In 1976, the World Health Organization defined psychosurgery as ‘the selective surgical removal or destruction of nerve pathways for the purposes of influencing behaviour’. Implicit within this definition was the assumption that so-called ‘psychosurgery’ held as its primary purpose the modification of behaviour by its effects on psychological processes. This no longer reflects appropriately the therapeutic aims of neurosurgical intervention for mental disorder. Such procedures are, today, more accurately described as neurosurgery for mental disorder. Neurosurgery for mental disorder has been defined by the Royal College of Psychiatrists (2000: p. 11) as
‘a surgical procedure for the destruction of brain tissue for the purposes of alleviating specific mental disorders carried out by a stereotactic or other method capable of making an accurate placement of the lesion’.
This definition emphasises important conceptual shifts, both in the manner in which psychological processes are now considered to be located within, and a product of, specific brain circuitry; and in the explicit focus on the alleviation of the symptoms of specific mental disorders.
Although it would be hoped that successful neurosurgery for mental disorder might indeed result in significant behavioural change associated with altered symptom burden, the primary aim is to engender a release of adaptive behaviour, not a suppression of an ‘undesirable’ or ‘unwanted’ behavioural repertoire, as has been extensively documented following earlier psychosurgical approaches.
Modern neurosurgery for mental disorder
A questionnaire survey of UK centres offering psychosurgery between 1974 and 1976, covering 431 patients, calculated that the yearly rate of leucotomy (a procedure that resulted in the widespread destruction of white matter connections between the frontal lobes and other brain areas) in the UK at that time was 3.4 operations per million population aged over 15. Depression was the most common indication, followed by anxiety, with ‘violence’ coming third (Reference Barraclough and Mitchell-HeggsBarraclough & Mitchell-Heggs, 1978). Within a relatively short period, there has been a dramatic reduction in rates of neurosurgery for mental disorder, a major change in both the selection of target sites and the techniques employed, and a contraction of the recognised indications for treatment.
The UK centre with the greatest clinical experience of neurosurgery for mental disorder, the Geoffrey Knight Unit (London), ceased service provision a number of years ago. With the closure of this service, the most commonly performed neurosurgical procedure for mental disorder in the UK – stereotactic subcaudate tractotomy – ceased to be available. Only two centres in the UK today offer neurosurgery for mental disorder. The service based at Ninewells Hospital in Dundee performed 33 procedures between 1990 and 2001 (Reference Matthews and EljamelMatthews & Eljamel, 2003). The service based at the University Hospital of Wales in Cardiff performed 39 procedures between January 1994 and June 2000 (Royal College of Psychiatrists, 2000: p. 49). Contemporary indications for neurosurgery are chronic, treatment-refractory depressive disorder (including bipolar depression) and obsessive–compulsive disorder. Some international centres, for example Stockholm's Karolinska Institute, also offer neurosurgery for non-obsessive–compulsive anxiety disorders.
Treatment-refractory depression, obsessive–compulsive disorder and anxiety disorders
In Western nations, depression in adults has a 12-month prevalence of 9.3% (Reference Lindeman, Hamalainen and IsometsaLindeman et al, 2000) and a lifetime prevalence of about 18% (Reference Kessler, McGonagle and ZhaoKessler et al, 1994). It is usually a recurrent disorder and it is often chronic. Treatment costs for depression and anxiety in the USA have been estimated at $42.3 billion in 1993 and $43.7 billion in 1999 (Reference Greenberg, Stiglin and FinkelsteinGreenberg et al, 1993, Reference Greenberg, Sisitsky and Kessler1999). Approximately 12% of patients will experience chronic (lasting more than 24 months) and unremitting symptoms despite treatment (Reference JuddJudd, 1997).
Anxiety has a prevalence rate of up to 17.2% (Reference Regier, Farmer and RaeRegier et al, 1993) and, in many cases, anxiety disorders can be chronic and disabling. Obsessive–compulsive disorder has a lifetime prevalence of 2.2–3.1% (Reference BebbingtonBebbington, 1998).
Long-term follow-up studies suggest that 48% of patients will still have ‘clinically relevant’ obsessive–compulsive disorder after 30 years, with a correlation between clinical symptoms and social functioning (Reference Skoog and SkoogSkoog & Skoog, 1999).
The increasing awareness of the problems of treatment resistance, coupled with the severity and chronicity of affective and anxiety disorders in some patients, has meant that neurosurgery for mental disorder has remained an option for a small number of patients for whom all other treatments have failed.
