan2011; Makhanon and Srisinlapakorn, Reference Makhanon and Srisinlapakorn2012). Two of the comparisons between an antibiotic and a non-treated control group had an RR of 1, and the remaining comparisons showed a numeric reduction in the risk of mortality in the antibiotic intervention group. However, there was insufficient replication of interventions to allow for a quantitative comparison of any specific antibiotic regime. Again, none of the trials measuring mortality in grower pigs adjusted for the pen effect.
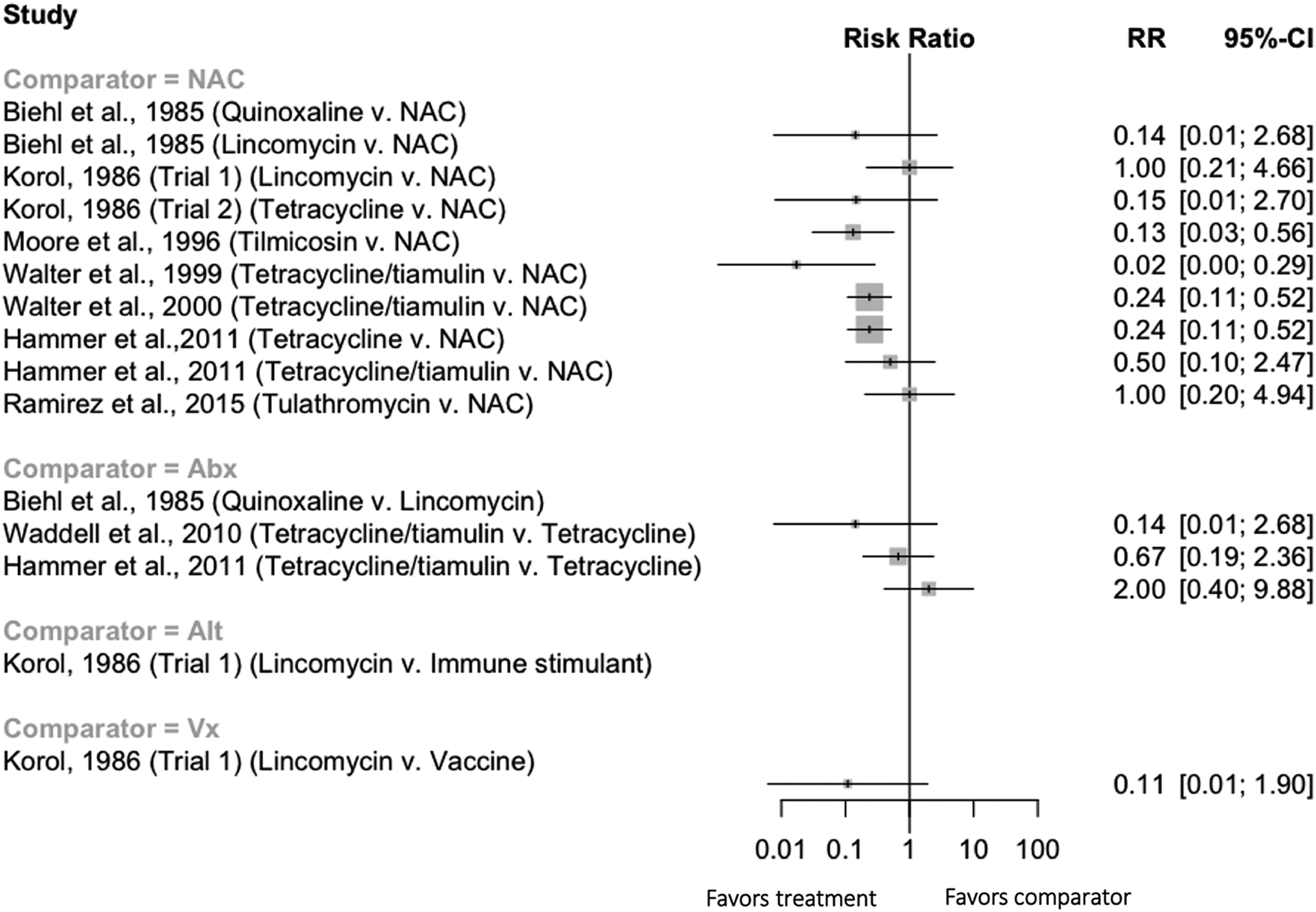
Fig. 7. Forest plot illustrating the efficacy of preventive antibiotics to reduce mortality when given to grower-finisher pigs. Trials where no estimate of RR is provided in the figure represent trials where zero cells (no events in one or more intervention groups) precluded estimation of an RR and confidence intervals. NAC = ‘non-active comparison,’ i.e. not treated.
