The key feature of psychosis lies in the misinterpretation of the nature of reality, which is reflected in impaired perceptions and interpretation of the environment, false beliefs, and disorganised patterns of speech and behaviour. In clinical practice the word ‘psychosis’ is commonly used to describe a severe mental illness in which delusions and hallucinations are prominent.
Among elderly patients psychotic symptoms can be seen in a wide range of conditions. The causes and clinical manifestations of the symptoms usually vary with the underlying condition. Psychotic symptoms of acute onset are usually seen in delirium secondary to a medical condition, drug misuse and drug-induced psychosis. Chronic and persistent psychotic symptoms may be due to a primary psychotic disorder (chronic schizophrenia, late-onset schizophrenia, delusional disorders, affective disorders), psychosis owing to neurodegenerative disorders (Alzheimer's disease, vascular dementia, dementia with Lewy bodies and Parkinson's disease) or chronic medical conditions.
Psychotic symptoms are not uncommon in the elderly population and prevalence figures in community samples range from 0.2 to 4.7% (Reference Targum and AbbottTargum & Abbott, 1999). In nursing homes prevalence rates from 10% to as high as 63% have been reported (Reference Zayas and GrossbergZayas & Grossberg, 1998). In a 3-year follow-up study of psychotic symptoms in a population-based sample of very old people (above 85 years of age) without dementia, Reference Östling and SkoogÖstling & Skoog (2002) reported a prevalence of 7.1–13.7%. They also reported that hallucinations and paranoid ideation were associated with increased incidence of dementia and mortality within 3 years.
Psychotic symptoms can be associated with aggressive or disruptive behaviour (Reference Gilley, Wilson and BeckettGilley et al, 1997) and are often a source of distress to caregivers (Reference Zarit, Todd and ZaritZarit et al, 1986; Reference Schneider, Olin and DoodySchneider et al, 1997). They can result in neglect and abuse of elderly patients (Reference Steele, Rovner and ChaseSteele et al, 1990) and persistent symptoms often result in institutionalisation, which imposes a heavy financial burden (Reference Stern, Tang and AlbertStern et al, 1997).
A number of factors have been hypothesised to contribute to an increased risk of psychosis in elderly people (Box 1), and the combination of these make its management complicated in older patients.
Box 1 Increased risk of psychosis in elderly people: contributing factors
-
• Age-related deterioration of frontal and temporal cortices
-
• Neurochemical changes associated with aging
-
• Social isolation
-
• Sensory deficits
-
• Cognitive decline
-
• Age-related pharmacokinetic and pharmaco-dynamic changes
-
• Polypharmacy
(Reference Targum and AbbottTargum & Abbott, 1999; Targum & Steven, 2001)
Use of antipsychotics in elderly people
Elderly people show variable responses and increased sensitivity to medications in general (Reference Avron, Gurwitz, Cassel, Reisenberg and SorensonAvron & Gurwitz, 1990) and to antipsychotics in particular. Age-related bodily changes affect the pharmacokinetics and pharmacodynamics of anti-psychotic drugs, which have numerous side-effects (Box 2) that can be more persistent and disabling in older people. Tardive dyskinesia, for example, can lead to a number of physical and psychological complications, including difficulty in eating and swallowing, weight loss, falls, difficulty in keeping balance and depression (Reference JesteJeste, 2004). The risk of developing tardive dyskinesia from typical (older) antipsychotics is 5–6 times higher in older people (Reference KaneKane, 1999), although recent studies indicate that the newer atypicals may pose a lower risk of this side-effect and may therefore be safer for older people (Reference JesteJeste, 2004).