Target sites for surgery
Compared with the relatively indiscriminate lesions of psychosurgery, where the aim was to divide substantial tracts of white matter connecting frontal lobes with other parts of the brain, the lesions generated by modern neurosurgery for mental disorder target specific sites in the limbic system. Historically (Reference Kelly and Granville-GrossmanKelly, 1985), the main target areas have been as follows:
-
• the frontolimbic connections, concentrated in the posterior orbital cortex and cingulate gyrus;
-
• the limbic circuitry, including the Papez circuit and the basolateral circuit; the Papez circuit, originally proposed as the mediating neural substrate for emotional responses, runs from the hippocampus, via the fornix, to the mamillary bodies in the hypothalamus, and back to the hippocampus via the anterior thalamic nuclei, cingulate cortex and parahippocampal gyrus; the basolateral circuit is associated with social behaviour, and connects the prefrontal cortex and temporal cortex with limbic regions;
-
• the limbic cortex, for example, the amygdala, hippocampus and cingulate gyrus.
The pathophysiology of obsessive–compulsive disorder is thought to involve dysfunction of frontostriatal–pallidal–thalamic circuits within the brain (for a review, see Reference Saxena, Brody and SchwartzSaxena et al, 1998). Similar circuits are also implicated in the pathology of depression. Neuropsychological studies have demonstrated abnormalities of the orbitofrontal cortex and anterior cingulate regions, a hypothesis supported by neuroimaging studies. Symptom provocation is correlated with hypermetabolism in these areas (Reference Breiter, Rauch and KwongBreiter et al, 1996).
The place of neurosurgery in modern psychiatric practice
Neurosurgery has been practised for millennia (Box 1). Psychosurgery, and subsequently neurosurgery for mental disorder, have always been contentious, particularly during the height of the antipsychiatry movement of the 1960s and ‘70s. Psychosurgery has been frequently portrayed in the popular arts as a punitive means of social control (One Flew Over the Cuckoo's Nest and A Clockwork Orange, for example). Much of the hostility towards neurosurgery for mental disorder has resulted from the historical application of crude procedures, together with a lack of rigorous investigation regarding effectiveness and a lack of detailed assessment of adverse effects on personality and cognition.
Box 1 Milestones in the history of neurosurgery for mental disorder

| c. 8000 BC | Trepanation practised in many cultures worldwide. Archaeological evidence shows that some patients survived. However, in the absence of evidence that these procedures were necessarily instituted for the treatment of mental disorder, there are no firm grounds to consider them a form of psychosurgery. |
| 1891 | Gottlieb Burckhardt reports on six patients, described as ‘demented and aggressive’, undergoing bilateral cortical excision. Two patients developed epilepsy (one died) and one suffered motor weakness. |
| 1908 | Robert Henry Clarke and Victor Horsley describe the principles of stereotaxis for recording cerebellar function in monkeys. |
| 1935 | Jacobsen and Fulton report that the ‘frustration’ response to the withholding of anticipated reward in chimpanzees is lost after prefrontal cortex ablation. |
| 1936 | Egas Moniz, a Portuguese neurologist working with neurosurgical colleague Almeida Lima, treats 20 patients with severe anxiety, obsessional behaviour and irrational fears. They sever fibres connecting subcortical areas and the frontal lobes – the ‘prefrontal leucotomy’. It is alleged that one-third improved, one-third worsened and one-third were no different. Egas Moniz is the first to use the term ‘psychosurgery’. |
| 1936 | Neurologist Walter Freeman and neurosurgeon James Watts begin to treat patients with depression in the USA, using bilateral frontal leucotomy, which became known as the Freeman–Watts lobotomy. |
| 1940 | Peyton develops the ‘frontal lobotomy’, involving extensive tissue destruction. The procedure carried a high rate of postoperative epilepsy. |
| 1945 | Walter Freeman develops the transorbital leucotomy (‘ice pick lobotomy’), which involved inserting an instrument under the eyelids, through the roof of the orbit and into the orbitofrontal cortex. A quick sweeping motion cut the cortical tissue and related frontothalamic tracts. It was essentially a non-sterile procedure, could be performed with minimal anaesthesia (often two electroconvulsive treatments were used) and was widely adopted. |
| 1947 | Ernest Spiegel, a neurologist, and Henry Wycis, a neurosurgeon, perform the first stereotactic neurosurgical operation in a patient with psychiatric disorder, in the USA. |
| 1947 | The Columbia–Greystone project in the USA fails to provide evidence of the benefits of lobotomies. |
| 1948 | William Scoville develops ‘orbital undercutting’, with the aim of severing the medial fibres connecting the frontal lobes with the thalamus. |
| 1949 | Egas Moniz wins the Nobel Prize for Physiology and Medicine for his work in establishing leucotomy as a procedure for treating psychotic illness, along with Walter Hess (who received it jointly for unrelated work). In the ensuing years, leucotomy rates rise. |
| 1962 | Foltz and White perform the first open anterior cingulotomy. |
| 1964 | Geoffrey Knight develops the subcaudate tractotomy, an extensive lesion of orbitofrontal cortex and frontostriatal thalamic tracts by focal irradiation. |
| 1968 | Lars Leksell develops the ‘gamma knife’ – a stereotactic method for creating lesions by targeted irradiation without the need for cranial burr holes. |
| 1972 | Lars Leksell performs the thermal anterior capsulotomy as a focal method of disconnecting ventral and medial frontal cortex from subcortical and limbic structures. |
| 1973 | Desmond Kelly and Alan Richardson perform the first limbic leucotomy. |
Recent recommendations from both the Clinical Resource and Audit Group (CRAG) and the Royal College of Psychiatrists propose that neurosurgery for mental disorder retains an important role in treating a small number of carefully selected patients (CRAG Working Group on Mental Illness, 1996; Royal College of Psychiatrists, 2000). Although previous reviews have recognised methodological difficulties, a systematic review and meta-analysis have yet to be performed.
