No CrossRef data available.
Published online by Cambridge University Press: 06 March 2019
To replace time-consuming chemical analysis, a procedure was developed for applying X-ray fluorescence to the analysis of a manganese ore. This X-ray fluorescence method is rapid; the total time for both sample preparation and analysis is ½ hr. The method is also simple enough for routine laboratory use. The components determined, the concentration ranges, and the agreement between chemical and X-ray analysis in terms of standard deviation are:
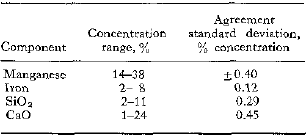
The agreements between chemical and X-ray analysis noted for manganese and iron are obtained by correcting for CaO concentration when determining the manganese and for manganese and SiO2 concentrations when determining the iron. The corrections employ empirical equations developed by a multiple regression technique.
Sample preparation is reduced to a minimum because it consists of only two steps, pulverization of the manganese ore followed by briquet ring. Four minutes of grinding with Boraxo as the grinding aid gives sufficient uniformity to minimize the effect of particle size variation. The ground mix of manganese ore and Boraxo is pressed into 1.25-in. briquettes for analysis.