Box 2 Potential side-effects of antipsychotics in elderly people
Extrapyramidal side-effects
-
• Pseudoparkinsonism
-
• Akathesia
-
• Acute dystonia
Tardive dyskinesia Anticholinergic effects
-
• Urinary hesitancy
-
• Constipation
-
• Blurred vision
-
• Dryness of mouth
-
• Delirium
Postural hypotention
Sedation
Hypersalivation
Gastrointestinal effects
-
• Nausea
-
• Constipation
-
• Diarrhoea
Liver effects
-
• Cholestatic jaundice
-
• Raised transaminase enzyme activities
Cardiovascular effects
-
• ECG abnormalities: QTc prolongation
Endocrine effects
-
• Weight gain
-
• Diabetes mellitus
Epilepsy
Antipsychotics can also increase the rate of cognitive decline (Reference Holmes, Fortenza and PowellHolmes et al, 1997; Reference McShane, Keene and GedlingMcShane et al, 1997); they have been associated with neuroleptic sensitivity syndrome, a potentially lethal adverse effect (Reference Byrne, Burns and WaiteByrne et al, 1992; Reference McKeith, Fairbairn and PerryMcKeith et al, 1992); and some are now subject to restrictions under the Committee on Safety of Medicines (CSM). Having reviewed the literature on the use of risperidone and olanzapine for the treatment of behavioural and psychological symptoms in dementia, the chairman of the CSM concluded that each was associated with at least a two-fold increase in the risk of stroke and therefore should no longer be used in dementia (Reference DuffDuff, 2004). Reference Herrmann, Mamdani and LanctotHerrmann et al (2004), however, found no difference in the risk of stroke between risperidone and olanzapine compared with typical neuroleptics used in the treatment of dementia (n=11400). Others have subsequently commented on the potential detrimental effects of such a blanket ban (Reference Mowat, Fowlie and MacEwanMowat et al, 2004).
Following the CSM restriction on risperidone and olanzapine, a Working Group for the Royal College of Psychiatrists’ Faculty of the Psychiatry of Old Age, the Royal College of General Practitioners, the British Geriatrics Society and the Alzheimer's Society also acknowledged a small but significant risk of cerebrovascular adverse events in elderly people, especially in people over 80 years of age, with the use of risperidone and olanzapine (Royal College of Psychiatrists et al, 2004). The Working Group advocated a more balanced approach to their prescription that involves weighing the risks and benefits for individual patients, since these drugs may still be worth using in some circumstances, particularly when alternative drug treatments have similar or worse side-effects and non-pharmacological approaches are not suitable. For other antipsychotics, both typical and atypical, the choice of prescription should be based on the side-effect profile and risk factors such as cerebrovascular events, postural hypotension and tardive dyskinesia. For people who have been stable on antipsychotics for more than 3 months, cautious withdrawal may be considered. The decision to withdraw or continue should be based on past history and the risks of recurrence. The reasons for using or continuing a particular antipsychotic must be clearly documented and the general practitioner should be involved in the decision-making process.
The discussions about the use and safety of antipsychotics will probably go on for some time, but they have highlighted the need for research on alternative forms of treatment. For the present, it is important to be careful not to do more harm than good when initiating antipsychotic medication for older people and to follow the principle ‘start low and go slow’ (Reference Zayas, Grossberg, Copeland, Abou-Saleh and BlazerZayas & Grossberg, 2002).
Schizophrenia
Older people with schizophrenia have traditionally been divided into two main groups, those who develop the illness in later life and those who have had it from an early age and have now grown old. Historically, it was Kraepelin in the early 20th century who recognised that the non-affective psychosis in young adults that he called ‘dementia praecox’ could also first become apparent in middle or old age. Bleuler subsequently coined the term ‘late-onset schizophrenia’ to describe this schizophrenia-like illness that arises in old age in the absence of organic brain disease or amnestic syndrome.
There has since been much debate about the nosology and classification of psychotic disorders in old age. Some emphasise the similarities between the early- and late-onset illnesses and others highlight the differences in aetiology, phenomenology and outcome. A consensus on nomenclature was reached in 1998, at a meeting of the International Late Onset Schizophrenia Group (Reference Howard, Rabins and SeemanHoward et al, 2000). On the basis of the research evidence on symptoms, family history, brain imaging studies and the nature of the cognitive deficits observed, it was agreed to retain the word schizophrenia for both the early- and late-onset illnesses. However, the late-onset illness was further subdivided into late onset (onset after 40 years of age) and very late onset (onset after 60 years of age). Some comparative features of early-and late-onset schizophrenia are shown in Box 3, and characteristics of the very-late-onset illness are listed in Box 4.
