The prevalence of dementia would be reduced by 50% if risk reduction strategies were successful in delaying its onset by 5 years (Reference Jorm, Korten and HendersonJorm et al, 1987). In this article we describe the potential for prevention of dementia. Our review does not attempt to be exhaustive: for example, we do not discuss the role of oestrogen or vaccines for Alzheimer’s disease. Instead, we focus on a few key areas that are particularly amenable to potential therapeutic intervention, giving possible pathophysiological mechanisms and a flavour of the available evidence.
Prevention strategies may be empirically grouped into three categories (although a crossover between categories is expected): treatment of vascular risk factors, neuroprotection and building up neuronal reserves. Each of the three categories includes a number of strategies relating to specific risk factors or mechanisms involved in neurodegeneration (Box 1).
Box 1 Strategies for prevention of dementia
Treatment of vascular risk factors
-
• Hypertension
-
• Hypercholesterolaemia
-
• Diabetes
-
• Carotid atherosclerosis
-
• Heart disease
-
• Smoking
Neuroprotection
-
• Folate and vitamin B12
-
• Antioxidants (vitamins C and E, alcohol)
-
• Anti-inflammatory agents
Building up neuronal reserves
-
• Cognitive activity
-
• Physical activity
-
• Social and leisure activity
Treatment of vascular risk factors
Hypertension
Hypertension is a known risk factor for stroke, both haemorrhagic and ischaemic, which itself increases the risk of dementia by five to ten times. Hypertension can cause hyalinisation of vessel walls, reducing cerebral blood flow and causing ischaemia in vulnerable areas such as deep white matter. This can result in the disconnection of subcortical–cortical association pathways, which is one of the likely mechanisms that lead to cognitive impairment. Hypertension is also thought to disrupt the blood–brain barrier, exposing the brain to systemic factors.
Several large epidemiological studies have now established hypertension (both systolic and diastolic) in middle age as a risk factor for Alzheimer’s disease and vascular dementia in later life. For example, an ageing study that followed over 3500 Japanese American men over 25 years found hypertension to increase the risk of dementia four- to fivefold in those who had not been treated with antihypertensives. The risk was found to be greatest in those with systolic hypertension and the apolipoprotein E4 (ApoE4) allele (after controlling for other risk factors; odds ratio, OR = 13.0) (Reference Launer, Ross and PetrovitchLauner et al, 2000). Reference Murray, Lane and GaoMurray et al(2002) followed 1900 African Americans over 5 years and found the use of antihypertensives to be associated with a 38% (OR = 0.62) reduction in incident Alzheimer’s disease and vascular dementia.
The results of randomised controlled trials (RCTs) studying the use of antihypertensives in individuals with hypertension have been variable. Earlier trials identified no significant impact of such drugs on cognition or incident dementia. The more recent Systolic Hypertension in Europe (Syst–Eur) study (Reference Forette, Seux and StaessenForette et al, 2003) reported that nitrendipine (a calcium channel blocker) reduced the risk of Alzheimer’s disease by 50% over 2 years. The results of RCTs involving people with multiple vascular risk factors in addition to hypertension have been more consistently encouraging. For example, the PROGRESS (Reference Tzourio, Anderson and ChapmanTzourio et al, 2003) trial found a significant reduction of incident dementia in patients who had had strokes treated with an angiotensin-converting enzyme (ACE) inhibitor. The statistical power of these studies is limited by the low incident risk of dementia in the study populations.
In all RCTs, incident dementia has been a secondary outcome, heart disease or stroke being the primary outcomes. The time lapse between the diagnosis of hypertension and onset of dementia can be over 15 years, and it is not known whether the protective effect on incident dementia of starting antihypertensives extends throughout this period. In this respect, it is interesting to note that blood pressure falls just before or at the onset of dementia, probably because of the central dysregulation of blood pressure control. In hypertensive patients with multi-infarct dementia, maintaining systolic blood pressure within the upper limits of normal (135–150 mmHg) was more beneficial in preserving cognitive function than reducing systolic blood pressure below this level (Reference Meyer, Judd and TawaklnaMeyer et al, 1986).
Another issue that needs further clarification is whether the risk reduction is related to actual reduction in blood pressure and/or some other neuroprotective action of the individual anti-hypertensive agent (e.g. a calcium channel antagonist or ACE inhibitor). For example, angiotensin II inhibits acetylcholine release and angiotensin II (AT1) receptor blockers improve cognitive performance (Reference Barnes, Barnes and CostallBarnes et al, 1990; Reference Fogari, Mugellini and ZoppiFogari et al, 2003).
