Pathogenic bacteria in swine feed and ingredients
Biological hazards that may be pathogenic to swine health include bacteria, such as Salmonella spp. and Escherichia coli, and viruses, such as PEDV, ASFV, SVA, Classical Swine Fever Virus (CSFV), Pseudorabies Virus (PRV), and Foot and Mouth Disease (FMD). These hazards differ in chemical and molecular structure, and therefore their prevalence may differ in feedstuffs. However, fecal contamination may lead to entry of many of these pathogens into ingredients, and the type of feedstuff and manner of contamination event may impact their survivability in feed and infectivity in swine.
Of the potential biological hazards in feed, Salmonella spp. is the most researched and understood. Feed-based transmission of Salmonella has been demonstrated to impact swine health, including a feed-based outbreak of Salmonella enterica subsp. enterica serovar Cubana in Sweden (Österberg et al., Reference Österberg, Vågsholm, Boqvist and Sternberg Lewerin2006). Furthermore, commercial feed was reported to have a high significance as a potential vehicle for Salmonella transmission in the United States (Molla et al., Reference Molla, Sterman, Mathews, Artuso-Ponte, Abley, Farmer, Rajala-Schultz, Morrow and Gebreyes2010). Researchers found 3.6% of feed samples and 17.2% of fecal samples positive for S. enterica subsp. enterica 36 barns and more than 6500 pigs. Of the Salmonella isolates, more than half were genotypically related to similar phenotypes and patterns of antimicrobial resistance. Currently, the United States Food and Drug Administration (FDA) considers S. enterica serotype Choleraesuis as an adulterant in swine feed, but adulteration by other serotypes is evaluated on a case-by-case basis (FDA, 2013). While Salmonella spp. has been reported by the FDA to be present in approximately 8% of animal feeds, neither Salmonella Cubana nor Choleraesuis are in the top 25 most prevalent serotypes found by the agency during routine surveillance (Li et al., Reference Li, Bethune, Jia, Lovell, Proescholdt, Benz, Schell, Kaplan and McChesney2012).
One of the emerging serotypes of concern for swine feed is S. enterica serovar 4,5,12:i:−, a monophasic variant of S. enterica serovar Typhimurium. This serotype was responsible for a recall of whole roaster hogs in the United States in 2016, and has been associated with resistance to many common antimicrobials (Moreno Switt et al., Reference Moreno Switt, Soyer, Warnick and Wiedmann2009; Centers for Disease Control, 2016). In 2012, Li et al. reported the serotype was the sixth most prevalent serotype found in animal feeds, and the seventh most common serotype in human infections. In a recent survey of 11 United States feed mills, S. enterica serovar 4,5,12:i:− was found in the manufacturing environment of two different mills (Magossi et al., Reference Magossi, Cernicchiaro, Dritz, Houser, Woodworth, Jones and Trinetta2019). Contaminated surfaces included the ingredient pit grating, floor dust in the ingredient receiving area, and floor dust in the control room (Magossi et al., Reference Magossi, Cernicchiaro, Dritz, Houser, Woodworth, Jones and Trinetta2019). Due to its multidrug resistance and links to both pork safety and prevalence in feed mills, S. enterica serovar 4,5,12:i:− is likely the key Salmonella serotype to control through future feed biosecurity.
The presence of other pathogenic bacteria in swine feed is less established. Tulayakul et al. (Reference Tulayakul, Boonsoongnern, Kasemsuwan, Ratanavanichrojn, Netvichian and Khaodiar2012) reported 17 of 24 nursery, finishing, and sow feed samples collected in central Thailand were positive for E. coli, but only one sample had >100 colony forming units (CFU) mL−1. Doane et al. (Reference Doane, Pangloli, Richard, Mount, Golden and Draughon2007) reported two of 24 United States swine feed samples contained E. coli O157:H7, both of which were obtained from the state of Washington. The recent survey of 11 United States feed mills described above also identified E. coli in one sample of finished swine feed (Magossi et al., Reference Magossi, Cernicchiaro, Dritz, Houser, Woodworth, Jones and Trinetta2019).