Box 3 Similarities and differences between early- and late-onset schizophrenia
Similarities
-
• Genetic risk
-
• The presence and severity of positive symptoms
-
• Early psychosocial maladjustments
-
• Subtle brain abnormalities revealed by imaging
Differences
Late-onset schizophrenia is characterised by:
-
• fewer negative symptoms
-
• better neuropsychological performance
-
• better response to antipsychotics
Box 4 Characteristic features of very-late-onset schizophrenia
Compared with early- or late-onset schizophrenia, very-late-onset schizophrenia is characterised by:
-
• associated sensory impairment
-
• social isolation
-
• a greater likelihood of visual hallucinations
-
• a lesser likelihood of formal thought disorder
-
• a lesser likelihood of affective blunting
-
• a lesser likelihood of family history of schizophrenia
-
• a greater risk of developing tardive dyskinesia
-
• the significantly higher number of females affected than males
(Reference Lisa, Zorrilla, Jeste, Copeland, Abou-Saleh and BlazerLisa et al, 2002; Reference Tune and SalzmanTune & Salzman, 2003)
This classification is, however, not considered final and there is much room for further debate and research. Moreover, there are no separate categories for late-onset and very-late-onset schizophrenia in either DSM–IV–TR (American Psychiatric Association, 2000) or ICD–10 (World Health Organization, 1992).
The prevalence of schizophrenia (early, late and very late onset combined) in the population aged 65 years and above is believed to be about 1% (Reference Cohen, Cohen and BlankCohen et al, 2000). Out of these, nearly 25% have late- or very-late-onset illness, and the remaining 75% are people with early-onset schizophrenia who have reached old age (Reference Jeste and TwamleyJeste & Twamley, 2003).
Pharmacological treatment
Antipsychotic medications are the most widely used pharmacological treatment for both early- and late-onset schizophrenia in elderly people. Although there is a dearth of well-conducted studies (with few randomised controlled trials), there is some evidence that these drugs improve acute symptoms and prevent relapse (Reference Jeste, Eastham and LacroJeste et al, 1996).
Conventional antipsychotics
The research literature on the use of conventional antipsychotics in elderly people with schizophrenia is sparse and there are very few recent studies. Significant improvement in psychotic symptoms with the use of haloperidol, trifluoperazine (10–30 mg/day) and thioridazine (40–50 mg/day) was reported in studies carried out in the 1960s (Reference PostPost, 1966; Reference Tsuang, Lu and StotskyTsuang et al, 1971). Thioridazine has since been shown to cause prolongation of the QT interval and its use in elderly people is not recommended.
Depot antipsychotic medication can be useful in elderly patients who have problems adhering to medication regimens. Reference Howard and LevyHoward & Levy (1992) reported that low doses of depot antipsychotics (14.4 mg of flupentixol decanoate or 9 mg of fluphenazine decanoate every 2 weeks) were associated with improved adherence and treatment outcome compared with oral medication.
Atypical antipsychotics
The newer atypical antipsychotics are currently considered the first-line treatment for older patients owing to their better side-effect profile in comparison with conventional antipsychotics (Reference Tune and SalzmanTune & Salzman, 2003). However, limited data are available from controlled trials showing their efficacy and safety in older people.
Clozapine
The usefulness of clozapine for treatment-resistant early-onset schizophrenia is well established, but concerns about toxicity and the need for monitoring white cell counts has led to limited use in older patients. A few small studies on its use at lower doses in this population have reported sedation, lethargy and postural hypotension as common side-effects (reviewed by Reference Barak, Wittenberg and NaorBarak et al, 1999). In their review Barak et al concluded that most showed moderate-to-marked improvement of psychotic features at a relatively low mean dose of 134 mg/day, but cautioned that agranulocytosis may occur more frequently in older people. In light of these risks, clozapine is not a first-line antipsychotic for elderly patients and should probably be used only in cases of treatment resistance and severe tardive dyskinesia (Reference Howard, Jacoby and OppenheimerHoward, 2002).
Risperidone and olanzapine
Of the atypicals, risperidone is the most extensively studied in the elderly population. It is effective, well tolerated in low doses (1.5–6 mg/day) and produces significant clinical improvement in elderly people with schizophrenia (Reference Katz, Jeste and MintzerKatz et al, 1999; Reference Madhusoodanan, Suresh and BrennerMadhusoodanan et al, 1999). Limited data are available on the use of olanzapine in treating older people with schizophrenia. Reference Madhusoodanan, Suresh and BrennerMadhusoodanan et al (1999) compared 151 hospitalised elderly psychiatric patients (mean age 71 years) who received either risperidone or olanzapine. Olanzapine therapy was found to be effective, with side-effects reported in 17% of the patients, and the authors concluded that the drug was safe and effective in that population. Reference Sajatovic, Perez and BrescanSajatovic et al (1998) studied olanzapine in an open-label trial with 22 older patients with schizophrenia. They found that it significantly improved symptoms of schizophrenia and had few extrapyramidal side-effects without adversely affecting comorbid medical problems. Owing to recent concerns about the side-effects of these two antipsychotics in people with dementia, their use is also likely to be restricted in people with schizophrenia.