Hypercholesterolaemia
Hypercholesterolaemia, especially in the form of increased low-density lipoproteins, is a known risk factor for coronary heart disease, atherosclerosis and stroke, which are all associated with increased risk of dementia. The ApoE4 allele, the best established genetic risk factor for Alzheimer’s disease, affects stabilisation of membrane lipoproteins and is now known to be a risk factor for hypercholesterolaemia. Cholesterol increases the production of β-amyloid, which is at the centre of senile plaques in Alzheimer’s disease. Lipid-lowering agents reduce cholesterol but lack the additional benefits of statins. Statins reduce cholesterol levels by inhibiting 3-hydroxy-3-methylglutaryl co-enzyme A (HMG-CoA). In addition, statins have an antioxidant action, decrease pro-inflammatory processes and improve cerebral blood flow. They have also been shown to reduce amyloid β-protein 42 (Aβ42) in hippocampal neurons and cerebrospinal fluid.
Reference Moroney, Tang and BerglundMoroney et al(1999) followed up more than 2000 older people in New York over 2 years and found those with cholesterol levels in the highest quartile to be at increased risk of dementia, especially vascular dementia (OR = 3–4), compared with those in the lowest quartile. A Finnish study of a similar size but with a much longer follow-up (21 years) reported that raised cholesterol in middle age (≥6.5 mmol/l) more than doubled the risk of dementia, including Alzheimer’s disease, independently of other risk factors such as hypertension and ApoE4 (Reference Kivipelto, Helkala and LaaksoKivipelto et al, 2002).
Review of case–control and retrospective cohort studies indicates that statins reduce the risk of dementia, but there are some important inconsistencies between individual reports. For example, some studies have found risk reduction with lipid-lowering agents in general; others suggest the risk reduction to be more specifically associated with the use of statins. A meta-analysis of seven observational studies found risk reduction in cognitive impairment to be significant for statins (OR = 0.43) but not for other lipid-lowering agents (Reference Etminan, Gill and SamiiEtminan et al, 2003). The results of intervention studies examining the effect of statins on cognitive functioning have, however, not been encouraging. Neither simvastatin (Heart Protection Study Collaborative Group, 2002) nor pravastatin (Reference Shepherd, Blauw and MurphyShepherd et al, 2002) were shown to have any positive effect on cognitive decline. Not all statins are the same, and whether individual statins would have a differential effect on cognition is not known. In this respect, it is noteworthy that simvastatin and lovastatin, but not mevastatin or pravastatin, significantly inhibit butylcholinesterase. In rare cases, statins have an adverse effect on cognition, although the underlying mechanism and causality are not certain (Reference Wagstaff, Mitton and ArvikWagstaff et al, 2003).
Diabetes
Diabetes is a known risk factor for cerebrovascular disease. Insulin receptors are abundant in the brain. Amyloid β-protein 42 reduces insulin binding, which may partially explain the insulin resistance observed in Alzheimer’s disease. Insulin reduces amyloid precursor protein and β-amyloid in Alzheimer’s disease, especially in those not carrying the ApoE4 allele.
Large epidemiological studies from the USA, Canada and The Netherlands, involving over 17 000 people followed for between 2 and 5 years, have shown diabetes to increase the risk of both Alzheimer’s disease and vascular dementia (OR = 2–4), independent of other risk factors. Diabetes may be a stronger risk factor for vascular dementia in general and for Alzheimer’s disease in ApoE4-negative individuals in particular, but this needs further confirmation. Whether treatment of diabetes can reduce the risk of dementia is unknown. A Cochrane review identified five trials in this field, but none was suitable for inclusion as each was either not double-blind or did not include both pre- and post-treatment cognitive measures (Reference Areosa and GrimleyAreosa & Grimley, 2002).