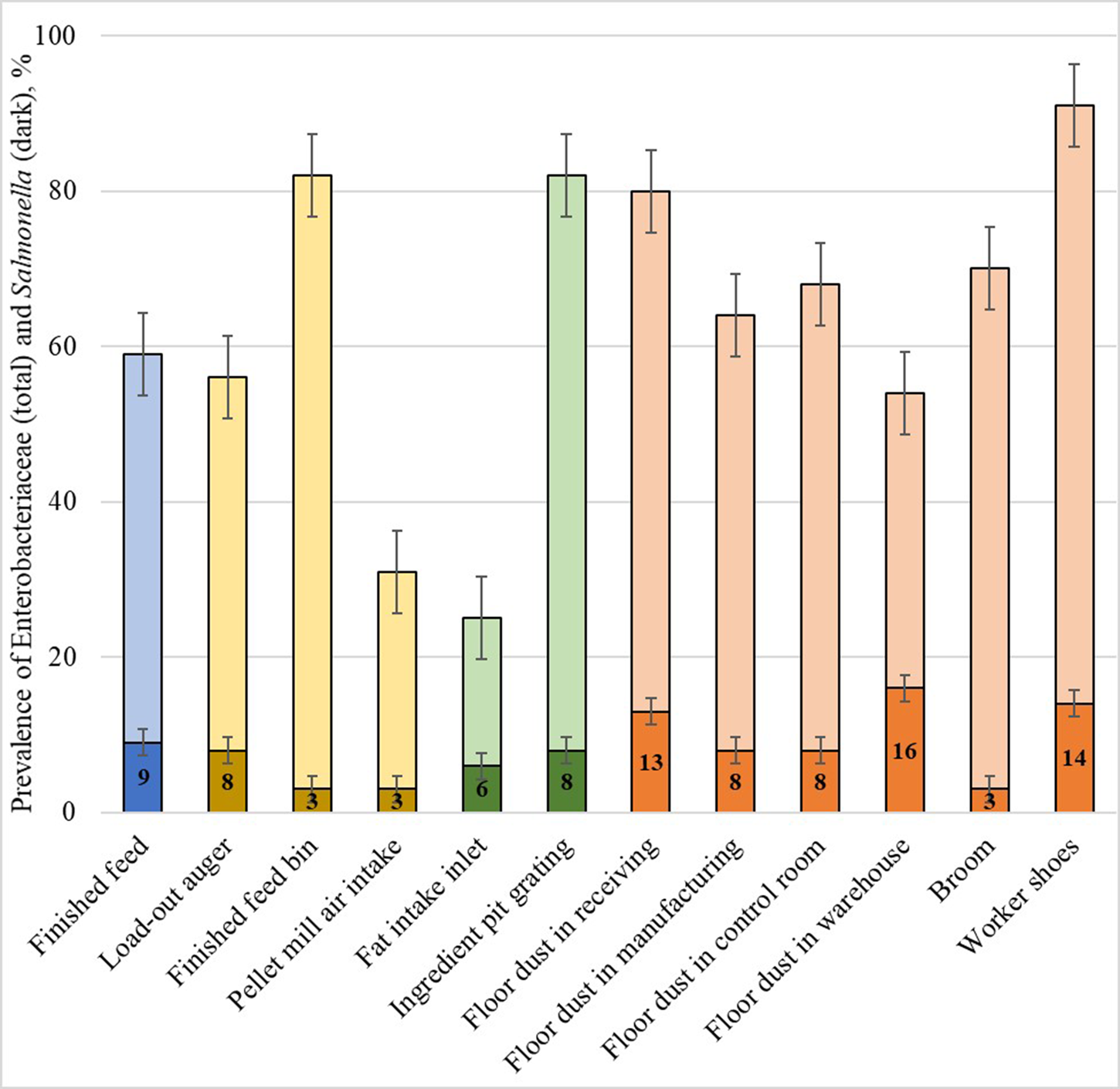
Both Salmonella and E. coli belong to a family of bacteria called Enterobacteriaceae. Active surveillance of this bacteria family may act as an indicator of biosecurity compliance and even predict future outbreaks. Enterobacteriaceae and Salmonella spp. in the 11 feed mills by Magossi et al. (Reference Magossi, Cernicchiaro, Dritz, Houser, Woodworth, Jones and Trinetta2019) are shown in Fig. 1. Most Enterobacteriaceae identified in feed or the manufacturing environment were generally non-pathogenic in nature, such as Enterobacter and Citrobacter. However, areas with high levels of Enterobacteriaceae also had high levels of Salmonella spp. (association P = 0.05). Analysis of retained samples showed that worker shoes also carried Senecavirus A in one feed mill. When another mill that was part of the surveillance was associated with an outbreak of Porcine Deltacoronavirus, the virus was found in the load-out auger, cooler air intake, ingredient pit grating, all locations of floor dust, broom, and worker shoes. Enterobacteriaceae is commonly used to indicate hygiene and/or biosecurity compliance in human food, rendering, and poultry feed manufacturing facilities (Van Schothorst and Oosterom, Reference Van Schothorst and Oosterom1984; Jones and Richardson, Reference Jones and Richardson2004; Nestle, 2014). The proactive monitoring of Enterobacteriaceae should be further evaluated and considered as a method to better identify and control the highest risk points of entry into the swine feed supply chain.

Fig. 1. Presence of Enterobacteriaceae in 11 United States feed mills. The levels of Enterobacteriaceae (total bars) vary across location, but are associated with Salmonella spp. (dark portion of bars) presence. High Enterobacteriaceae levels may indicate biosecurity compliance and even predict future outbreaks.