Quetiapine
On the basis of their review of the literature, Reference Zayas, Grossberg, Copeland, Abou-Saleh and BlazerZayas & Grossberg (2002) have suggested that quetiapine is safe for use in elderly people and is not associated with weight gain. To avoid the common side-effects of postural hypotention, dizziness and agitation, they recommend starting with the lowest possible dose (25 mg) and slowly titrating up to 100–300 mg/day. More recently, Reference Jaskiw, Thyrum and FullerJaskiw et al (2004), in a multicentred open-label trial, have reported safe use in dosages up to 750 mg/day, given in divided doses. As no other study has reported use of quetiapine in such high doses for elderly people, we suspect that only an occasional patient would require a very high dose.
Aripiprazole
The latest of the atypical anti-psychotics aripiprazole, with its unique mode of action as a partial agonist at receptors can be D2 effective in improving both positive and negative symptoms. Furthermore, it is less likely than the other atypicals to cause extrapyramidal symptoms, sedation, weight gain and cardiovascular side-effects (Reference Hirose, Uwahodo and YamadaHirose et al, 2004). It probably holds promise for both young and older people with schizophrenia, but there are few data on its use, safety and dosing strategies in older people. Reference Madhusoodanan, Brenner and GuptaMadhusoodanan et al (2004) described their clinical experience of aripiprazole in ten elderly people with schizophrenia. They concluded that it is safe, improved both positive and negative symptoms and caused fewer side-effects.
Dosage
Suggested daily doses of various atypicals for elderly people are given in Table 1 (no data are available for aripiprazole). These should be taken as a guideline only and the dosing regimen should be tailored to the needs of individual patients. The already mentioned strategy of starting low and going slow is probably the safest way of using the newer anti-psychotics for which robust safety data are lacking.
Table 1 Recommended doses of atypical anti-psychotics for elderly people
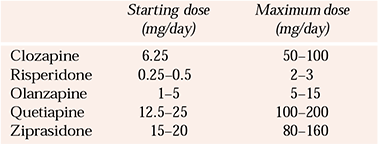
| Starting dose (mg/day) | Maximum dose (mg/day) | |
|---|---|---|
| Clozapine | 6.25 | 50–100 |
| Risperidone | 0.25–0.5 | 2–3 |
| Olanzapine | 1–5 | 5–15 |
| Quetiapine | 12.5–25 | 100–200 |
| Ziprasidone | 15–20 | 80–160 |
Electroconvulsive therapy
Most research on the use of electroconvulsive therapy (ECT) on elderly patients with schizophrenia was conducted during the 1950s and 1960s. Reference Kay and RothKay & Roth (1961) reported temporary remission following the use of ECT or neuroleptics in about 25% of their patients. A better response to ECT in patients with late paraphrenia presenting with prominent affective symptoms was reported by Reference FrostFrost (1969). It appears that, with the introduction of a variety of typical and atypical antipsychotics, the use of ECT on elderly patients with schizophrenia has declined in clinical practice.
Cognitive–behavioural therapy
The usefulness of cognitive–behavioural techniques in modifying delusional beliefs and controlling hallucinations has been widely reported in younger people (Reference Garety, Kuipers and FowlerGarety et al, 1994; Reference Fowler, Garety and KuipersFowler et al, 1995). Unfortunately, there have been few attempts to study their use with elderly patients. Reference Agüera-Ortiz, Reneses-Prieto, Howard, Rabins and CastleAgüera-Ortiz et al (1999) have suggested that they might help elderly people gain insight into their illness and provide them with coping strategies to help them live a meaningful life.
Reference McQuaid, Granhoolm and McClureMcQuaid et al (2000) have developed a novel intervention for older people with schizophrenia that integrates cognitive–behavioural techniques and social skills training. This approach suits the needs of elderly people and aims at reducing their cognitive vulnerabilities and improving their ability to cope with stress and to adhere to other forms of treatment.