Carotid atherosclerosis
Hypertension, hypercholesterolaemia, diabetes and smoking are known risk factors for carotid atherosclerosis. Atherosclerosis of carotid arteries is a major determinant of stroke, and the latter increases the risk of dementia five- to tenfold. The mechanism involved is thought to be ischaemia or embolism of a large thrombus. In our pilot study, spontaneous cerebral emboli were detected in 11/41 (27.5%) individuals with dementia, compared with 1/16 (7%) controls. Emboli were most frequent in vascular dementia (7/17, 41%) compared with controls (P = 0.04) (Reference Purandare, Welsh and HutchinsonPurandare et al, 2005). We found no relationship between the presence of spontaneous cerebral emboli and carotid disease (probably because of the small sample size), but as most people with severe carotid stenosis are likely to produce spontaneous cerebral emboli (Reference Hutchinson, Riding and CoullHutchinson et al, 2002), it is possible that repeated subclinical microembolisation of cerebral circulation may be another mechanism of brain damage in patients with carotid disease.
Reference Hofman, Ott and BretelerHofman et al(1997), in their population-based study involving 284 individuals with dementia (207 with Alzheimer’s disease) and 1698 controls, found both Alzheimer’s disease and vascular dementia to be associated with the presence of carotid atherosclerosis. In participants with severe atherosclerosis (compared with those without atherosclerosis), the odds ratios for Alzheimer’s disease and vascular dementia were 3.0 (95% CI 1.5–6.0) and 9.5 (95% CI 3.0–30.0) respectively. Whether controlling spontaneous cerebral emboli in patients with moderate-to-severe carotid atherosclerosis will delay cognitive decline is not known.
Heart disease
Myocardial infarction and atrial fibrillation are known risk factors for both Alzheimer’s disease and vascular dementia. Senile plaques are more common in people with severe cardiovascular disease than in those without a history of heart disease. A prospective cohort study with up to 7-year follow-up found older women with a history of myocardial infarction to be at five times greater risk of developing dementia (Reference Aronson, Ooi and MorgensternAronson et al, 1990). Similarly, a population-based cohort study found atrial fibrillation to double the risk of all dementia, independent of clinical stroke (Reference Ott, Breteler and de BruyneOtt et al, 1997).
Smoking
Smoking, an established cardiovascular risk factor, is considered to be one of the prime targets in prevention of vascular cognitive impairment and vascular dementia. An aging study of elderly Japanese American men found that smoking during middle age was associated with later risk of cognitive impairment, and that the risk was higher for those who continued to smoke into old age than it was for those who no longer smoked (Reference Galanis, Petrovitch and LaunerGalanis et al, 1997). Smoking is also a risk factor for Alzheimer’s disease, and current smoking has been shown to double the incidence of dementia and Alzheimer’s disease (Reference Ott, Slooter and HofmanOtt et al, 1998). In contrast, some epidemiological reviews suggest that smoking may be protective against Alzheimer’s disease, but this finding might be attributable to the underappreciated influence of differential survival. Although smoking appears overall to be associated with a doubling of the risk of Alzheimer’s disease (presumably because of cardiovascular factors), nicotine itself may have neuroprotective properties, possibly reducing amyloid burden (Reference Nordberg, Hellstrom-Lindahl and LeeNordberg et al, 2002).
Neuroprotection
Folate and vitamin B 12
Folate and vitamin B12 are required both in the methylation of homocysteine to methionine and in the synthesis of S-adenosylmethionine, a methyl donor. Hyperhomocysteinaemia damages vascular endothelium and is an independent risk factor for coronary artery disease, peripheral vascular disease and cerebrovascular disease, especially extracranial carotid atherosclerosis. In addition, metabolites of homocysteine have an excitotoxic effect on N-methyl-d-aspartate (NMDA) receptors. An elevated homocysteine level is a more accurate marker of folate or vitamin B12 deficiency at tissue level than are serum folate or vitamin B12 levels. Hyperhomocysteinaemia is common in older people, and is present in about 30% of individuals with mild cognitive impairment, 45% of those with Alzheimer’s disease and 60% of those with vascular dementia.
In older people without dementia, elevated plasma homocysteine is associated with some indicators of cognitive impairment. Reference Dufouil, Alperovitch and DucrosDufouil et al(2003) followed 1241 people aged between 61 and 73 years over a 4-year period. They found an association between higher concentrations of homocysteine and impaired neuropsychological performance, with the odds of cognitive decline 2.8-fold (P<0.05) higher in individuals with homocysteine levels above 15 μmol/l compared with those with levels below 10 μmol/l.
Elevated total homocysteine is associated with Alzheimer’s disease and predicts radiological evidence of disease progression. A large epidemiological study in the USA, involving 1092 older people followed over 8 years, found that the risk of Alzheimer’s disease nearly doubled (relative risk 1.8, 95% CI 1.3–2.5) with an increase of one standard deviation in homocysteine levels at baseline (Reference Seshadri, Beiser and SelhubSeshadri et al, 2002).
