Neuroimaging is traditionally divided into structural and functional imaging. Structural imaging looks at brain structure or anatomy and includes computed tomography (CT) and magnetic resonance imaging (MRI). Functional techniques seek to examine the physiological functioning of the brain, either at rest or during activation, and include single photon emission computed tomography (SPECT), positron emission tomography (PET), MRI spectroscopy, functional MRI (fMRI) and encephalographic brain mapping. Although fMRI, MRI spectroscopy and brain mapping are likely to have clinical applications in the near future, the main imaging modalities of current clinical relevance to psychiatrists are CT, MRI and SPECT, which will be the focus of this article.
Computed tomography
Computed tomography scanning is widely available, inexpensive (approximately £80 per scan) and quick (a whole-head CT can be performed in under 30 seconds using modern scanners). This means it is very well-tolerated by patients and also suitable for restless and uncooperative patients, such as those in the moderate to severe stages of dementia. It is good for excluding most space-occupying brain lesions, such as tumours or subdural haematomas. However, images are limited to axial (or angled axial) views, soft tissue lesions are not always well-visualised, and patients are exposed to radiation. Nevertheless, because of wide availability, good tolerability and (relatively) low cost, CT is almost universally the first imaging modality to which most psychiatrists have access.
Magnetic resonance imaging
Magnetic resonance imaging utilises the electromagnetic properties of protons to construct a spatial representation of tissue. The main types of MRI sequences and their relative advantages are summarised in Box 1. Good anatomical resolution is provided by heavily T1-weighted images, whereas proton density- and T2-weighted images are sensitive to changes in water content and provide good visualisation of white matter lesions and smaller infarcts. The cerebrospinal fluid spaces appear dark on proton density- and T1-weighted images (as on CT) and bright on T2-weighted images. In practice, a combination of sequences is often used to provide optimum anatomical and pathological differentiation.
Box 1. Main types of MRI sequences
T1-weighted
Provides good definition of anatomy
T2-weighted
Provides high contrast and definition of soft tissue pathology
Proton density-weighted
Provides good brain/cerebrospinal fluid contrast
The different advantages and disadvantages of MRI and CT are summarised in Box 2. Magnetic resonance imaging provides a sensitive method to study brain morphology, white matter and vascular pathology and abnormalities associated with epilepsy, such as medial temporal sclerosis. The improved resolution and superior soft tissue contrast of MRI may offer greater potential for early diagnosis. Moreover, MRI can examine the structure of the whole brain in any plane and generate three-dimensional images suitable for detailed volumetric quantification. As MRI does not use ionising radiation it is more suited for serial monitoring and the study of normal controls. The noise and confinement of the scanner is poorly tolerated in approximately 1 in 20 subjects. The main contraindication of MRI is the presence of ferromagnetic materials such as cardiac pacemakers and intracranial aneurysm clips. Orthopaedic implants are not contraindicated although may introduce local artefacts.
Box 2. Comparison between MRI and CT
MRI CT
Higher spatial and anatomical resolution Shorter scan times
Superior soft tissue definition and contrast Widely available
Image in multiple planes; therefore Lower cost
superior views of middle and posterior Better tolerated
fossa, pituitary, brain stem and spinal cord Limited to axial plane
Uses non-ionising radiation Good for bone abnormalities
Wide application for quantitative analysis Better for lesions with little or no water
Greater sensitivity to detect white mattercontent (e.g. meningiomas) and detection
pathology and lesions causing epilepsy of acute intracerebral haemorrhage
SPECT scanning
This is an emission, rather than transmission, technique and involves the injection of a radioisotope that distributes in brain and emits gamma rays (photons), which are detected by gamma cameras. Depending on the choice of radioligand (or tracer), different brain functions can be demonstrated, and SPECT has the advantage over structural imaging techniques that the images provide some measure of cerebral function. The most common SPECT tracer is Tc-HMPAO (technetium hexamethylpropylene amine oxime; Ceretec), which is taken up in brain within 2–5 minutes of intravenous injection and distributes in proportion to blood flow. Once in the brain, the ligand undergoes a transformation that fixes it for several hours until imaging can be performed, therefore Tc-HMPAO SPECT provides a ‘snapshot’ of cerebral blood flow a few minutes after injection. Brain blood flow has been shown to be closely coupled with brain metabolism as determined by glucose and oxygen utilisation and so Tc-HMPAO SPECT scans are regarded as a good measure of brain function.