Indications for surgery
Three broad categories of psychiatric disorder may benefit from modern neurosurgery: obsessive–compulsive disorder, anxiety disorders and depressive disorders. For each category, it is generally accepted that only patients with illness of substantial chronicity and treatment refractoriness should be considered for neurosurgery. Other procedures, such as bilateral amygdalotomy, thalamotomy and hypothalamotomy, were performed during the 1960s and ‘70s for the treatment of aggression and hypersexuality, but these conditions are no longer considered to represent legitimate indications for neurosurgery.
Contraindications to surgery
Provided the diagnosis is secure, there are few absolute contraindications to neurosurgery for mental disorder other than incapacity to provide informed consent. In all cases, such surgery can only be offered following careful and detailed consideration of the potential costs and benefits to the individual on a case-by-case basis. Where affective or obsessional symptoms are the product of active organic or degenerative brain disease, or where pervasive developmental disorder is likely, neurosurgery would not be considered. A history of cerebrovascular disease or pre-existing epilepsy does not necessarily preclude neurosurgery for mental disorder. There is no evidence that personality disorders, anorexia nervosa or schizophrenia respond to neurosurgery, and patients with these disorders should not be considered unless the aim of the surgery is restricted to chronic intractable affective or obsessional comorbid symptoms. Difficulties can arise in determining the suitability of patients where illness onset was at a sufficiently early age to have had an adverse impact on personality development. Neurosurgery for mental disorder is contraindicated if the patient is not fit for surgery because of a tendency to bleed, local infection or a high anaesthetic risk.
Neurosurgical procedures
Four main procedures have been used to treat mental disorder, although only the first two are currently performed in the UK. Each procedure is conducted bilaterally:
-
• anterior capsulotomy
-
• anterior cingulotomy
-
• stereotactic subcaudate tractotomy
-
• limbic leucotomy.
Most modern procedures use an electrical current to generate heat (radio-frequency thermocoagulation) or employ gamma radiation to ablate target areas (the ‘gamma knife’). The gamma knife uses 201 distinct cobalt gamma rays that converge to deliver radiation to an accuracy of about 0.5 mm, destroying tissue only at the convergence of the beams. Among earlier techniques that are no longer employed, one of the most favoured was the implantation of radioactive yttrium seeds, which caused highly localised radioactive damage to target brain areas through the emission of beta radiation for a limited time before decaying to inert zirconium. This was the primary technique used to generate the lesions of the stereotactic subcaudate tractotomy procedure.
Anterior capsulotomy
Anterior capsulotomy (also known as ACAPS) may be performed under general or local anaesthesia, with intravenous sedation. Lesions are generated by thermal damage or focal gamma radiation. The lesions are targeted within the fibres connecting the ventromedial and orbitofrontal cortex and anterior cingulate gyrus with the thalamus, amygdala and hippocampus (Fig. 1). These fibres pass through the anterior one-third of the anterior limb of the internal capsule. Capsulotomy interrupts broadly similar frontothalamic fibres of connection, as did the stereotactic subcaudate tractotomy. Indications for anterior capsulotomy vary across Europe. In Sweden, it is used for generalised anxiety disorder, agoraphobia with panic disorder, and obsessive–compulsive disorder, whereas in the UK it is mainly used for depression and obsessive–compulsive disorder.

Fig. 1 Transverse section through human brain, showing the two capsulotomy lesions (cross-hatched areas).
Anterior cingulotomy
Anterior cingulotomy (or ACING) may be performed under general or local anaesthesia, with intravenous sedation.