Although an association between elevated homocysteine and cognitive impairment in Alzheimer’s disease has been found, the effect of reducing homocysteine levels on cognition and global functioning is not clear. A case–control study from Ireland found moderately high homocysteine levels to be associated with stroke, vascular dementia and Alzheimer’s disease, independent of vascular risk factors and nutritional status (Reference McIlroy, Dynan and LawsonMcIlroy et al, 2002). A meta-analysis from the Homocysteine Studies Collaboration Group (2002), which included individual participant data from 30 prospective or retrospective studies, found that after adjusting for known cardiovascular risk factors, low homocysteine levels (3.0 (s.d. = 0.41) μmol/l) were associated with an 11% lower risk of ischaemic heart disease and a 19% lower risk of stroke. In a small prospective study involving 33 patients, Reference Nilsson, Gustafson and HultbergNilsson et al(2001) reported an improvement in cognitive function after 2 months of vitamin B12 or folate in individuals with mild-to-moderate dementia who had elevated homocysteine levels.
Antioxidants (vitamins C and E, alcohol and green tea)
In vitro studies suggest that ApoE4 reduces the antioxidant capacity of neurons. Oxidative stress can cause lipoprotein oxidation, β-amyloid polymerisation and generation of excessive free radicals. Vitamins C and E, along with alcohol (in moderation), scavenge these free radicals. Furthermore, micronutrients in red wine, especially resveratrol, have been shown to protect against β-amyloid toxicity, an effect also observed with green tea (Reference Choi, Jung and LeeChoi et al, 2001; Reference Russo, Palumbo and AlianoRusso et al, 2003; Reference Savaskan, Olivieri and MeierSavaskan et al, 2003).
Low levels of vitamins C and E have been found in both the blood and cerebrospinal fluid of people with Alzheimer’s disease. Epidemiological evidence suggests that higher dietary intake of these vitamins is associated with lower risk of dementia. An RCT in which 341 people with moderate-to-severe Alzheimer’s disease were randomised to receive selegiline (a monoamine oxidase inhibitor) and vitamin E (either alone or in combination) or placebo found all active treatments, including vitamin E alone, to slow the progression of Alzheimer’s disease over 2 years, as measured by time to occurrence of death, institutionalisation, loss of ability to perform basic activities of daily living or severe dementia (Reference Sano, Ernesto and ThomasSano et al, 1997). In a study that followed over 5000 people for an average of 6 years, moderate alcohol consumption (1–3 drinks per day) was associated with reduced risk of dementia, including both Alzheimer’s disease and vascular dementia (relative risk RR = 0.58) (Reference Ruitenberg, van Swieten and WittemanRuitenberg et al, 2002). Reference Orgogozo, Dartigues and LafontOrgogozo et al(1997) found 250–500 ml of wine daily to reduce the risk of dementia even more (OR = 0.19).
Additional studies are needed to examine the effect of type of alcohol on risk reduction.
Anti-inflammatory agents
The importance of inflammatory processes in causation of dementia is now well established, with evidence from biochemical, neuropathological and epidemiological studies. Primary inflammatory cytokines such as the interleukins IL-1β and IL-6 and α2-macroglobulin are increased in the brains of patients with Alzheimer’s disease. The neuropathological features of Alzheimer’s disease include accumulation of microglia and astrocytes around senile plaques. Both express human leukocyte antigens (HLAs) and are involved in activation of the complement cascade. These inflammatory changes are thought to lead to further amyloid deposition and neuronal damage. Non-steroidal anti-inflammatory drugs (NSAIDs) may offer a neuroprotective effect by inhibition of both cyclo-oxygenases COX-1 and COX-2. There are interesting differences between locations and actions of COX-1 and COX-2, but all the epidemiological evidence is based on traditional NSAIDs with mixed action.
A number of epidemiological studies have shown long-term use of NSAIDs to be associated with a two- to fourfold reduced risk of Alzheimer’s disease, but others have been inconclusive.Reference In't Veld, Ruitenberg and HofmanIn’t Veld et al(2001) suggest that the conflicting evidence may be related to inaccurate recording and reliance on patients’ and/or relatives’ memory or incomplete medical records. They examined computerised pharmacy records in a follow-up study of almost 7000 residents of Rotterdam over 7 years. The study found use of NSAIDs to be associated with reduced risk of Alzheimer’s disease that depended on the duration of the use (<1 month: RR = 0.95; 1–24 months: RR = 0.83; >24 months: RR = 0.20). The results of intervention trials in Alzheimer’s disease have been disappointing. It may be that some NSAIDs influence amyloid deposition by inhibiting γ-secretase, independent of cyclo-oxygenase activity (Reference Weggen, Eriksen and DasWeggen et al, 2001). Ongoing trials are investigating the potential effects of such agents.