Disadvantages of SPECT include the fact that, unlike PET, imaging is not performed in real time. Resolution is poor (10–15 mm) and, like CT, there is a need for exposure to radiation. Scanning takes 10–40 minutes, limiting its utility for restless or uncooperative patients. Because of the poor resolution and the similar appearances of some lesions, despite different pathologies (i.e. severe hypoperfusion on SPECT may be due to either infarction or degenerative change), accurate interpretation of a SPECT image almost invariably requires availability of a contemporary CT scan. Other applications of SPECT are hampered by the lack of suitable radioligands, although one for the dopaminergic D2-receptor (IBZM) and one for the dopamine transporter (FP–CIT) may become available in clinical practice this year.
Uses of neuroimaging in psychiatry
The primary purpose of performing a brain scan on someone presenting with cognitive impairment, depression or other psychiatric disorder is to exclude an intracerebral lesion as a cause for his or her symptoms. Such lesions include subdural haematomas (e.g. those which can occur even without a history of head injury), space-occupying lesions (tumours and abscesses), normal pressure hydrocephalus, infarcts and haemorrhages. Neuroimaging changes can also be supportive, not only by excluding conditions that may present with psychiatric disturbance but also, particularly for the different types of dementia, by providing supportive features that may assist with diagnosis. Although the syndrome of dementia is a clinical diagnosis, classification of the cause (e.g. Alzheimer's disease, vascular dementia, dementia with Lewy bodies, frontotemporal dementia and others) can ultimately be made only at post-mortem. Diagnosis during life is made after a careful assessment process that includes full history, physical and mental state examination, detailed cognitive assessment and appropriate use of investigations, which increasingly include neuroimaging. Current clinical diagnostic criteria contain all these elements. Although the availability of neuroimaging is increasingly important for the application of such criteria (see below), it should be emphasised that no imaging marker should be regarded as an absolute diagnostic test for any subtype of dementia at the current time.
Typical neuroimaging changes in the different subtypes of dementia are summarised in Table 1.
Alzheimer's disease
CT scanning
Although atrophy on CT is seen in around two-thirds of cases, CT appearance can be normal, particularly in the early stages of the disorder. As such, CT is more often used to exclude the diagnosis of Alzheimer's disease (for example, if infarcts are seen then a diagnosis of only ‘possible’ rather than ‘probable’ Alzheimer's disease could be made) than to support it. Standard CT cannot reliably differentiate Alzheimer's disease from normal ageing or other causes of cognitive impairment. However, standard CT involves imaging in the axial plane parallel to the orbito-meatal line. It is possible to perform angled CT, at 20–25° to this line, through the temporal lobes, and if thin (2–4 mm) slices are performed a good view of the medial and lateral temporal lobe structures can be obtained (Fig. 1). It has been suggested that a measurement of the minimal width of the medial temporal lobe on CT (between the anterior and posterior margins of the brain stem) is a sensitive and specific marker for Alzheimer's disease (Reference Jobst, Barnetson and ShepstoneJobst et al, 1998). A cut-off of around 11 mm was found to differentiate those with Alzheimer's disease from normal controls. However, although this may be useful in separating those with dementia from normal controls and non-organic causes of cognitive impairment (e.g. depression), its usefulness in separating Alzheimer's disease from other types of dementia such as vascular dementia and dementia with Lewy bodies remains to be shown (Reference O'Brien, Metcalfe and BallardO'Brien et al, 1999). Cross-sectional measures are invariably limited by the huge variability between individuals that often masks important differences between disease states. Several studies suggest that longitudinal changes between successive scans (e.g. an increase in atrophy) are useful in identifying those with Alzheimer's disease (Reference Jobst, Smith and SzatmariJobst et al, 1994; Reference Fox, Freeborough and RossorFox et al, 1996).