Psychosocial therapies
The effectiveness of psychosocial interventions in improving independent living and social skills in younger people with schizophrenia is well established (Reference Kopelowicz, Liberman, Nathan and GormanKopelowicz & Liberman, 1998). Such interventions may also be of importance for elderly patients, a significant number of whom fail to show a complete response to antipsychotics (Reference Howard, Jacoby and OppenheimerHoward, 2002). Reference Bartels, Forester and MueserBartels et al (2004), in a pilot study of elderly people with severe mental illness, found that a combination of interpersonal and independent skills training, together with standard occupational therapy, was associated with improved social functioning and independent living.
Neurodegenerative disorders
Among the neurodegenerative disorders, psychotic symptoms are commonly seen in Alzheimer's disease, dementia with Lewy bodies and Parkinson's disease. In Alzheimer's disease and Lewy body dementia, psychotic symptoms are thought to be related to the underlying pathophysiology of the condition. In Parkinson's disease, which commonly presents with motor symptoms and dementia, anti-Parkinsonian medication is the most frequent cause of psychotic symptoms (Reference Mintzer and TargumMintzer & Targum, 2003).
Alzheimer's disease
The prevalence of psychosis in people with Alzheimer's disease ranges between 30 and 50% (Reference Jeste and FinkelJeste & Finkel, 2000). Reference Bassiony, Steinberg and WarrenBassiony et al (2000), in a community-based study of Alzheimer's disease, reported that about one-third of the participants showed evidence of psychotic symptoms and that delusions were more common than hallucinations.
The question of whether delusions in Alzheimer's disease (Box 5) are secondary to the cognitive deficits or are true psychotic phenomena remains unanswered. Hallucinations in Alzheimer's disease can occur in any sensory modality, but visual and auditory hallucinations are the most common (Reference TariotTariot, 1995). There is some evidence of the association of psychotic symptoms with a rapid decline in cognition in Alzheimer's disease (Reference Förstl, Burns and LevyFörstl et al, 1994; Reference Levy, Cummings and FairbanksLevy et al, 1999).
Box 5 Four common types of misidentifying delusion seen in individuals with Alzheimer's disease
-
• The Capgras type The false belief that previously known people (e.g. wife or care-giver) have been replaced by impostors
-
• The phantom boarder symptom A false belief that guests are living in the person's house
-
• The mirror sign The individual misidentifies his or her own mirror image as someone else
-
• The TV sign Misidentification of television images as real (a variant of this is the magazine sign, in which magazine images on a table are perceived as being real and existing in three-dimentional space (Reference Karim and BurnsKarim & Burns, 2003))
Pharmacological treatment of psychosis
Antipsychotics
Antipsychotics have been the most widely used form of treatment for psychosis in Alzheimer's disease (Reference Margallo-Lana, Swann and O'BrienMargallo-Lana et al, 2001), although not without concerns about the safety of their use, as discussed above. A number of fairly recent studies have demonstrated the efficacy of antipsychotics in controlling psychotic symptoms. However, most of these were designed to look at the usefulness of these drugs in controlling the behavioural and psychological symptoms of dementia, not its psychotic symptoms.
Reference SchneiderSchneider (1996), in a meta-analysis of seven placebo-controlled trials of the use of typical anti-psychotics, reported significant but modest efficacy. Reference Devanand, Marder and MichaelsDevanand et al (1998), in a randomised placebo-controlled dose-comparison trial of haloperidol, reported superior efficacy of doses of 2–3 mg/day, with moderate-to-severe extrapyramidal symptoms occurring in 20% of patients; a lower dose (0.5–0.75 mg/day) was no better than placebo.
Of the atypical antipsychotics, there have been a number of randomised placebo-controlled trials of risperidone and olanzapine.
Reference Katz, Jeste and MintzerKatz et al (1999), in a randomised double-blind trial comparing risperidone with placebo in nursing-home patients, demonstrated the efficacy of risperidone over placebo; the optimal dose was 1 mg/day. Other studies (Reference De Deyn, Rabheru and RasmussenDe Deyn et al, 1999; Reference Brodaty, Ames and SnowdonBrodaty et al, 2003) have confirmed the efficacy of low doses of risperidone for controlling psychotic symptoms in Alzheimer's disease.
Olanzapine in a dose of 5 mg/day significantly improved psychotic symptoms in Alzheimer's disease in a double-blind placebo-controlled trial of 6 weeks’ duration. Higher doses (10 and 15 mg) showed no added benefit. An open-label follow-up showed that the improvement could be maintained (Reference Street, Clark and GannonStreet et al, 2000, Reference Street, Clark and Kadam2001).