Building up neuronal reserves
Cognitive activity
A number of cross-sectional and retrospective studies have found an inverse relationship between cognitive activity and risk of dementia. The repetition of cognitive activities may improve processing functions such as working memory and perceptual speed or, with repetition, certain cognitive skills may become more efficient (possibly owing to an increased dendritic network) and less susceptible to the pathology of Alzheimer’s disease. On the other hand, cognitive activity may be a proxy for some other factors such as education and socio-economic status. It is also possible that a reduction in cognitive activity may be an early consequence rather than a cause of dementia.
Reference Wilson, Mendes De Leon and BarnesWilson et al(2002) followed 801 older members of the clergy over 4 years. One-hundred and eleven developed Alzheimer’s disease. They recorded seven common activities that involved information processing as a core component (e.g. reading, watching the television and doing crosswords). The frequency of each activity was rated on a 5-point scale (1 = once a year, 5 = every day) and responses to each item were averaged to calculate a composite score (range: 1–5). After controlling for age, gender and education, a 1-point increase in cognitive activity was associated with a 33% reduction in risk of Alzheimer’s disease (RR = 0.67). A similar finding has emerged from other cohort studies, but there have been no RCTs.
Physical activity
The importance of exercise in cardiovascular risk reduction is already established. Physical exercise can also increase insulin-like growth factor (IGF-1), which has been shown to reduce τ-phosphorylation, a process implicated in Alzheimer’s disease.
No clinical studies have specifically examined the role of physical exercise in prevention of dementia. All epidemiological evidence relates to physical activity rather than physical exercise. Reference Wilson, Mendes De Leon and BarnesWilson et al(2002) suggested that risk reduction was due to cognitively stimulating activities rather than just physical activity, while Reference Friedland, Fritsch and SmythFriedland et al(2001) found that reduced activity (whether intellectual or physical) in middle age increased the risk of developing Alzheimer’s disease by 250%. The mixed results could be partly explained by arbitrary division of activities into physical or cognitive categories. For example, dancing or going to a museum, included as cognitive activities by Reference Wilson, Mendes De Leon and BarnesWilson et al(2002), also include an strong physical component.
Social and leisure activity
Activities, either cognitive or physical, often include social aspects and are leisure pursuits. The social aspect of an activity or a rich social network in itself may be beneficial in reducing risk of dementia. Reference Wang, Karp and WinbladWang et al(2002) found that engagement in stimulating activities (mental, social or productive) reduced the risk of developing dementia over 6 years by 50% (RR = 0.54–0.58). In experiments on rats, environmental enrichment has been shown to inhibit spontaneous apoptosis, increase neurogenesis in the dentate gyrus and improve spatial memory (Reference Nilsson, Perfilieva and JohanssonNilsson et al, 1999; Reference Young, Lawlor and LeoneYoung et al, 1999). This suggests a possible link between environment/social stimulation and regenerative brain processes.
Target populations
Dementia typically presents clinically in the sixth or seventh decade of life. However, a number of risk factors, such as hypertension and cholesterol in middle age (15–20 years earlier), are now known to be involved, suggesting that the disease process (e.g. amyloid deposition) may have continued at a sub-clinical level for a number of years before clinical presentation and diagnosis. Perhaps prevention strategies should therefore be targeted at middle-aged people who may not have any cognitive impairment. This may be economically impractical, as the actual number developing dementia would be small, requiring a very long follow-up. We would need better identification of subgroups at highest risk and surrogate markers of sub-clinical disease progression.