MRI scanning
Because of the increased resolution of MRI, interest has shifted to its use in the early diagnosis of Alzheimer's disease, the detection of subjects at risk of developing Alzheimer's disease, and the differentiation of Alzheimer's disease from other disorders. The most consistent and widely described structural change in patients with a clinical diagnosis of Alzheimer's disease is atrophy of medial temporal lobe structures, as shown in Fig. 2. Patients with Alzheimer's disease have significantly smaller hippocampi (10–50% reduction), amygdalae (up to 40% reduction) and parahippocampi (up to 40%) than age-matched controls.
There is convincing evidence that atrophy of medial temporal lobe structures, especially hippocampus and entorhinal cortex, occurs early in the natural history of the illness. By the time mild symptoms are apparent, hippocampal volume reductions may have already exceeded 25%. Clinically, in vivo hippocampal volume loss correlates with symptoms of memory loss and pathologically with underlying neurofibrillary tangle pathology.
However, although medial temporal lobe atrophy provides good discrimination between patients with Alzheimer's disease and controls, as with CT it is not specific to Alzheimer's disease and has been described in a number of other conditions including other dementias, Parkinson's disease (Reference Laakso, Partanen and RiekkinenLaakso et al, 1996), temporal lobe epilepsy and schizophrenia. However, the volume reductions are less extensive than with Alzheimer's disease.
SPECT scanning
Characteristic SPECT changes associated with Alzheimer's disease include temporo-parietal hypoperfusion (Reference O'Brien, Eagger and SyedO'Brien et al, 1992; Reference Jobst, Barnetson and ShepstoneJobst et al, 1998). Such change can separate those with Alzheimer's disease from normal controls and those with other causes of cognitive impairment in 60–90% of cases (Reference O'Brien, Eagger and SyedO'Brien et al, 1992; Reference Read, Miller and MenaRead et al, 1995; Reference Jobst, Barnetson and ShepstoneJobst et al, 1998).
The relative place of structural and functional imaging in the diagnosis of Alzheimer's disease remains to be determined. In practice, structural imaging is usually performed first and may be helpful in supporting the diagnosis of Alzheimer's disease if temporal lobe atrophy is seen (especially on MRI). However, SPECT may be helpful in supporting the diagnosis of Alzheimer's disease either in cases where structural imaging is normal or equivocal or when MRI is not available.
Vascular dementia
CT and MRI
Several studies have compared structural imaging changes between patients with vascular dementia and Alzheimer's disease. Degree of cortical atrophy or ventricular enlargement does not seem to separate the groups, although vascular dementia is invariably associated with an increased prevalence of infarcts and more extensive white matter change. Evidence from studies of patients with established vascular dementia suggests that vascular disease is associated with cortical and central atrophy and that both the volume and location of lesions are important. In particular, bilateral, left-sided lesions, diffuse white matter change and small infarcts in strategic areas may all be important in causing cognitive impairment.
SPECT scanning
In vascular dementia, a variable pattern of perfusion on SPECT is seen. Classically, a ‘patchy’ multi-focal pattern is seen, corresponding to perfusion deficits, but that depends on the actual site of vascular lesions (Reference Read, Miller and MenaRead et al, 1995). Large cortical infarcts are represented by areas of absent blood flow. Greater diffuse white matter change can be associated with greater generalised cortical reduction in blood flow, with some regional variations that depend both on the site of lesion and projection pathways. For example, infarcts in the basal ganglia can result in reduced cortical blood flow to frontal lobes, consistent with deafferentation of the main projection pathways. At present, it is probably sensible to reserve the use of SPECT as an investigation when information from clinical assessment and structural imaging is not definitive enough to confirm or refute a diagnosis of vascular dementia.
Imaging changes needed for diagnosis
With vascular dementia, even more so than with Alzheimer's disease, the extent of imaging that may be considered necessary in individual patients very much depends on which set of diagnostic criteria is being applied. Imaging requirements for the application of several current sets of diagnostic criteria for vascular dementia are summarised in Box 3. The NINDS/AIREN criteria (National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l'Enseignement Neurosciences; Reference Roman, Tatemichi and ErkinjunttiRoman et al, 1993) are perhaps the most rigorous with regard to the need for neuroimaging confirmation. The criteria for probable vascular dementia include multiple infarcts or strategic single infarcts as well as multiple subcortical or white matter lesions. The criteria specifically state that the absence of cerebrovascular lesions on brain CT or MRI makes the diagnosis of vascular dementia uncertain (Box 4).