The targets for cingulotomy are the supracallosal fibres of the cingulum bundle (part of the Papez circuit) as it travels through the anterior cingulate gyrus (Fig. 2). The lesion procedure also results in damage to a localised area of anterior cingulate cortex. The target site for the lesion is 20–25 mm posterior to the anterior horn of the lateral ventricles, 7 mm from the midline (Reference Spangler, Cosgrove and BallantineSpangler et al, 1996).

Fig. 2 Sagittal section through human brain, showing a cingulotomy lesion (cross-hatched area).
The procedure was initially developed for the treatment of intractable pain, but its other indications include anxiety disorders, depressive disorders and obsessive–compulsive disorder.
Stereotactic subcaudate tractotomy
Stereotactic subcaudate tractotomy represents a stereotactic modification of Scoville's ‘orbital undercutting’ method, and was developed by Geoffrey Knight in the UK (Reference KnightKnight, 1965). It is performed under general anaesthesia and involves the division of fibres connecting the orbital cortex to subcortical and limbic areas (e.g. thalamus, basal ganglia, amygdala). Lesions are placed in the white matter of the substantia innominata, below the head of the caudate nucleus. Typically, the lesions would be created using radioactive yttrium-90 rods inserted using stereotactic guidance. This procedure has been used to treat depression, obsessive–compulsive disorder, anxiety disorder and chronic pain, although it is no longer offered within the UK.
Limbic leucotomy
This procedure was first developed by Kelly and Richardson in 1973. It is performed under local anaesthesia with intravenous sedation. The fibres targeted are those of the anterior cingulate cortex, the cingulum bundle and frontostriatolimbic circuits.
Essentially, limbic leucotomy is a combination of anterior cingulotomy and stereotactic subcaudate tractotomy, although the frontal lesions are slightly smaller than those conventionally created using stereotactic subcaudate tractotomy. Its main indications are for depression, obsessive–compulsive disorder and anxiety disorder. Its place in contemporary neurosurgery for mental disorder may be as a treatment alternative where anterior cingulotomy has resulted in non-sustained or partial benefit.
Outcomes
When assessing outcomes, it is important to remember that research on neurosurgery for mental disorder has been hindered by numerous methodological difficulties – some of which have remained unresolved. Challenges include: limitations of diagnostic processes and the rigour of classification systems; low numbers treated at any single site and small populations in many studies; the ethical considerations in conducting sham or other control procedures; difficulties in identifying appropriate control groups; and the logistical challenges that tertiary referral centres experience when following up patients over many years. For these reasons, at least in part, research into neurosurgery for mental disorder tends to have presented incomplete data-sets, with an absence of independently derived outcome measures. There has probably also been a marked positive publication bias.
Outcome by procedure
Defining success as a score of 1 or 2 on the Clinical Global Impression – Improvement Score (CGI–I; Reference GuyGuy, 1976), Reference Spangler, Cosgrove and BallantineSpangler et al(1996) collated composite results for published reports up to 1996 (Table 1).
Table 1 Combined outcomes per procedure for neurosurgical procedures for mental disorder (Reference Spangler, Cosgrove and BallantineSpangler et al, 1996)
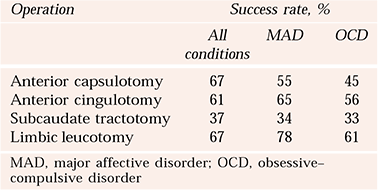
| Operation | Success rate, % | ||
|---|---|---|---|
| All conditions | MAD | OCD | |
| Anterior capsulotomy | 67 | 55 | 45 |
| Anterior cingulotomy | 61 | 65 | 56 |
| Subcaudate tractotomy | 37 | 34 | 33 |
| Limbic leucotomy | 67 | 78 | 61 |
Outcome by condition
Table 2 shows a summary of the Royal College of Psychiatrists’ (2000) report into neurosurgery for mental disorder.
Table 2 Combined outcomes by diagnosis (Royal College of Psychiatrists, 2000)
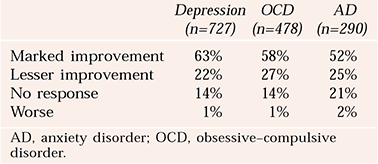
| Depression (n=727) | OCD (n=478) | AD (n=290) | |
|---|---|---|---|
| Marked improvement | 63% | 58% | 52% |
| Lesser improvement | 22% | 27% | 25% |
| No response | 14% | 14% | 21% |
| Worse | 1% | 1% | 2% |
Depression
More recent studies include one report of 6 patients with major depressive disorder (part of a group of 21 patients) who underwent limbic leucotomy. Of these 6 individuals, 2 (33%) met criteria for response when measured as a 50% reduction in Beck Depression Inventory score (Reference Montoya, Weiss and PriceMontoya et al, 2002).