A more feasible approach would be to target older people who have developed cognitive impairment that is not as yet severe enough to cause functional impairment and warrant a diagnosis of dementia. The somewhat loose and controversial term ‘mild cognitive impairment’ is most widely used to describe this population, which has a community prevalence of 17–34% and an annual rate of conversion to dementia of 10–15% (Reference Burns and ZaudigBurns & Zaudig, 2002). An intervention trial would still need to recruit large numbers, but this would be achievable by including 10–15 study centres. The required sample size could be reduced further by identifying a subgroup within the ‘mild cognitive impairment’ category which reflects ‘preclinical dementia’, with higher conversion rates to dementia. In this respect, ‘vascular cognitive impairment’, which describes early cognitive impairment related to cerebrovascular disease, may be a better target. One problem in targeting older patients with preclinical dementia/mild cognitive impairment/vascular cognitive impairment is that we do not know whether prevention strategies, especially those directed at vascular risk factors, will have an effect similar to that observed in cohort studies of people followed since middle age. There may be a threshold period beyond which disease progression continues unabated independent of triggering or predisposing risk factors. In this respect, it is worth noting that most of the trials (and there are not that many) on people with dementia involving treatment of cardiovascular risk factors, vitamin supplementation, hormone-replacement therapy or anti-inflammatory therapy show at most a very modest effect on progression of dementia.
Conclusions
The evidence from large cohort studies (Table 1) suggests that it is possible to prevent or delay onset of dementia, but evidence from RCTs is limited. Currently ongoing RCTs, which tend to focus on one or two vascular risk factors, may strengthen the evidence, although they do not focus on dementia as a primary outcome. There is a need for RCTs, preferably with incident dementia as a primary outcome, that target multiple risk factors in at-risk people with mild cognitive impairment. Such trials would need to be multi-centred and of sufficient duration, possibly requiring pooled resources from two or more funding bodies.
Table 1 Summary of epidemiological evidence for treatable risk factors in prevention of dementia
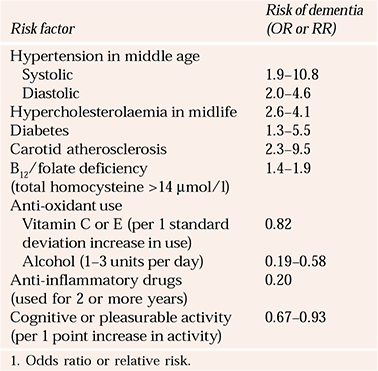
| Risk factor | Risk of dementia (OR or RR) |
|---|---|
| Hypertension in middle age | |
| Systolic | 1.9–10.8 |
| Diastolic | 2.0–4.6 |
| Hypercholesterolaemia in midlife | 2.6–4.1 |
| Diabetes | 1.3–5.5 |
| Carotid atherosclerosis | 2.3–9.5 |
| B12/folate deficiency (total homocysteine >14 μmol/l) | 1.4–1.9 |
| Anti-oxidant use | |
| Vitamin C or E (per 1 standard deviation increase in use) | 0.82 |
| Alcohol (1–3 units per day) | 0.19–0.58 |
| Anti-inflammatory drugs (used for 2 or more years) | 0.20 |
| Cognitive or pleasurable activity (per 1 point increase in activity) | 0.67–0.93 |
MCQs
-
1 The following are preventable risk factors for dementia:
-
a hypertension
-
b inactivity
-
c Apoe E4
-
d raised homocysteine
-
e age.
-
-
2 Delaying onset of dementia by 5 years may reduce its prevalence by:
-
a 10%
-
b 20%
-
c 30%
-
d 40%
-
e 50%.
-
-
3 Homocysteine levels above 15 μmol/l increase the risk of developing cognitive impairment over 4 years by a factor of:
-
a 1.8
-
b 2.8
-
c 3.8
-
d 4.8
-
e 5.8.
-
-
4 Reduced activities in middle age have been shown to increase risk of Alzheimer’s disease by:
-
a 50%
-
b 100%
-
c 200%
-
d 250%
-
e 300%.
-
-
5 The annual conversion rate to dementia in patients with ‘mild cognitive impairment’ is:
-
a 10%
-
b 20%
-
c 30%
-
d 40%
-
e 50%.
-
MCQ answers
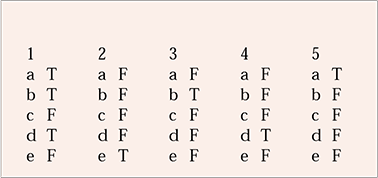
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | T | a | F | a | F | a | F | a | T |
| b | T | b | F | b | T | b | F | b | F |
| c | F | c | F | c | F | c | F | c | F |
| d | T | d | F | d | F | d | T | d | F |
| e | F | e | T | e | F | e | F | e | F |




eLetters
No eLetters have been published for this article.