Pathogenic viruses in swine feed and ingredients
Research has demonstrated that viruses, such as PEDV, ASFV, SVA, CSFV, PRV, and FMD, are able to survive in at least some commonly imported feed ingredients (Dee et al., Reference Dee, Bauermann, Niederwerder, Singrey, Clement, DeLima, Long, Patterson, Shehan, Stoian, Petrovan, Jones, De Jong, Ji, Spronk, Hennings, Zimmerman, Rowland, Nelson, Sundberg, Diel and Minion2018). Modeling performed to simulate the environmental conditions during transport of ingredients from China to the United States has shown that a viable PEDV sample is able to survive in certain ingredients, including soybean meal (both conventional and organic), vitamin D, lysine hydrochloride, and choline chloride (Dee et al., Reference Dee, Neill, Clement, Nelson, Singrey, Christopher-Hennings, Jones, Cochrane, Patterson and Spronk2016). In addition to PEDV, 11 other pathogens have been subjected to a similar modeling procedure in a variety of different ingredients (Dee et al., Reference Dee, Bauermann, Niederwerder, Singrey, Clement, DeLima, Long, Patterson, Shehan, Stoian, Petrovan, Jones, De Jong, Ji, Spronk, Hennings, Zimmerman, Rowland, Nelson, Sundberg, Diel and Minion2018). The survivability of a pathogen varied depending on the genetic and physicochemical properties of the virus, and differed between pathogens and the feed ingredients tested. Certain feed ingredients or feed products presented a better matrix for virus survival than the others and select ingredient matrices seemed to enhance the survival of multiple viruses. For example, conventional soybean meal had a higher level of virus survival in comparison with organic soybean meal. The exact reason for this difference in survivability in sources of soybean meal is unknown, but could be attributed to the higher levels of fat present in the organic variety used in the trial, as there has been some evidence that medium chain fatty acid (MCFA) blends have viricidal effects (Cochrane, Reference Cochrane2018). It has also been hypothesized that higher protein ingredients have greater capability of retaining viral infectivity, but the mechanism is not yet understood. Overall, laboratory simulations have indicated that certain feed ingredients exhibit a higher risk of transporting viral pathogens (Dee et al., Reference Dee, Bauermann, Niederwerder, Singrey, Clement, DeLima, Long, Patterson, Shehan, Stoian, Petrovan, Jones, De Jong, Ji, Spronk, Hennings, Zimmerman, Rowland, Nelson, Sundberg, Diel and Minion2018). Additional research is needed to better understand what ingredient attributes are associated with enhanced survivability.
Infectivity of biological hazards in swine feed and ingredients
Once it has been established that biological hazards can survive in feed and ingredients, it is important to understand their infectivity at a dose that may cause infection. Infectivity frequently relies on ensuring the viral capsids or bacteria lipid membranes are intact as they protect the pathogen from deterioration during storage. Sufficient numbers of intact particles are needed to cause infection in otherwise healthy animals, and this is known as the minimum infectious dose. Loynachan and Harris (Reference Loynachan and Harris2005) first published the minimum infectious dose of S. enterica serovar Typhimurium in pigs as >103 CFU g−1 of feed. Cornick and Helgerson (Reference Cornick and Helgerson2004) reported the infectious dose of E. coli O157:H7 is 6 × 103 CFU g−1 in 3-month old pigs. As Österberg et al. (Reference Österberg, Vågsholm, Boqvist and Sternberg Lewerin2006) reported, infectious dose is difficult to determine, especially in bacteria, because challenge doses are strongly associated with fecal shedding, but weakly associated with infection.
Schumacher et al. (Reference Schumacher, Woodworth, Jones, Chen, Zhang, Gauger, Stark, Main, Hesse, Tokach and Dritz2016) reported the minimum infectious dose for PEDV-inoculated feed is 5.6 × 101 TCID50, equivalent to antiprimer-based quantitative real-time polymerase chain reaction (aqRT-PCR) cycle threshold (CT) of 37.1. Notably, this was above the threshold of many PEDV PCR assays in diagnostic laboratories. This research helped demonstrate why PEDV was so easily spread through a feed matrix, as 1 g of feces from an acutely infected pig could infect 500 ton of feed, with all the feed being infected at a dose capable of causing illness.
Ongoing research focuses on determining the median infectious dose of African Swine Fever Virus in both feed and water (Niederwerder, Reference Niederwerder2018). Additional research is needed to determine the minimum or median infectious dose for a number of bacteria and viruses, including Enterotoxigenic E. coli, SVA, CSF, and PRV. These doses are necessary as they become targets for mitigation measures. While ideally there is no detectable pathogen in feed or ingredients, it must at least be prevented or reduced to levels below an infectious dose to sustain animal health.
Once biological hazards that are considered a risk have been identified, procedures should be created that prevent entry of the hazard into the mill, as well as procedures for mitigation and decontamination in case hazard entry cannot be prevented. Cochrane et al. (Reference Cochrane, Huss, Aldrich, Stark and Jones2016a, Reference Cochrane, Dritz, Woodworth, Stark, Huss and Jones2016b) published an overview of a feed mill biosecurity plan that can easily serve as the foundation for developing a mill-specific biosecurity plan. Some of their recommendations are highlighted below.
Preventing biological hazards in swine feed and ingredients
The most effective component of a feed mill biosecurity plan is prevention of hazard entry. There is incentive to prevent a hazard's entry into a facility altogether because it has been shown that the introduction of a contaminated material into a feed mill can lead to the mill being contaminated for an extended period (EFSA, 2008). Controlling the entry of biological hazards into a facility should begin with evaluation of the ingredient suppliers. The development of a supplier verification program that includes specific requirements for ingredients being purchased, as well as communicating safety expectations to the supplier of an inbound ingredient is an important step in preventing the entry of a biological hazard. This may also include verification of ingredient-supplier protocols and on-site manufacturing facility reviews and assessments. As mentioned in the previous section, some ingredients have the potential to maintain bacteria or virus survivability and infectivity more than others. As a result, the best way to prevent hazard entry into the mill is to eliminate high risk ingredients from diet formulations. Thus, coordinated efforts between nutritionists, formulators, purchasers, and the rest of the integrated feed supply team is essential for maintaining an effective feed mill biosecurity plan.