Quetiapine in a dose of 100–300 mg/day has been reported to be well tolerated and to improve psychotic symptoms and hostility in people with Alzheimer's disease (Reference McManus, Arvanitis and KowalcykMcManus et al, 1999; Reference Tariot, Salzman and YeungTariot et al, 2000; Reference Yeung, Tariot and SchneiderYeung et al, 2000).
Cholinesterase inhibitors These are routinely used for cognitive deficits in Alzheimer's disease, and more recently their possible usefulness in improving psychotic symptoms has been investigated. Although there have been no prospective double-blind studies, reviews of current data (most studies have been on rivastigmine, donepezil and galantamine) suggest that these drugs are well tolerated and may be of value in preventing or reducing psychotic symptoms in Alzheimer ’s disease (Reference FinkelFinkel, 2004; Reference Wynn and CummingsWynn & Cummings, 2004).
Dementia with Lewy bodies
Dementia with Lewy bodies is probably a part of the spectrum of Lewy body disorders (Reference ByrneByrne, 1997). Its clinical presentation usually varies according to the site of Lewy body formation and associated neuronal pathology. Psychotic symptoms are seen more frequently in Lewy body dementia than in Alzheimer's disease. Visual hallucinations are the most common symptom and have been reported in up to 80% of cases; other classic symptoms include fluctuating cognition, Parkinsonian motor symptoms, frequent falls and sensitivity to neuroleptic medication (Reference McKeith, Fairbairn and BothwellMcKeith et al, 1994). Auditory hallucinations and paranoid delusions are also common, with prevalence rates of 20% and 65%, respectively (Reference McKeith, Galasko and KosakaMcKeith et al, 1996).
The treatment of psychotic symptoms in Lewy body dementia remains a challenge and most often requires a treatment plan tailored to the characteristics of individual patients. This should strike a balance between use of anti-Parkinsonian medication, which improves motor disorder but may induce psychotic symptoms, or not treating motor symptoms and cautiously treating the psychotic symptoms. This challenge also highlights the importance of non-pharmacological interventions (see below).
Pharmacological treatment
Antipsychotics
People with Lewy body dementia are extremely sensitive to antipsychotics. Small doses can lead to extreme worsening of Parkinsonian symptoms, and about 50% of individuals experience life-threatening adverse effects (Reference McKeith, Fairbairn and PerryMcKeith et al, 1992). Severe reactions may be dose related (Reference Byrne, Burns and WaiteByrne et al, 1992). The above-mentioned adverse effects of neuroleptics in older people have discouraged their use and consequently no robustly designed studies of antipsychotics in Lewy body dementia have been carried out. However, some reports on the use of olanzapine in this population have been published (Reference Walker, Grace and OvershotWalker et al, 1999; Reference Cummings, Street and MastermanCummings et al, 2002).
Cholinesterase inhibitors
A number of studies have reported improvement of psychotic symptoms with the use of cholinesterase inhibitors in Lewy body dementia. A large multicentre double-blind trial comparing rivastigmine with placebo showed significant improvements in delusions and hallucinations (Reference McKeith, Del Ser and SpanoMcKeith et al, 2000). Beneficial effects of the use of donepezil have also been reported (Reference Fergusson and HowardFergusson & Howard, 2000).
Cholinesterase inhibitors are not yet licensed for the treatment of Lewy body dementia in the UK.
Parkinson's disease
Psychotic symptoms in Parkinson's disease are most commonly extrinsic (resulting from treatment with anti-Parkinsonian drugs) and only occasionally intrinsic (secondary to the neurodegenerative process involving dopamine-producing cells in other parts of the brain) (Reference WoltersWolters, 2001). Most anti-Parkinsonian drugs (including levodopa, dopamine receptor agonists, dopamine release enhancers such as amantadine, and monoamine oxidase inhibitors such as selegiline) can cause psychotic symptoms.
Between 20 and 60% of people with Parkinson's disease develop psychotic symptoms (Reference KuzuharaKuzuhara, 2001; Reference Wolters and BerendseWolters & Berendse, 2001). Hallucinations are more frequent than delusions in extrinsic cases (Reference Aarsland, Larsen and CumminsAarsland et al, 1999) and visual hallucinations are more common than hallucinations in other sensory modalities (Reference Hoeh, Gyalai and WeistraubHoeh et al, 2003). Epidemiological studies have found that the risk of psychotic symptoms in Parkinson's disease is higher in later stages of the disease and when there is concurrent dementia or depressive illness (Reference Aarsland, Larsen and CumminsAarsland et al, 1999; Reference Giladi, Treves and PaleacuGiladi et al, 2000).