Box 3. Neuroimaging requirements for application of current diagnostic systems
Diagnostic system Vascular brain changes required on structural imaging criteria
Hachinski Ischaemic Scale (Reference Hachinski, Iliff and ZilhkaHachinski et al, 1975)
No requirement for brain imaging.
ICD–10 (World Health Organization, 1992)
In some cases, confirmation can be provided only by computerised axial tomography or, ultimately, neuropathological examination.
In addition, the ICD–10 research diagnostic criteria require evidence from the history, examination, or tests of significant cerebrovascular disease that may be reasonably be judged to be aetiologically related to the dementia (e.g. a history of stroke or evidence of cerebral infarction).
DSM–IV (American Psychiatric Association, 1994)
There must be evidence of cerebrovascular disease (i.e. focal neurological signs and symptoms or laboratory evidence) that is judged to be aetiologically related to the dementia. CT of the head and MRI usually demonstrate multiple vascular lesions of the cerebral cortex and subcortical structures. The extent of central nervous system lesions detected by CT and MRI in vascular dementia typically exceeds the extent of changes detected in the brains of healthy elderly persons (e.g. periventricular and white matter hyperintensities noted on MRI scans). Lesions often appear in both white matter and grey matter structures, including subcortical regions and nuclei. Evidence of old infarctions (e.g. focal atrophy) as well as of more recent disease may be detected.
California Criteria (Reference Chui, Victoroff and MargolinChui et al, 1992)
The criteria for the clinical diagnosis of probable ischaemic vascular dementia (IVD) include evidence of two or more ischaemic strokes by history, neurological signs and/or neuroimaging studies (CT or T1-weighted MRI) and evidence of at least one infarct outside the cerebellum on CT or T1-weighted MRI.
The diagnosis of probable IVD is supported by evidence of multiple infarcts in brain regions known to affect cognition. Features that are thought to be associated with IVD, but await further research, include periventricular and deep white matter changes on T2-weighted MRI that are excessive for age.
A clinical diagnosis of possible IVD may be made when there is dementia and Binswanger's syndrome (without multiple strokes) that includes extensive white matter changes on neuroimaging.
NINDS/AIREN Criteria (Reference Roman, Tatemichi and ErkinjunttiRoman et al, 1993)
The criteria for the clinical diagnosis of probable vascular dementia require the presence of cerebrovascular disease (CVD), defined by the presence of focal signs on neurological examination and evidence of relevant CVD by brain imaging (CT or MRI) including multiple large-vessel infarcts (angular gyrus, thalamus, basal forebrain, or posterior or anterior cerebral artery territories), as well as multiple basal ganglia and white matter lesions, or combinations thereof.
Features that make the diagnosis of vascular dementia uncertain or unlikely include absence of cerebrovascular lesions on brain CT or MRI.
Box 4. Brain imaging lesions associated with vascular dementia (NINDS/AIREN Criteria)
Topography
Radiological lesions associated with dementia include any of the following or combinations thereof:
Large-vessel strokes in the following territories:
Bilateral anterior cerebral artery
Posterior cerebral artery, including paramedian thalamic infarctions, inferior medial temporal lobe lesions
Association areas: parietotemporal, temporo-occipital territories (including angular gyrus)
Watershed carotid territories: superior frontal and parietal regions
Small-vessel disease:
Basal ganglia and frontal white matter lacunes
Extensive periventricular white matter lesions
Bilateral thalamic lesions
Severity
In addition to the above, relevant radiological lesions associated with dementia include:
Large-vessel lesions of the dominant hemisphere
Bilateral large-vessel hemispheric strokes
Leukoencephalopathy involving at least one-quarter of the total white matter
Although volume of lesion is weakly related to dementia, an additive effect may be present.