While having a supplier control program is an important step when controlling the entry of a biological hazard into a facility, routine sampling and analysis of bagged, bulk, or liquid ingredients that are considered high-risk for certain pathogens is a valuable tool. All samples’ collection should be performed by using an aseptic method, as cross-contamination of samples during the collection process needs to be prevented. If an ingredient is considered high risk, every lot should be analyzed separately. If it is lower risk, it may be more practical to collect samples and pool them for more intermittent analysis as a way to reduce analytical cost. Determining and setting a schedule for sampling of ingredients that are considered higher risk, as well as defining an inventory holding procedure until analytical results are obtained can help lower the potential of a biological hazard being introduced into the mill. Traceability of ingredients is essential, and maintaining records that indicate information such as the receiving date, time, lot number during unloading, and prior haul data that is connected to specific batches of finished feed allows for a quick response if a biological hazard is suspected.
Movement of people or vehicles in or out of a facility also has the potential to introduce biological hazards. Employees in the feed mill and visitors, such as guests, truck drivers, and subcontractors have the ability to introduce contaminants into a feed manufacturing facility. People may unknowingly carry fecal, dirt, or dust particles contaminated with undesirable microorganisms on the bottoms of their shoes or on clothing and are at a particularly higher risk if they are coming from another farm or feed mill where the hazard is present. The risk of people introducing biological hazards is easily illustrated in Fig. 1 (Magossi et al., Reference Magossi, Cernicchiaro, Dritz, Houser, Woodworth, Jones and Trinetta2019), as 91% of samples collected from worker boots were contaminated with Enterobacteriaceae. Controlling and minimizing foot traffic across receiving pit grates or around hand-add port grates is a logical, low-cost method to reduce the risk of a biological pathogen being introduced into the manufacturing system, and can easily be accomplished by covering the grates when not in use. No-walk zones or even hygienic zoning may be appropriate to include in biosecurity plans in feed mills that have a higher risk of biological pathogen introduction. Procedures requiring that all visitors must be accompanied at all times by a trained employee can to help prevent biosecurity breaches. Visitors should be provided clean footwear, plastic boots, or boot cover-ups to limit the entry of outside hazards. This includes the drivers of inbound trucks. Ideally, drivers should stay inside their trucks at all times to minimize foot traffic, especially over the receiving grates. If the driver must exit the vehicle, wearing disposable plastic boots or covers will limit the potential of hazards being introduced from their shoes. Trucks entering the feed mill should have mud and sludge removed from the trailer opening before the vehicle reaches the receiving pit, and the pit should remain covered until the truck is ready to unload. Ingredients may be contaminated prior to unloading, but they may also be contaminated during the unloading process due to mud or floor sweepings intermingling with ingredients at the point of entry. Ensuring the receiving pit remains covered while trucks are being moved reduces the risk of contamination during unloading, which is important considering the impracticality of thoroughly cleaning conveying equipment such as the central pit or, bucket elevators. Use of cones and funneling devices can also be used to limit the quantity of material that spills during unloading and prevents mill employees from sweeping spilled ingredients into the pit.
Floor sweepings, including those from the unloading process, should be disposed of and not swept into the pit. In addition, many feed manufacturing facilities have grain cleaners and dust collection equipment in place, and it has been well established that dust and other screened particles can act as a carrier for biological hazards including PEDV (Gebhardt et al., Reference Gebhardt, Woodworth, Jones, Gauger, Tokach, DeRouchey, Goodband, Muckey, Cochrane, Niederwerder, Stark, Bai, Chen, Zhang, Ramirez, Derscheid, Main and Dritz2016) or mycotoxins (Yoder et al., Reference Yoder, Stark, DeRouchey, Tokach and Jones2018), among others. Many feed manufacturers have the mentality that adding back the dust or screened material to the finished feed is acceptable because it will reduce ingredient shrink. However, the cost associated with reduced animal performance and/or increased mortality is much greater than the loss of mill efficiency, and therefore all dust and screened materials should always be disposed of compared to being added back into the feed.
Reducing biological hazards in swine feed and ingredients
Once a biological hazard is introduced into a facility, it can be almost impossible to control because most feed manufacturing facilities were not hygienically designed. Furthermore, mitigation strategies that may be possible in some systems may not work in others because of differences in facility design and equipment, manufacturing operations, and other associated risk factors among feed mills. For instance, Muckey (Reference Muckey2016) reported that the surface type (concrete, plastic, rubber, stainless steel, etc.) impacts pathogen survivability in the presence of different decontamination procedures. Stainless steel and smooth plastic surfaces, while easier to clean than tires, rubber belts, or polyethylene totes, are more difficult to sanitize due to the formation of biofilms that protect the bacteria or virus from a chemical sanitizer. Therefore, both cleaning and sanitizing is often necessary, and nearly impossible based on current equipment design constraints.