Bipolar disorder
There are few studies looking at bipolar disorder specifically. Reference Lovett and ShawLovett & Shaw (1987) followed up nine patients for 5 years after stereotactic subcaudate tractotomy, showing a reduction in frequency of mood cycling. A greater response to drugs was reported after the surgery. Reference Poynton, Bridges and BartlettPoynton et al(1988) reported positive improvements in mood cycling in nine female patients after stereotactic subcaudate tractotomy, but 3 (33%) patients had ‘mild to moderate impairment of cognitive function’.
Obsessive–compulsive disorder
In the years since the College's report (Royal College of Psychiatrists, 2000), there have been further studies of the use of neurosurgery for obsessive–compulsive disorder. A study of 44 patients with obsessive–compulsive disorder undergoing stereotactic cingulotomy reported that 19 (44%) demonstrated a response or partial response (Reference Dougherty, Baer and CosgroveDougherty et al, 2002). There was an average reduction of 28.7% on the Yale–Brown Obsessive Compulsive Scale (Y–BOCS). Reference Montoya, Weiss and PriceMontoya et al(2002) report that, of 15 patients who underwent limbic leucotomy, 6 patients (42%) were ‘responders’ on the basis of improvement on the CGI, and 5 patients (33%) achieved a 35% reduction in their Y–BOCS score. A more recent study of anterior cingulotomy (n=14) revealed an average improvement of 36% on the Y–BOCS after 12 months (Reference Kim, Chang and KooKim et al, 2003).
Anxiety disorder
A recent study of thermocapsulotomy in 26 patients with generalised anxiety disorder, social phobia or panic disorder reported a response in 23 of 26 patients (92%) after 1 year, with 12 of 18 patients (67%) showing response at long-term (mean=13 years) follow-up. However, 5 patients (28%) had clinical symptoms indicating frontal lobe dysfunction, suggesting that procedure- and disorder-related factors may have featured in these cases (Reference Rück, Andreewitch and FlycktRück et al, 2003).
Side-effects
Headache (responsive to analgesia) and nausea, typically lasting a few days, are common following neurosurgical procedures for mental disorder. Confusion occurs in about 10% of cases (Reference Bridges, Bartlett and HaleBridges et al, 1994), and more frequently in those aged over 50. It usually lasts for a few days post-operatively, but can last up to 1 month before resolving. Incontinence (both urinary and faecal) occurred in up to 30% of patients after bimedial leucotomy (Reference Tan, Marks and MarsetTan et al, 1971). It has been reported after limbic leucotomy and anterior capsulotomy, but rates are lower. It usually resolves within a week or so, but it can persist for up to a month.
Insomnia is not uncommon and transient somnolence may also occur. Apathy and anergia are often reported in the post-operative period in up to 24% of patients (Reference Montoya, Weiss and PriceMontoya et al, 2002), in some cases enduring for over 12 months (Reference Mitchell-Heggs, Kelly and RichardsonMitchell-Heggs et al, 1976).
Seizures occur in about 1–6% of cases (Reference Ström-Olsen and CarlisleStröm-Olsen & Carlisle, 1971; Reference Ballantine, Bouckoms and ThomasBallantine et al, 1987; Reference Spangler, Cosgrove and BallantineSpangler et al, 1996), rarely progressing to epilepsy. In some cases, the seizures only occur in the first week post-operatively. Patients are advised not to drive for 6 months after the procedure.
Weight gain has been reported in 12.5% (3/24) after bimedial leucotomy (Reference Tan, Marks and MarsetTan et al, 1971), 6.2% (13/210) after stereotactic tractotomy (Reference Ström-Olsen and CarlisleStröm-Olsen & Carlisle, 1971), 21% (3/14) after anterior capsulotomy (Reference Kim, Chang and KooKim et al, 2003) and 5.6% (1/18) after anterior cingulotomy (Reference Baer, Rauch and BallantineBaer et al, 1995). In many cases, the weight gain resolves by 3 months, but the gain can be up to 10–15 kg and may persist. It tends to occur more frequently in women.
Memory and concentration problems are reported as occurring more commonly in older studies involving procedures such as prefrontal leucotomy. There are a few later reports of memory deficits occurring in 5% of patients, which take 6–12 months to resolve (Reference Dougherty, Baer and CosgroveDougherty et al, 2002). A study of cognitive function in 23 patients who underwent subcaudate tractotomy found significant impairment of cognitive function 2 weeks post-operatively, which had largely resolved after 6 months (Reference Kartsounis, Poynton and BridgesKartsounis et al, 1991). An earlier series of 66 patients undergoing limbic leucotomy found that WAIS–IQ scores increased 6 weeks post-operatively, possibly owing to the impairing effects of symptoms on previous cognitive performance (Reference Mitchell-Heggs, Kelly and RichardsonMitchell-Heggs et al, 1976). There were no clear personality deficits noted.