White matter changes observed only on T2- but not on T1-weighted MRI, or CT, may not be significant
Absence of vascular lesions on brain CT/MRI rules out probable vascular dementia
Perhaps one of the more difficult areas is the role of white matter change alone. Although white matter changes seen on imaging can be caused by vascular disease, they are in fact pathologically heterogeneous. The NINDS/AIREN criteria deal with this by stating that white matter lesions on CT or MRI may be considered as evidence for cerebrovascular disease, but for this to be the case they must be diffuse and extensive, extending to deep white matter. The differences between infarcts and white matter changes on MRI are summarised in Box 5 and Fig. 3. Changes observed only on T2-weighted MRI are felt to be of doubtful significance. Importantly, the criteria suggest that the changes should involve at least a one-quarter of the total of white matter, based on previous studies (Reference Erkinjuntti, Ketonen and SulkavaErkinjuntti et al, 1987). The discussion emphasises the need to correlate radiological images with clinical and neuropsychological changes combined with neuropathology in patients with vascular dementia, and prospective validation of the NINDS/AIREN and other criteria is certainly needed.
Box 5. Differences between cerebral infarcts and deep white matter hyperintensities on MRI
Infarcts Deep white matter hyperintensities
Hypointense on T1 and proton density-Hyperintense on T2- and proton density- weighted imagesweighted images
Hyperintense on T2-weighted images No change/invisible on T1-weighted images
Well-defined; wedge shape if peripheral Range from punctuate to diffuse confluent lesions
Often single or low numbers Often multiple
Often evidence of cortical extension Restricted to white matter with no cortical extension
May be associated with focal enlargement of ventricles and sulci Ventricles and sulci unchanged
Dementia with Lewy bodies
CT and MRI
So far, MRI studies have shown dementia with Lewy bodies to be associated with relative preservation of temporal lobe structures compared with Alzheimer's disease (Reference Hashimoto, Kitagaki and ImamuraHashimoto et al, 1998; Reference Barber, Gholkar and ScheltensBarber et al, 1999a ; Reference Harvey, O'Brien and HughesHarvey et al, 1999). Initial reports of frontal atrophy in subjects with Lewy body pathology were not confirmed by a recent study (Reference Harvey, O'Brien and HughesHarvey et al, 1999). Dementia with Lewy bodies does not seem to differ from Alzheimer's disease in terms of white matter changes on MRI (Reference Barber, Scheltens and GholkarBarber et al, 1999b ).
In summary, the limited evidence available suggests that structural imaging in dementia with Lewy bodies reveals similar generalised atrophic changes to Alzheimer's disease in most cases, although approximately 40% of subjects show preservation of medial temporal lobe structures.
Functional imaging changes in dementia with Lewy bodies
In Parkinson's disease, decreased blood flow in basal ganglia occurs and, when associated with dementia, biparietal changes similar to those seen in Alzheimer's disease are reported. Similarly, in the few SPECT studies of dementia with Lewy bodies, similar patterns of blood flow changes to Alzheimer's disease have been found – although Donnemiller et al (1997) also found a subtle difference in perfusion patterns with a greater degree of occipital hypoperfusion in dementia with Lewy bodies compared with Alzheimer's disease.
The more powerful, although still research-based, use of SPECT involves specific ligands for different neurochemical systems. Ligands have been developed for pre- and post-synaptic dopaminergic and cholinergic systems. Donnemiller et al (1997) found significant differences between dementia with Lewy bodies and Alzheimer's disease in beta-CIT binding (a ligand for the dopamine transporter) – differences that would be predicted from known neurochemical differences between Alzheimer's disease and dementia with Lewy bodies. Reduced D2-receptor density in basal ganglia using IBZM has also been reported in dementia with Lewy bodies (Reference Walker, Costa and JanssenWalker et al, 1997). Using a marker of the choline transporter, significant differences between Alzheimer's disease subjects and controls as well as between Parkinson's disease subjects with and without dementia have been found (Reference Kuhl, Minoshima and FesslerKuhl et al, 1996). In summary, current evidence suggests that blood flow SPECT shows similar appearances in dementia with Lewy bodies to those seen in Alzheimer's disease although, as with Alzheimer's disease, SPECT may still be useful in distinguishing dementia with Lewy bodies from frontal lobe dementia or vascular dementia. However, new chemical imaging techniques, although not yet available in practice, show great promise in differentiating dementia with Lewy bodies from other disorders and are an exciting area of current research.