Physical prevention of hazard spread via cross-contamination is especially difficult due to the highly infective nature of contaminated dust and the impracticality of physical clean-out in most mills (Fig. 2). In Schumacher et al. (Reference Schumacher, Cochrane, Huss, Stark, Woodworth, Bai, Poulsen, Chen, Main, Zhang, Gauger, Derscheid, Magstadt, Dritz and Jones2017), the role of PEDV cross-contamination in feed mills was evaluated. Initially, a PEDV-negative corn- and soybean meal-based nursery pig diet was mixed, conveyed, and discharged using pilot scale feed manufacturing equipment. Next, a diet was manufactured, including an ingredient that had been spiked with infectious PEDV. Subsequently, four separate PEDV-negative diets were mixed, conveyed, discharged to test how many negative diets were necessary to ‘flush’ contamination from the manufacturing surface. Environmental swabs were collected prior to and after each batch of feed by swabbing direct feed contact surfaces, adjacent surfaces located within 1 m of manufacturing equipment, and other surfaces located at least 1 m away from manufacturing equipment. The presence of PEDV RNA was reported in CT of qRT-PCR using PROC GLIMMIX (SAS Institute, Inc., Cary, NC). The statistical model evaluated the effect of manufacturing sequence (negative, positive, flush 1, flush 2, flush 3, and flush 4) and location (direct feed contact, adjacent, or other surface) and the associated interaction. The LSMEANS procedure compared surface type among treatments within animal food-contact surfaces by pairwise comparison. Both main effects and the interaction were significant (P < 0.05). Subsequently, Gebhardt et al. (Reference Gebhardt, Woodworth, Tokach, DeRouchey, Goodband, Jones and Dritz2018) demonstrated that dust collected from feed manufacturing surfaces can cause infectivity in a swine bioassay. Therefore, limiting and controlling dust created during manufacturing should be a priority, as it can serve as a vector in viral disease transmission such as PEDV. Sequencing procedures in order to minimize risk to the most sensitive phases of production should be utilized. Furthermore, flushing protocols should be established to help minimize cross contamination risk. Gebhardt et al. (Reference Gebhardt, Woodworth, Jones, Gauger, Tokach, DeRouchey, Goodband, Muckey, Cochrane, Niederwerder, Stark, Bai, Chen, Zhang, Ramirez, Derscheid, Main and Dritz2016) showed in a PEDV model that rice hull flushes can be a cost-effective strategy to reduce cross-contamination risk.

Fig. 2. Schumacher et al. (Reference Schumacher, Cochrane, Huss, Stark, Woodworth, Bai, Poulsen, Chen, Main, Zhang, Gauger, Derscheid, Magstadt, Dritz and Jones2017) reported the role of PEDV cross-contamination in feed mills. Initially, a PEDV-negative swine diet was manufactured, followed by a diet that included an ingredient that had been spiked with infectious PEDV, then four subsequent PEDV-negative diets in an attempt to ‘flush’ contamination from the manufacturing surfaces. Environmental swabs were collected prior to and after each batch of feed by swabbing direct feed contact surfaces, adjacent surfaces located within 1 m of manufacturing equipment, and other surfaces located at least 1 m away from manufacturing equipment. Both main effects and the interaction were significant (P < 0.05). Subsequently, Gebhardt et al. (Reference Gebhardt, Woodworth, Tokach, DeRouchey, Goodband, Jones and Dritz2018) demonstrated that dust collected from feed manufacturing surfaces can cause infectivity in a swine bioassay. Therefore, limiting and controlling dust created during manufacturing should be a priority, as it can serve as a vector in viral disease transmission such as PEDV. Sequencing procedures in order to minimize risk to the most sensitive phases of production should be utilized.
For RNA viruses in particular mitigation techniques depend on disrupting the viral capsid which removes the protective shell around the virus (Cliver, Reference Cliver2009). Three main categories of mitigation strategies have been identified and include biological, physical, and chemical. Deng and Cliver (Reference Deng and Cliver1995) reported that biological inactivation typically occurs with the use of specific enzymes or other products of microbial origin that attack viruses or bacteria, but research is lacking to determine if this is a feasible mitigation strategy for the feed manufacturing industry. Physical inactivation in feed manufacturing is most commonly achieved thermally, but should be considered a point-in-time mitigation strategy, because it would not prevent post-processing contamination risk. The use of chemical agents, such as formaldehyde or medium-chain fatty acids as feed additives has been shown to have excellent potential to inhibit virus and bacterial hazards in feed. The benefit of these chemical agents is that they have the potential to have immediate as well as residual efficacy which could help with mitigation from the point of application until the time the feed is consumed. Specific research identifying mitigation strategies that can be used in the feed manufacturing process are reviewed below.