Pharmacological treatment
Antipsychotics
Treatment of psychotic symptoms in Parkinson's disease is difficult owing to older people's sensitivity to antipsychotics in general and to typical antipsychotics in particular. Clozapine has been the most widely used and studied anti-psychotic. Several double-blind controlled trials have established its efficacy. The optimal dose to reduce symptoms and minimise side-effects is 6.25–50 mg/day (Reference Hoeh, Gyalai and WeistraubHoeh et al, 2003).
There have been several retrospective reports and open-label trials on other atypicals such as risperidone and olanzapine, but none has been shown to improve psychotic symptoms without worsening extrapyramidal symptoms (Reference Breier, Sutton and FeldmanBreier et al, 2002; Reference Ondo, Levy and VuongOndo et al, 2002).
Cholinesterase inhibitors
There have been encouraging reports on the success of cholinesterase inhibitors such as donepezil and rivastigmine in improving both psychotic symptoms and cognitive deficits in Parkinson's disease (Reference Bergman and LernerBergman & Lerner, 2002; Reference Bullock and CameronBullock & Cameron, 2001; Reference Fabbrini, Barbanti and AuriliaFabbrini et al, 2002).
Should antipsychotics be used in dementia?
In addition to general concerns about the saftey of neuroleptics for older people, the use of any medication to treat psychotic symptoms in dementia is increasingly being questioned. Do psychotic symptoms that are not distressing or adversely affecting the patient require treatment with medication (Reference KidderKidder, 2003)? A careful assessment of potentially remediable environmental causes such as sensory deprivation, poor lighting and social isolation can prevent use of antipsychotics. Addressing other contributory and causal factors such as physical illness and side-effects of medication (Box 6) is equally important.
Box 6 Medications that can cause psychotic symptoms in elderly people during use or on withdrawal
Benzodiazepines
Antihistamines
-
• Cimetidine
Anti-Parkinsonian drugs
-
• Levodopa
-
• Amantadine
-
• Bromocriptine
-
• Procyclidine
Anti-arrhythmics
-
• Digoxin
-
• Propranolol
-
• Quinidine
-
• Procainamide
Anti-inflammatory drugs
-
• Aspirin
-
• Indomethacin
Anticonvulsants
-
• Phenytoin
-
• Primidone
-
• Carbamazepine
Steroids
-
• Prednisolone
Anti-cancer drugs
(Reference Wood, Harris and MorrealeWood et al, 1988; Reference Targum and AbbottTargum & Abbott, 1999; Reference TargumTargum, 2001)
Non-pharmacological treatment of psychotic symptoms in dementia
Non-pharmacological treatment of behavioural and psychological symptoms (including psychosis) in dementia has been the subject of increasing research in recent years (Reference Overshott, Byrne and BurnsOvershott et al, 2004).The non-pharmacological approach requires a detailed knowledge of the patient's personality and past psychiatric history, careful listening, observation of the current situation, and effective verbal and non-verbal communication. Reference Cohen-MansfieldCohen-Mansfield (2003) has described a three-stage (Box 7) approach involving assessment, ascertainment of causes of symptoms and planning an intervention such as the following.
Box 7 The stages of a non-pharmacological approach to symptoms of dementia (Reference Cohen-MansfieldCohen-Mansfield, 2003)
Assessment
This includes systematic observation of the patient and should concentrate on the following areas:
-
• identification of the problem through assessment of the symptoms
-
• assessment of the interaction of symptoms with the environment by dividing them into antecedents and consequences
-
• clarification of the negative effect of the symptoms, i.e. whether the patient and caregivers are negatively affected by them. This area is important because psychotic symptoms that do not have a negative impact may not require treatment (Reference KidderKidder, 2003).