Specific lobar atrophies
Focal atrophy of the frontal or frontotemporal lobes on CT or MRI may be helpful in the diagnosis of frontotemporal dementia and has recently been incorporated into consensus diagnostic criteria (Reference Neary, Snowden and GustafsonNeary et al, 1998). However, in general, although such lobar atrophy is a fairly specific finding (i.e. when it occurs it lends strong support to the diagnosis), it is not particularly sensitive and may be lacking in up to 50% of cases that have the diagnosis. Similarly, anterior, rather than posterior, perfusion deficits are characteristic of frontotemporal dementia and are thought to be a more sensitive marker of the disorder than structural changes and similarly form part of recently proposed diagnostic criteria (Reference Neary, Snowden and GustafsonNeary et al, 1998).
Depression
Studies in the early 1980s demonstrated that depression in late life is associated with structural changes, including ventricular enlargement on CT. These findings have generally been confirmed and similar changes are also known to occur in younger patients with unipolar and bipolar disorder (Reference VidebechVidebech, 1997). These abnormalities are non-specific and recent interest has focused on other changes, such as the presence of volume reductions in the caudate (Reference Krishnan, McDonald and EscalonaKrishnan et al, 1992) and frontal lobe (Reference Coffey, Wilkinson and WeinerCoffey et al, 1993; Reference Drevets, Price and SimpsonDrevets et al, 1997). However, the most consistent abnormality described in depression, in particular late-life depression, comes from the many studies that have repeatedly shown an increase in the number and/or severity of signal hyperintensities in the white matter in both unipolar and bipolar disorder (Reference Rabins, Pearlson and AylwardRabins et al, 1991; Reference O'Brien, Desmond and AmesO'Brien et al, 1996). White matter lesions are found scattered in the deep white matter, the periventricular white matter, the basal ganglia and the pons. They may be divided on the basis of scan appearance and neuropathological substrate into those adjacent to the ventricular system (periventricular lesions; see Fig. 3) and those distinct from the ventricles and located in deep white matter (deep white matter lesions; see Fig. 3). Rabins et al (1991) showed a particular increase in deep white matter lesions in elderly patients with unipolar disorder, with the predominance of lesions in the frontal lobes and basal ganglia. The location of these lesions fits with functional imaging changes suggesting frontal and caudate abnormalities in depression and supports the notion of ‘frontostriatal dysfunction’ in depression. The increase in white matter lesions in those with depression may be most marked for those presenting with their first ever depression in late life (Reference O'Brien, Desmond and AmesO'Brien et al, 1996). Such findings add credence to the long-held view that subtle organic changes may be one important reason why elderly patients develop their first ever depression in late life. However, the clinical relevance of such deep white matter lesions remains to be fully determined. They may be associated with poor initial treatment response (Reference Simpson, Baldwin and JacksonSimpson et al, 1998) and poor long-term outcome. In a three-year study, O'Brien et al (1998) found that every single elderly patient with depression who had severe (confluent) deep white matter change relapsed. Similar findings with regard to an association between white matter lesions and poor treatment response have been reported for younger patients with bipolar disorder. This emphasises the need for careful clinical assessment and follow-up of cases with lesions and indicates that the presence of lesions may be one consideration when deciding upon long-term prophylactic therapy.
Improving access to neuroimaging
Although psychiatrists deal with diseases of the brain, access to investigative facilities has traditionally not been easy. Historically, this was often because large psychiatric units were geographically distant from general hospitals with investigative facilities. However, even today, psychiatrists often appear to have more difficulty accessing investigative facilities than other specialists. Some centres, for example, may insist that an MRI scan is approved by a neurologist or other specialist before it can be requested. It is certainly the case that psychiatrists must make informed and reasonable requests for imaging, based on what may be appropriate for a particular diagnosis. However, there is equally a need for others to be educated regarding the importance of imaging in psychiatry and the degree to which it influences management. The old argument that imaging patients with dementia to determine causal diagnosis is unimportant, because it does not change management, is easily rebuffed now that specific management options are available.