Thermal Processing: In a benchtop model, Goyal (Reference Goyal2013) confirmed that PEDV is a heat-sensitive virus and that a temperature × time relationship could be used as a guide for PEDV inactivation. Based on this information, two studies were conducted to determine if passing feed through a pellet mill would be sufficient to apply thermal insult to a great enough extent to prevent PEDV infectivity. Cochrane et al. (Reference Cochrane, Schumacher, Dritz, Woodworth, Huss, Stark, DeRouchey, Tokach, Goodband, Bai, Chen, Zhang, Gauger, Derscheid, Magstadt, Main and Jones2017) showed in the first trial that when a low or high dose of PEDV was used to inoculate feed, with the resulting feed subsequently processed at one of nine combinations of conditioning temperature (68, 79, or 90 °C) or conditioner retention time (45, 90, or 180 s) all processed batches of feed were unable to generate infectivity in a pig bioassay model, even though the unprocessed feed did lead to PEDV infectivity. In a subsequent trial, the same researchers processed feed through a conditioner utilizing a 30 s retention time and one of five condition temperatures (38, 46, 54, 63, or 71 °C) and observed that feed processed at or above 54 °C was able to prevent PEDV infectivity, while feed that was processed at the two low temperatures did lead to PEDV infection when fed to pigs. This series of trials demonstrated that thermal mitigation is a possible means of minimizing PEDV-associated risk, and more importantly demonstrated that equipment commonly found in commercial feed mills was effective at applying the thermal stress. However, it is important to remember that even though the feed mill may target a specific processing temperature adequate to inactivate PEDV, there are times during the feed manufacturing process (such as at equipment startup, or if steam flow is turned off to ameliorate a plugged die) that the feed may not be processed at a high enough temperature to effectively eliminate all virus transmission risk. Furthermore, the research demonstrates that the pellet mill is an effective point-in-time mitigation strategy, but it cannot prevent post-processing recontamination risk.
Residual control measures
The use of chemical feed additives as strategies to reduce biological hazards in feed is appealing because they allow for efficacy throughout the remainder of the feed supply chain, with the potential to also influence animal performance once consumed. As a result, a number of different products have been tested as chemical-based feed hazard mitigants. Some compounds that have shown mixed efficacy at reducing or eliminating virus or bacterial risk include organic acids (Eklund Reference Eklund1985), essential oils (Orhan et al., Reference Orhan, Özçelik, Kartal and Kan2012), sodium bisulfate (Knueven, Reference Knueven1998), or sodium chlorate (Smith et al., Reference Smith, Oliver, Taylor and Anderson2012); however, the cumulative data suggest that the effectiveness of any chemical-based feed mitigant is not only target specific but also feed ingredient/matrix specific (Cochrane, Reference Cochrane2018). Of all the potential chemical mitigants available, the two that have garnered the most commercial interest are formaldehyde and MCFAs.
Formaldehyde has been shown to be effective at preventing risk associated with PEDV (Dee et al., Reference Dee, Neil, Clement, Christopher-Hennings and Nelson2014, Reference Dee, Neill, Clement, Singrey, Christopher-Hennings and Nelson2015; Cochrane, Reference Cochrane2018) as well as Salmonella (Cochrane et al., Reference Cochrane, Huss, Aldrich, Stark and Jones2016a, Reference Cochrane, Dritz, Woodworth, Stark, Huss and Jones2016b). However, regulatory restrictions can limit some applications as the product is only approved for use to prevent contamination with Salmonella. Additionally, specialized equipment must be used for accurate application, and there are worker health concerns as well as negative perception by some consumers, which can lead to formaldehyde being limited in its commercial application. Furthermore, the use of formaldehyde in feed may lead to detrimental bacterial shifts in the pig gut (Williams et al., Reference Williams, Cochrane, Woodworth, DeRouchey, Dritz, Tokach, Jones, Fernando, Burkey, Li, Goodband and Amachawadi2018).
The use of MCFAs as chemical-based feed mitigants was reviewed by Cochrane et al. (Reference Cochrane, Amachawadi, Remfry, Lerner, Gebhardt, Nagaraha, Pluske, Niederwerder, Woodworth, Dritz and Jones2018). They observed that MCFA are effective at preventing risk associated with feed contaminated with PEDV in addition to their effectiveness against Salmonella (Cochrane et al., Reference Cochrane, Huss, Aldrich, Stark and Jones2016a, Reference Cochrane, Dritz, Woodworth, Stark, Huss and Jones2016b). Through a series of trials this group of researchers has shown that combinations of caproic, caprylic, and capric acid are the most effective with little efficacy of lauric acid against PEDV. Interestingly, the same group of researchers also showed that increasing concentrations of a 1:1:1 blend of caproic, caprylic, and capric acid also resulted in a linear increase in growth performance with a 1.50% inclusion resulting in an almost 2 kg BW advantage compared to a diet with no MCFA after feeding nursery pigs for 35 days (Thomson et al., Reference Thomson, Gebhardt, Lerner, Woodworth, Tokach, DeRouchey, Goodband and Dritz2018). Furthermore, Gebhardt et al. (Reference Gebhardt, Woodworth, Tokach, DeRouchey, Goodband, Jones and Dritz2018) showed that feed used in this trial that was collected 40 days after MCFA application was still successful at reducing PEDV risk which demonstrates the residual mitigation potential of MCFA.