Personality change
Personality change is often quoted to occur after neurosurgery for mental disorder, and undoubtedly it occurred after the indiscriminate and crude procedures of the first half of the 20th century. Whether it happens now is less certain. Valid, sensitive measures of personality that are designed for repeated testing are unavailable. Also, it is helpful to consider that some apparent personality changes described post-surgery can be due to the return of premorbid personality function. Recovery after many years of illness can pose as great a challenge to the established roles of carers and relatives as it does for the patient.
In a series of more than 1300 patients who underwent subcaudate tractotomy, there was no report of significant personality change (Reference Bridges, Bartlett and HaleBridges et al, 1994). ‘Significant’ was not defined. Reference Goktepe, Young and BridgesGoktepe et al(1975) reported rates of personality change of 6.7% when reported by relatives, but it is difficult to differentiate between irreversible personality damage and unmasking of premorbid personality traits.
Suicide
Reported suicide rates in depression range from 6% to 15%. Most of the patients undergoing neurosurgery for mental disorder have had chronic severe illness, often with a history of suicide attempts. Suicide rates per procedure are as follows:
-
• anterior capsulotomy: 4% (Reference Rück, Andreewitch and FlycktRück et al, 2003)
-
• anterior cingulotomy: 2% (Reference Dougherty, Baer and CosgroveDougherty et al, 2002); 9% (Reference Ballantine, Bouckoms and ThomasBallantine et al, 1987)
-
• subcaudate tractotomy: 1% (Reference Bridges, Bartlett and HaleBridges et al, 1994)
-
• limbic leucotomy: 10% (Reference Montoya, Weiss and PriceMontoya et al, 2002).
Who should be referred for neurosurgery?
Adequate treatment trials are those that have involved prescription of drugs shown to be effective, at doses at or above the British National Formulary (BNF) dose range, for at least 6 weeks in depression and 12–16 weeks in obsessive–compulsive disorder. Patients should also have undergone trials of appropriate psychological therapy. In the case of depression, patients should also have had at least two separate courses of electroconvulsive treatment (Reference Matthews and EljamelMatthews & Eljamel, 2003). For most patients, this means at least 5 years of continuous, vigorous treatment of their illness, using a combination of pharmacological, psychological and socially based treatment strategies.
Post-surgery
Most patients spend at least 2–3 weeks in hospital following neurosurgery for mental disorder. Thereafter, they return to their hospital or service of referral for a period of agreed rehabilitation. Unless comprehensive rehabilitation plans have been agreed, surgery will not be carried out. For the majority of patients, response and/or recovery following neurosurgery for mental disorder (if it takes place) is slow, although most patients who do eventually demonstrate a clinical response will do so within the first 9–12 months. Some patients have difficulty with the lack of dramatic response, given that they have been unwell for so long, and the recovery phase may involve set-backs.
Like other providers of neurosurgery for mental disorder, we believe that, for some patients, neurosurgery modifies responsiveness to other ‘standard’ treatments such that they may merit a revisit during the post-operative period. Whenever possible, patients at Ninewells Hospital undergo detailed review 12 and 24 months after the procedure. Longer-term follow-up, although desirable, is not always possible. Following surgery, a shared-care arrangement is negotiated such that the neurosurgery for mental disorder (NMD) service retains an interest and involvement in ongoing management.
New developments in functional neurosurgery
Neurosurgical interventions for psychiatric disorders have always been influenced by advances in other therapeutic areas. Recently, two potentially attractive developments have emerged from the neurosurgical management of epilepsy and movement disorders respectively. Vagus nerve stimulation, a treatment for intractable epilepsy, involves the direct electrical stimulation of the vagus nerve in the neck with the intention of modifying activity in central brain circuitry. Deep brain stimulation, extensively used in the management of intractable movement disorders, entails direct electrical stimulation and modulation of specific brain areas. Both procedures are potentially reversible and do not involve the destruction of brain tissue. As the stimulation can be switched on or off, it is, potentially, possible to conduct relatively pure double-blind controlled studies of efficacy.
Vagus nerve stimulation
Neuroscientists have tried to develop non-invasive methods of stimulating the brain for over a century. Vagus nerve stimulation was postulated as a method of stimulating higher brain activity as early as 1938. The first human vagus nerve stimulation implant was performed in 1988 for treatment of epilepsy. Since that time, the procedure has become established for treatment-resistant, partial-onset seizure disorder, and over 15 000 patients worldwide had received implants by mid-2003.