Ascertaining possible causes for the symptoms
Some symptoms have environmental causes, and important areas to explore are:
-
• whether the patient has a negative view of the caregiver
-
• whether the patient is unable to understand the intentions of caregivers
-
• whether the patient suffers from social isolation or sensory deprivation
-
• the patient's misinterpretations of the environment and situations
Planning an intervention
The following points should be kept in mind:
-
• the intervention should be tailored to the needs of the particular patient and should address the cause of the symptoms
-
• the intervention may be directed at the patient, or at the environment, members of staff or the general system of care
-
• the need for regular assessment and re-evaluation of the intervention, to monitor symptom improvement
Reducing sensory deprivation
Practical measures aimed directly at the patient might include a hearing aid or glasses. External measures such as improving lighting, providing enhanced-contrast materials, and larger type faces and objects may also help. An increase in positive stimulation through auditory sensations such as music and tactile sensations such as touch and massage may also prove useful.
Reducing inappropriate inner sensory stimulation
Simple practical measures can reduce stimulations that produce psychotic symptoms. Examples include removing mirrors if reflections cause the delusion of having phantom boarders in the house, or drawing a curtains over windows if the patient has a delusion of being spied on or followed.
Measures for specific symptoms
Misinterpretation of reality is the basis of a number of psychotic symptoms in dementia. A common symptom such as seeing caregivers as impostors can be addressed by training them to establish a positive relationship with the patient, introduce themselves with each encounter and clearly explain what they are going to do before doing it.
Delusions of infidelity or abandonment in institutionalised patients can be addressed by arranging frequent contact with their families. This can be real or simulated (by using videotapes of family members or simulated presence therapy; Reference Hall and HareHall & Hare, 1997; Reference Camberg, Woods and OoiCamberg et al, 1999). Measures such as frequent telephone calls and bringing familiar items from the patient's home can also be helpful in countering feelings of abandonment and betrayal.
The delusion that other people are stealing belongings can be addressed by providing duplicates of items that are easily mislaid (such as reading glasses), providing a remote control finder or using methods such retrieval, which teaches the patient always to return certain items to particular places (Reference McKitrick, Camo and BlackMcKitrick et al, 1992).
Conclusions
Antipsychotic drugs commonly and successfully used in younger populations can be prescribed only cautiously for elderly people. Some have been inadequately studied in older age groups and many carry increased risk of dangerous or debilitating side-effects. However, they remain the mainstay of treatment for schizophrenia and related psychotic disorders in elderly people. Cholinesterase inhibitors show some promise for the psychotic symptoms of neurodegenerative disorders, but they have yet to be licensed in the UK to treat all dementias.
Drugs, however, are not the only option for psychotic symptoms, particularly those that neither distress nor endanger the patient. Much can be achieved with non-pharmacological interventions such as environmental changes, sensitive staff training and a patient-centred approach, which offer a safe augmentation of, or even alternative to, medication for a growing population of vulnerable people.
MCQs
-
1 Psychotic symptoms in elderly people:
-
a are diagnostic of schizophrenia
-
b can be seen in dementia
-
c are always secondary to a medical illness
-
d carry a higher caregiver burden
-
e can result in neglect of patients.
-
-
2 Schizophrenia in elderly people :
-
a always presents with visual hallucinations
-
b requires CT brain scan for diagnosis
-
c can be diagnosed at any age
-
d should be treated by isolating the patient
-
e can have associated cognitive deficits.
-
-
3 In people with Alzheimer's disease:
-
a psychotic symptoms are rare
-
b delusions are the most common psychotic symptoms
-
c antipsychotics are not effective for psychotic symptoms
-
d psychotic symptoms can be managed without medication
-
e psychotic symptoms can be secondary to cognitive deficits.
-
-
4 Regarding psychotic symptoms in Lewy body dementia:
-
a visual hallucinations are the most common feature
-
b antipsychotics are the first line of treatment
-
c can be induced by anti-Parkinsonian medication
-
d have been shown to improve with cholinesterase inhibitors
-
e rivastigmine is licensed in UK for their treatment.
-
-
5 Regarding use of antipsychotics in elderly people with schizophrenia:
-
a the risk of developing tardive dyskinesia is higher with conventional antipsychotics
-
b can increase the risk of falls
-
c depot antipsychotics are contraindicated
-
d clozapine is the first-line choice
-
e the usual starting dose is half the adult dose.
-
MCQ answers

| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | F | a | F | a | F | a | T | a | T |
| b | T | b | F | b | T | b | F | b | T |
| c | F | c | T | c | F | c | T | c | F |
| d | T | d | F | d | T | d | T | d | F |
| e | T | e | T | e | T | e | F | e | T |




eLetters
No eLetters have been published for this article.