Synthesis of results
NA
Risk of bias across studies
NA
Additional analysis
NA
Discussion
Summary of evidence
Given the global imperative to use antibiotics judiciously, it is important to consider the scientific evidence for their efficacy and, if antibiotic options have similar efficacy, to select the antibiotic of less importance to human medicine. In this review, the antibiotics that were evaluated ranged from those of critical importance to human medicine (such as ceftiofur) to quinoxaline, which is not currently used in humans. Ideally then, it would be useful to know the relative efficacy of different antibiotics. This can be accomplished by comparing antibiotic products to each other using pairwise meta-analysis when head-to-head comparisons are published in the literature, or using both direct and indirect comparisons in an NMA. The intention of the current review was to conduct an NMA to evaluate comparative efficacy; however, the data were not sufficient to allow this analytical approach. Overall, the results of this review suggest that antibiotics may be more efficacious than no treatment. However, the body of literature was too heterogeneous to allow quantification.
The most common comparison group was to a non-treated group. Across all of the outcomes, the results of this review suggest that antibiotics may be more efficacious than no treatment. However, given the need to reduce antibiotic use in animals, this currently may not be the most relevant comparison group for the swine industry. In addition to comparative efficacy between antibiotic options, comparisons to alternative treatments may be of high relevance to the industry. The data comparing antibiotics to other treatment options was sparse, and this may be an area where additional research is warranted. Of note, the aim of this study was not to evaluate the efficacy of non-antibiotic interventions to prevent respiratory disease per se and therefore the search was not designed to identify studies evaluating these options unless they also evaluated an antibiotic intervention. Therefore, it was not possible to assess the efficacy of non-antibiotic interventions compared to no treatment or to each other.
Limitations of the data
A major limitation of the data was its scarcity; there was a lot of heterogeneity between the interventions and comparison groups and almost no replication of specific intervention/comparisons across studies. The results of a comparison in a single trial represent a random result from a distribution of possible results, because of nuanced differences in trial conditions and as a result of sampling variation. Therefore, it is necessary to replicate comparisons across multiple studies to have an accurate and precise estimate of the intervention efficacy. Thus, additional trials are needed to evaluate the efficacy of antibiotics to prevent respiratory disease in swine, to support veterinarians’ recommendation on the use of preventive antibiotics or alternatives.
It is not possible to estimate RR or ORs for outcomes measured on a binary scale when there are no events in one or more of the intervention groups. Therefore, for the purposes of plotting the results, in trials where a single intervention arm had no events, we added 0.5 to demonstrate the results. However, this has the potential to provide an inaccurate representation of the results, particularly when sample sizes are small. Thus, it is important that, if rare outcomes such as mortality are important in decision-making, trials are adequately powered to be able to make meaningful comparisons. Specifically, rare outcomes will require large sample sizes.
Another limitation was the lack of adjustment for pen effects across all trials. When animals are housed in groups, observations on individual animals within the group are not independent, regardless whether interventions are allocated at the animal or group level. Adjusting for the pen effect reduces the effective sample size, with the magnitude of that reduction depending on the magnitude of the pen effect. This means that when a pen effect is present but not controlled for in the analysis, the confidence intervals on the estimate of treatment efficacy are inappropriately narrow. The issue of ‘clustering’ of observations in livestock studies has been discussed in the veterinary literature for decades (e.g. see McDermott and Schukken, Reference McDermott and Schukken1994; McDermott et al., Reference McDermott, Schukken and Shoukri1994). However, adjustment is still not ubiquitous, as evidenced by this review. Methods are available to adjust for pen effects (Schukken et al., Reference Schukken, Grohn, McDermott and McDermott2003) and should be used when animals are housed in groups.