The vagus nerve
The vagus nerve is not only (as most will recall from medical school days) a parasympathetic efferent nerve – around 80% of its fibres are afferent sensory fibres transmitting information to the brain. There are sensory afferent connections of the vagus nerve in the nucleus tractus solitarius that, in turn, send ascending projections to the forebrain, mainly through the parabrachial nucleus and locus ceruleus. Further connections offer potential routes of communication with the amygdala, hypothalamus, insula, thalamus, orbitofrontal cortex and other important limbic regions linked to mood regulation.
Practicalities of vagus nerve stimulation
In humans almost all reference to vagus nerve stimulation involves stimulation of the left cervical vagus nerve using a commercial device, the NeuroCybernetic Prosthesis (NCP®) system, manufactured by Firma Cyberonics Inc. (Webster, Texas, USA). Vagus nerve stimulation has been available commercially for treatment of resistant, partial-onset epileptic seizures since 1994 in Europe and 1997 in the USA. The procedure involves subcutaneous implantation of a pulse generator similar to a cardiac pacemaker in the left anterior chest wall. Bipolar electrodes are wrapped around the left vagus nerve in the neck, near to the carotid artery, and are connected to the stimulus generator. Thereafter, the treatment involves an intermittent stimulation of the afferent fibres within the vagus nerve, with the intensity and frequency of generator firing being controllable through the use of a telemetric wand connected to a personal computer.
Outcomes in treatment-resistant depression
Two multi-centre open pilot studies have been performed looking at treatment of refractory depression using the NCP® system (Reference Rush, George and SackeimRush et al, 2000; Reference Sackeim, Rush and GeorgeSackeim et al, 2001). Combining the two studies, 18 of 59 subjects (31%) were deemed to have shown a ‘clinical response’ within 12 weeks (defined as a ≥50% reduction in score on the 28-item Hamilton Rating Scale for Depression (HRSD28)). Of these, 9 (15%) met criteria for ‘complete response’ (HRSD28≤10). Three (5%) participants worsened during the treatment period. A follow-up study found that 13 of 28 subjects (46%) met criteria for ‘clinical response’ after 12 months of vagus nerve stimulation, 10 of whom were also ‘responders’ at 12 weeks (Reference Marangell, Rush and GeorgeMarangell et al, 2002). Early indications from these open, non-controlled studies suggest that vagus nerve stimulation may be most effective in patients with less chronic and treatment-refractory forms of depression.
Adverse effects
Treatment-related adverse effects usually relate directly to times when the vagus nerve is being stimulated. Hoarseness is common, but throat or neck pain, cough, dyspnoea and headache can also occur. Reference Marangell, Rush and GeorgeMarangell et al(2002) reported significant reduction in side-effects over the course of 12 months’ stimulation and also that no patient discontinued the stimulation because of adverse effects. Reducing the intensity or frequency of stimulation may improve adverse effects. Patients are also supplied with magnets that can turn off stimulation temporarily when held over the pulse generator.
The only reports of cardiac effects are of 6 instances of asystole lasting 10–20 s during implantation and first stimulation, none of which resulted in long-term sequelae. There have been isolated reports of hypomania occurring during vagus nerve stimulation (Reference Marangell, Rush and GeorgeMarangell et al, 2002).
The future for vagus nerve stimulation
Vagus nerve stimulation remains an experimental treatment and definitive treatment outcome studies are awaited. The long-term effects of the procedure, and its place in the treatment armamentarium, are uncertain, although initial signs are encouraging and the adverse effect profile appears to be favourable.
Deep brain stimulation
Deep brain stimulation is an established treatment method for movement disorders, especially Parkinson's disease, where stimulation of the subthalamic nucleus or the globus pallidus can alleviate symptoms. Deep brain stimulation has also been used to treat chronic pain, and it may be helpful for patients with intractable epilepsy (Reference Loddenkemper, Pan and NemeLoddenkemper et al, 2001).
Experience of deep brain stimulation as a form of neurosurgery for mental disorder has confirmed that chronic obsessive–compulsive disorder symptoms can be temporarily alleviated by electrical stimulation of the anterior limbs of the internal capsules (Reference Nuttin, Cosyns and DemeulemeesterNuttin et al, 1999).
Practicalities of deep brain stimulation
Electrodes are implanted bilaterally in the anterior limbs of the internal capsule under anaesthesia using stereotactic guidance, positioning being confirmed by post-operative computerised tomography or magnetic resonance imaging. The stimulation parameters are optimised in the weeks after surgery on the basis of symptomatic reduction in obsessive thoughts or anxiety and improvements in mood. Battery-operated programmable generators are then implanted subcutaneously in the abdomen and connected to the stimulating electrodes by subcutaneous wires.