Addressing feed mills contaminated with biological hazards
Due to the high quantity of airborne particulates in animal food manufacturing facilities, dust contamination is a widespread mechanism for both viral and bacterial hazard transmission (Fig. 2). This can be specifically challenging because of the difficulties associated with physical cleaning (Muckey, Reference Muckey2016). Highly aggressive procedures, such as the use of liquid chemical sanitizers and heat have been shown to be necessary when reducing bacteria on environmental surfaces to completely decontaminate manufacturing surfaces (Fig. 2; Huss et al., Reference Huss, Schumacher, Cochrane, Poulsen, Bai, Woodworth, Dritz, Stark and Jones2017; Schumacher et al., Reference Schumacher, Cochrane, Huss, Stark, Woodworth, Bai, Poulsen, Chen, Main, Zhang, Gauger, Derscheid, Magstadt, Dritz and Jones2017). Effective cleaning, which may require both physical cleaning and the use of cleaning solutions, removes biofilm formations that will allow for subsequent penetration and removal of vegetative bacteria by a sanitizer. Both steps are necessary, but can prove to be difficult in many feed manufacturing systems due to a lack of access or ability to thoroughly clean out or safely sanitize dry bulk manufacturing systems. Cleaning of non-animal food-contact surfaces should not be overlooked as biological hazards can efficiently spread throughout a facility through dust and other airborne particulates. This contamination is not mitigated during flushing procedures, and can contaminate subsequent feed batches (Schumacher et al., Reference Schumacher, Cochrane, Huss, Stark, Woodworth, Bai, Poulsen, Chen, Main, Zhang, Gauger, Derscheid, Magstadt, Dritz and Jones2017).
Because complete physical clean-out of feed manufacturing systems can prove to be difficult, flushing procedures including the use of added substances such as formaldehyde, MCFA, and dry essential oil blends may be used to help reduce the presence of biological hazards on feed-contact surfaces. Data suggest that biological hazard risk can be reduced after a third flush, or after the use of a chemically enhanced flush (Gebhardt et al., Reference Gebhardt, Woodworth, Jones, Gauger, Tokach, DeRouchey, Goodband, Muckey, Cochrane, Niederwerder, Stark, Bai, Chen, Zhang, Ramirez, Derscheid, Main and Dritz2016; Muckey, Reference Muckey2016; Schumacher et al., Reference Schumacher, Cochrane, Huss, Stark, Woodworth, Bai, Poulsen, Chen, Main, Zhang, Gauger, Derscheid, Magstadt, Dritz and Jones2017). Formaldehyde-based products and an MCFA blend have been shown to reduce the presence of PEDV on these surfaces when used in conjunction with a rice hull flush. Similarly, MCFA blends have been found to be effective at reducing S. enterica serovar Typhimurium on stainless steel surfaces, in addition to reducing the quantity of post-processing S. enterica serovar Typhimurium contamination if 2% is applied to swine feed prior to its inoculation with bacteria (Cochrane, Reference Cochrane, Huss, Aldrich, Stark and Jones2016a).
Future directions for swine feed safety
Clearly, additional research is necessary to better understand both the risk and prevention of biological hazards in swine feed and ingredients. Our knowledge of survivability, infectivity, mitigation, and decontamination strategies all must be improved to maintain the safety of swine feed in the future. Additional research is warranted to evaluate the role of beneficial bacteria to competitively exclude pathogens in feed manufacturing environments, such as those described by Zhao et al. (Reference Zhao, Podtburg, Zhao, Chen, Baker, Cords and Doyle2013) for controlling Listeria monocytogenes and Listeria in poultry processing plants. The concept of competitive bacteria for inhibitory exclusion is being tested for controlling Salmonella and other pathogenic bacteria in meat processing and rendering facilities, and may be viable to consider for feedstuff production facilities. Furthermore, it will be important to understand hygienic design for retrofits and new construction of feed mills in the future.
One of the items that limits the ability to make faster progress on feed safety is that few molecular diagnostics methods have been appropriately validated for feedstuffs. Our team has consistently witnessed lower recovery rates of viral nucleic acids when moving from inoculant (virus stock) into dry feed or ingredients. We reported this challenge in Schumacher et al. (Reference Schumacher, Woodworth, Jones, Chen, Zhang, Gauger, Stark, Main, Hesse, Tokach and Dritz2016), where we established the infectious dose of PEDV via feed. Increasing levels of virus were associated with lower qRT-PCR CT in the inoculum. However, the CT value of the same virus dose in feed was, on average, 9.5 CT higher than that in the inoculum after correcting for the dilution. The loss in viral RNA and diagnostic sensitivity did not appear to be dose-related, since a similar CT was detected despite different amounts of PEDV were spiked into the feed. This was later confirmed by Cochrane et al. (Reference Cochrane, Schumacher, Dritz, Woodworth, Huss, Stark, DeRouchey, Tokach, Goodband, Bai, Chen, Zhang, Gauger, Derscheid, Magstadt, Main and Jones2017), where we reported a consistent loss in PEDV detectability by RT-PCR when feed was inoculated with a low (103 TCID50 g−1) or high (105 TCID50 g−1) dose of PEDV. Tissue culture medium inoculum was 20 or 13 CT, while the detectable PEDV in feed was 30.7 or 23.9 CT, leading to a 10.7 or 10.9 CT loss in sensitivity for both the low and high PEDV doses, respectively. This loss in viral RNA and diagnostic sensitivity is not isolated to our laboratories. A similar loss in sensitivity was found by Iowa State University, when our samples