Finally, there were some issues identified that related to the potential for risk of bias. Two issues of note are the failure to randomly allocate to intervention groups, and inadequate reporting of key features necessary for the assessment of the potential for bias. Numerous studies, both in human and veterinary medicine, have documented that treatment benefits are exaggerated in trials where allocation to the treatment group is not random (Moher et al., Reference Moher, Pham, Jones, Cook, Jadad, Moher, Tugwell and Klassen1998; Burns and O'Connor, Reference Burns and O'Connor2008; Sargeant et al., Reference Sargeant, Elgie, Valcour, Saint-Onge, Thompson, Marcynuk and Snedeker2009a, Reference Sargeant, Saint-Onge, Valcour, Thompson, Elgie, Snedeker and Marcynuk2009b). In this review, although there were trials where allocation to the intervention group was not random, the more common issue was trials where the authors referred to allocation as ‘random,’ but did not provide a description as to how the allocation sequence was generated, counter to recommended reporting standards (Schulz et al., Reference Schulz, Altman and Moher2010; O'Connor et al., Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010a). Failure to report key features related to the potential for bias were also noted in this review, which is consistent with other publications which have evaluated the quality of reporting in livestock trials (Wellman and O'Connor, Reference Wellman and O'Connor2007; Burns and O'Connor, Reference Burns and O'Connor2008; Sargeant et al., Reference Sargeant, Elgie, Valcour, Saint-Onge, Thompson, Marcynuk and Snedeker2009a, Reference Sargeant, Saint-Onge, Valcour, Thompson, Elgie, Snedeker and Marcynuk2009b; Brace et al., Reference Brace, Taylor and O'Connor2010; Winder et al., Reference Winder, Churchill, Sargeant, LeBlanc, O'Connor and Renaud2019). Some study characteristics were also not well reported in the trials included in this review, such as month of study conduct and location of the study. This information is helpful in providing context to the reader, allowing them to better judge external validity. To address concerns related to the quality of reporting in trials in livestock, the REFLECT statement was co-published in multiple journals (O'Connor et al., Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010a, Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010b, Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010c, Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010d, Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010e). The REFLECT statement, created by expert consensus, comprises a checklist of 22 items that it is recommended trial authors describe in publications and reports for all trials in livestock. A companion article, co-published in two journals, provides an explanation and elaboration for each of the items, as well as examples from the published literature of good reporting for each item (Sargeant et al., Reference Sargeant, O'Connor, Gardner, Dickson, Torrence, Dohoo, Lefebvre, Morley, Ramirez and Snedeker2010a, Reference Sargeant, O'Connor, Gardner, Dickson, Torrence, Dohoo, Lefebvre, Morley, Ramirez and Snedeker2010b). Adhering to recommendations to transparently and fully report the design, conduct, and results of trials will improve the ability of journal editors, reviewers, and readers to understand and interpret research findings.
Limitations of the review
Despite conducting a comprehensive search for relevant literature, it is possible that not all trials evaluating preventive uses of antibiotics were identified by our search. Also, we choose to include only trials with natural exposure to the infectious disease agents of interest; this removed 35 challenge trials from eligibility. Challenge trials can provide useful proof of concept for intervention efficacy, but the disease challenge may not reflect actual exposure conditions in the field (Sargeant et al., Reference Sargeant, Kelton and O'Connor2014a, Reference Sargeant, Kelton and O'Connor2014b). Also, there is some empirical evidence that challenge trials are more likely to show beneficial treatment results compared to natural exposure trials (Wisener et al., Reference Wisener, Sargeant, O'Connor, Faires and Glass-Kaastra2014). Therefore, we felt that including only natural disease exposure trials would more accurately represent the efficacy of preventive antibiotics to reduce respiratory disease in swine under field conditions.
Conclusions
Based on a heterogeneous body of research with little replication, it was not possible to quantify the efficacy, nor relative efficacy, of antibiotics to prevent respiratory disease in swine. There is a need for additional trials, preferably comparing antibiotics to each other or to a non-antibiotic alternative, to further our understanding of antibiotic efficacy. Improvements in statistical control of non-independence of observations within pens and in reporting of key trial features will improve the rigor and transparency of trials addressing this topic.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1466252319000185
Author contributions
JMS developed the review protocol, coordinated the project team, assisted with data analysis, interpreting the results, and wrote the manuscript drafts. BD, MB, KC, KD, JD, CM, MR, and BW conducted relevance screening, extracted data, conducted risk of bias assessments, commented on manuscript drafts, and approved the final manuscript version. DH conducted the data analysis, provided guidance for the interpretation of the results, commented on manuscript drafts, and approved the final manuscript draft. CW assisted with the development of the review protocol, provided guidance on the conduct of the analysis and interpretation of the results, and approved the final manuscript draft. AMOC and TLOS assisted with the development of the review protocol, provided guidance on the interpretation of the results, commented on manuscript drafts, and approved the final manuscript draft. CBW assisted with the development of the review protocol, co-coordinated the research team, assisted with data screening, data extraction and risk of bias assessment, provided guidance on the interpretation of the results, commented on manuscript drafts and approved the final manuscript draft.
Financial support
Support for this project was provided by The Pew Charitable Trusts.
Conflict of interest
None of the authors have conflicts to declare.