Outcomes in treatment-resistant obsessive–compulsive disorder
By mid-2003, about 20 patients worldwide had received deep brain stimulation implants in the anterior internal capsule for treatment-resistant obsessive–compulsive disorder (Reference Gabriëls, Cosyns and MeyersonGabriëls et al, 2003). Follow-up of four patients with obsessive–compulsive disorder who had electrode implants in both anterior limbs of the internal capsules found that three showed at least 35% reduction in their baseline Y–BOCS scores with treatment. Mean Clinical Global Impression – Severity scores for the four subjects decreased from 5 to 3.3 during the ‘stimulation on’ period (Reference Gabriëls, Cosyns and MeyersonGabriëls et al, 2003). In one patient, there was marked improvement in depressive symptoms, which returned intensely with suicidality after stimulation was discontinued. Comparing the cases, there was wide variation in stimulation parameters, and comment was made regarding problems with battery life.
Reference Mallet, Mesnage and HouetoMallet et al(2002) reported significant improvement in obsessive and compulsive symptoms in two patients with obsessive–compulsive disorder and Parkinson's disease who had electrodes implanted in the area of the anteromedial part of the subthalamic nucleus and zona incerta. This raises interesting questions about selecting the optimal sites for deep brain stimulation in psychiatric disorders.
Deep brain stimulation and mood
There are few studies on the effect of deep brain stimulation on mood. A study of 12 patients with Parkinson's disease who received deep brain stimulation of the subthalamic nucleus reported improvements in self-reported mood, HRSD scores and emotional memory during stimulation, with no change in cognitive performance (Reference Schneider, Habel and VolkmannSchneider et al, 2003). Conversely, Reference Berney, Vingerhoets and PerrinBerney et al(2002) reported a significant worsening of mood state in 6 of 24 patients who received subthalamic nucleus deep brain stimulation for Parkinson's disease. There have been reports, during deep brain stimulation, of transient depression (Reference Bejjani, Damier and ArnulfBejjani et al, 1999), involuntary laughter (Reference Kumar, Krack and PollakKumar et al, 1999) and mania (Reference Kulisevsky, Berthier and GironellKulisevsky et al, 2002). There are, as yet, no published reports of deep brain stimulation as a treatment for depression.
Conclusions
In the foreseeable future, neurosurgery for mental disorder will remain a highly specialised treatment alternative for a small number of patients with severe and chronic treatment-refractory anxiety and depressive disorders. Ablative neurosurgery for mental disorder will never be validated by classical randomised controlled trials, but can be evaluated by careful prospective audit. Detailed pre- and post-operative assessments, including advanced neuropsychology and neuroimaging, will help us to evaluate neurosurgical procedures and their outcomes. Mechanisms of therapeutic action may be identified. The use of irreversible ablative treatments may diminish with the introduction of potentially less hazardous reversible interventions such as vagus nerve stimulation and deep brain stimulation. Substantive, robust, controlled and blinded trials involving vagus nerve stimulation and deep brain stimulation may be possible.
Multiple choice questions
-
1 The following are current indications for neurosurgery for mental disorder:
-
a obsessive–compulsive disorder
-
b schizophrenia
-
c social phobia
-
d major depression
-
e bipolar affective disorder.
-
-
2 Neurosurgical procedures for mental disorder performed in the UK today include:
-
a limbic leucotomy
-
b anterior capsulotomy
-
c subcaudate tractotomy
-
d cingulotomy
-
e amygdalotomy.
-
-
3 The following names are correctly associated with the procedure they pioneered:
-
a Lars Leksell – limbic leucotomy
-
b Geoffrey Knight – subcaudate tractotomy
-
c Desmond Kelly – anterior capsulotomy
-
d Foltz and White – anterior cingulotomy
-
e Walter Freeman – transorbital leucotomy.
-
-
4 The following statements are correct:
-
a anterior capsulotomy targets frontothalamic circuits
-
b anterior cingulotomy interrupts fibres just below the head of the caudate nucleus
-
c confusion occurs in 80% of patients following surgery
-
d all procedures have a high intra-operative mortality
-
e seizures occur in about 2% of patients after surgery.
-
-
5 Rate the following statements as true or false:
-
a in vagus nerve stimulation, the vagus nerve is stimulated in the brain-stem
-
b a frequent side-effect of vagus nerve stimulation is altered voice
-
c deep brain stimulation causes impaired cognitive performance
-
d deep brain stimulation for obsessive–compulsive disorder involves implanted electrodes in the same area as the lesions in anterior capsulotomy
-
e deep brain stimulation can be switched on and off.
-
MCQ answers

| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | T | a | F | a | F | a | T | a | F |
| b | F | b | T | b | T | b | F | b | T |
| c | F | c | F | c | F | c | F | c | F |
| d | T | d | T | d | T | d | F | d | T |
| e | T | e | F | e | T | e | T | e | T |







eLetters
No eLetters have been published for this article.