Article contents
Re: Crystal Structures of Pyroaurite and Sjögrenite
Published online by Cambridge University Press: 06 March 2019
Abstract
Pyroaurite and sjogrenite belong to the group of sandwiched lamellar metal hydroxides which have a fixed metallic ions MII:MIII ratio for a particular class. Their crystal structure consists of positively charged metal hydroxide blocks intercalated with negatively charged interlayers. The atomic positions for the interlayer are definite for a particular class. The exact chemical formula of the pyroaurite class is determined from crystal structure analysis to be MII6MIII2(OH)16-CO3-4.5H2O; it crystallizes in the space group R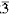 m with a = 12.4376 Å and c = 23.4126 Å. Sjogrenite, MII6 MIII2(OH)16-CO3-4H2O crystallizes in the space group P63/mcm. The crystallogiraphy and structural relationship between these classes are discussed. Previous discussion on these compounds did not give any conclusion on the exact chemical formula and the atomic positions.
m with a = 12.4376 Å and c = 23.4126 Å. Sjogrenite, MII6 MIII2(OH)16-CO3-4H2O crystallizes in the space group P63/mcm. The crystallogiraphy and structural relationship between these classes are discussed. Previous discussion on these compounds did not give any conclusion on the exact chemical formula and the atomic positions.
- Type
- X. Structural and Other Applications of Powder Diffraction
- Information
- Advances in X-Ray Analysis , Volume 38: Forty-third Annual Conference on Applications of X-ray Analysis , 1994 , pp. 749 - 755
- Copyright
- Copyright © International Centre for Diffraction Data 1994
References
- 2
- Cited by