were tested in their diagnostic laboratory. In our collaboration with Pipestone Systems and SDSU, we (Dee et al., Reference Dee, Neill, Clement, Nelson, Singrey, Christopher-Hennings, Jones, Cochrane, Patterson and Spronk2016) reported an up to 11.4 CT loss when moving from stock virus to feed or ingredients using diagnostic assays developed at the SDSU Veterinary Diagnostic Laboratory. The challenge in loss of sensitivity is not confined to PEDV. We recently observed a similar CT sensitivity loss between liquid inoculum and dry feed with Senecavirus A (SVA) (Sardella et al., Reference Sardella, Petrovan, Davis, Stewart, Niederwerder, Woodworth, Dritz, Rowland and Jones2019). Furthermore, Dee et al. (Reference Dee, Bauermann, Niederwerder, Singrey, Clement, DeLima, Long, Patterson, Shehan, Stoian, Petrovan, Jones, De Jong, Ji, Spronk, Hennings, Zimmerman, Rowland, Nelson, Sundberg, Diel and Minion2018) reported a loss in sensitivity between stock virus and various ingredients or complete feed for SVA, BVDV (surrogate for CSFV), PRRSV, and ASFV. Finally, the loss in diagnostic sensitivity appears to vary from one feed or ingredient matrix to another. Cochrane et al. (Reference Cochrane, Huss, Aldrich, Stark and Jones2016a, Reference Cochrane, Dritz, Woodworth, Stark, Huss and Jones2016b) demonstrated that complete swine diet, blood meal, meat and bone meal, and spray-dried porcine plasma inoculated with the same quantity of PEDV inoculum resulted in CTs ranging from 26 to 31 using identical sample preparation, extraction and RT-PCR conditions. Furthermore, Dee et al. (Reference Dee, Neill, Clement, Nelson, Singrey, Christopher-Hennings, Jones, Cochrane, Patterson and Spronk2016) reported a loss of sensitivity ranging from 1.4 to 11.4 CT for PEDV, depending upon the ingredient matrix.
We initially accepted this loss in sensitivity was inherently part of the assay, and that it posed a problem for veterinary diagnostic laboratories, but had limited biological relevance. However, we have recognized that the poor recovery of nucleic acids using the current methods in ingredients has substantial ramifications: it leads to false negative results via a type-II error. Natural fecal contamination in ingredients or from cross-contamination due to poor biosecurity is likely to have low levels of virus, and a 10-CT reduction in sensitivity may lead to the determination that a contaminated product is actually safe. For example, we reported in Schumacher et al. (Reference Schumacher, Cochrane, Huss, Gebhardt, Woodworth, Stark, Jones, Bai, Main, Chen, Zhang, Gauger, DeRouchey, Goodband, Tokach and Dritz2018) that a pig gavaged with a PEDV-spiked feed sample that was qRT-PCR-negative (>40 CT) became infected and symptomatic, i.e. presence of infectious PEDV was confirmed in the bioassay.
Presently, the current methods for sample preparation, extraction, and detection of nucleic acids in feedstuffs are too variable and pose too high of a risk for a false negative, which negates the value of the test. Urgent research is needed to validate molecular detection methods in feedstuffs, which can then be used to create an appropriate sampling method and point-of-use diagnostic devices. Until then, environmental monitoring and product testing is not a viable option for ensuring feed safety; instead efforts must be more preventative than reactive. The swine feed industry must embrace feed biosecurity as regulators and consumers shift their thinking of our product as swine feed to swine food.
Recommendations to maximize swine feed biosecurity
In conclusion, biosecurity is a well-known topic at the farm level, but only recently has begun to gain importance in the feed manufacturing process. Evidence demonstrating the ability of feed and feed ingredients to support virus infectivity and bacterial survivability has been collected which points to the fact that feed and ingredients can be a vector for biological hazard transmission. Consequently, a series of steps should be taken to help maximize feed biosecurity:
(1) Assess biological hazard risk: feed manufacturing facilities must take a proactive approach to understanding biological hazards for their own operations and the security of their customers. The biosecurity procedures employed by a specific mill may not be the same as other mills depending on the customers they serve and the associated risk tolerance versus price for mitigation strategies that are employed.
(2) Define protocols to prevent entry of hazard into the mill: the most important part of a feed mill biosecurity plan is to prevent hazards from entering the mill. Identifying and eliminating high risk ingredients, minimizing entry via people and equipment, covering all open points of entry when not being used, and other strategies can be used to prevent hazard entry into the mill.
(3) Utilize mitigation strategies to prevent risk: not all hazards can be prevented from entering the mill and consequently mitigation strategies should be utilized. The best option is to identify the mitigation strategies that are effective against the specific hazards of concern and utilize a combination of point-in-time mitigants as well as those that have residual effectiveness for continue protection through the remainder of the feed supply chain. Some mitigation strategies have multiple benefits. As an example, dust collection and elimination not only create a safer and better environment for the workers, but also can eliminate a major point of contamination.
(4) Feed mill decontamination: while it is extremely difficult to completely accomplish, a feed mill decontamination strategy must be developed and should include a combination of physical cleaning, chemical cleaning, and if applicable the use of high heat as the final step.