Another problem is in scan interpretation, with which psychiatrists are involved far too rarely. Many imaging changes of particular interest to psychiatrists, such as relatively subtle degrees of atrophy or white matter change, may not be particularly commented on in a standardised way, with traditional reporting methods being more concerned with the presence of gross structural pathology. Dialogue between the main disciplines involved (psychiatry, neuroradiology, medical physics, neurology, and others) is important and, from our own experience, joint meetings to discuss cases and view scans are invaluable in terms of clarifying areas of uncertainty and providing a focus for education, audit and research.
Conclusions
The primary use of neuroimaging in psychiatry is to exclude a structural brain lesion or some other pathology as a cause for the clinical presentation. “When should I request a scan?” is an important question often asked by trainees and consultants alike, although in truth the threshold that should be adopted for performing a scan is difficult to determine and no clear consensus exists. Some sensible approaches have been described (e.g. Reference Jacoby, Ames and ChiuJacoby, 1997) and guidelines from the Royal College of Psychiatrists in 1995 suggested that, with regard to patients with suspected cognitive impairment, CT should be performed “if practicable unless the patient has a history of more than one year and there is a typical clinical picture. Age should not be a bar”. Undoubtedly, the presence of cognitive impairment, an atypical presentation or course and focal neurology or other features suggestive of a cerebral lesion highlight the need for neuroimaging in individual cases. Increasingly, however, particularly with regard to dementia, there is a need to perform imaging to apply certain sets of clinical diagnostic criteria.
One sensible way forward may not be to ask “When should I do a scan?” since there can never be a comprehensive list of all indications for any investigation. The question may be more appropriately phrased: “If I need to make a diagnosis using a certain set of diagnostic criteria, what sort of imaging do I need to perform?”.
Computed tomography will, for cost and practical reasons, invariably be the imaging modality of first choice, although an MRI, if accessible, is almost always the superior imaging modality. Outside of research settings, it is more difficult to be clear when functional imaging such as SPECT scanning should be performed. SPECT changes can add support to a diagnosis of Alzheimer's disease, vascular dementia or frontotemporal dementia and may be very helpful in excluding these diagnoses in those presenting with a functional psychiatric disorder and cognitive impairment. SPECT may also be helpful in difficult cases where there is diagnostic doubt, for example in a patient with a seemingly clear history of a stroke with cognitive impairment in whom a CT is normal.
Increasingly, as further therapeutic options become available and imaging techniques become more able to provide useful diagnostic and prognostic information, structural and functional neuroimaging will become an important part of the clinical work-up in psychiatry.
Multiple choice questions
-
1. Comparing MRI and CT, MRI is:
-
a more sensitive in detecting white matter changes
-
b better tolerated
-
c dependent on electron density
-
d capable of generating multi-plane images
-
e more suitable for studying temporal lobe sclerosis.
-
-
2. Common neuroimaging changes in Alzheimer's disease are:
-
a medial temporal lobe atrophy
-
b extensive deep white matter change
-
c frontotemporal deficits on SPECT scanning
-
d ventricular enlargement
-
e periventricular hyperintensities on MRI.
-
-
3. Patients with dementia with Lewy bodies:
-
a are less likely to have medial temporal lobe atrophy than those with Alzheimer's disease
-
b have posterior deficits on SPECT scanning
-
c have increased D2-receptor density
-
d have reduced dopamine transporter
-
e have similar white matter changes to patients with Alzheimer's disease.
-
-
4. Neuroimaging changes associated with depression are:
-
a ventricular enlargement
-
b increased signal hyperintensities on MRI that have little, if any, clinical significance
-
c frontal predominance of white matter lesions
-
d prominent atrophy of the temporal lobes
-
e normal caudate and frontal lobe volumes.
-
-
5. Concerning vascular dementia:
-
a according to NINDS/AIREN criteria for vascular dementia, the absence of cerebrovascular changes on CT or MRI constitutes the most important brain imaging change to distinguish Alzheimer's disease from vascular dementia
-
b white matter changes seen on MRI are the most important diagnostic feature
-
c functional SPECT imaging is typically normal
-
d a single infarct may cause dementia
-
e white matter lesions are always due to vascular pathology.
-
Table 1. Typical neuroimaging findings in the major types of dementia
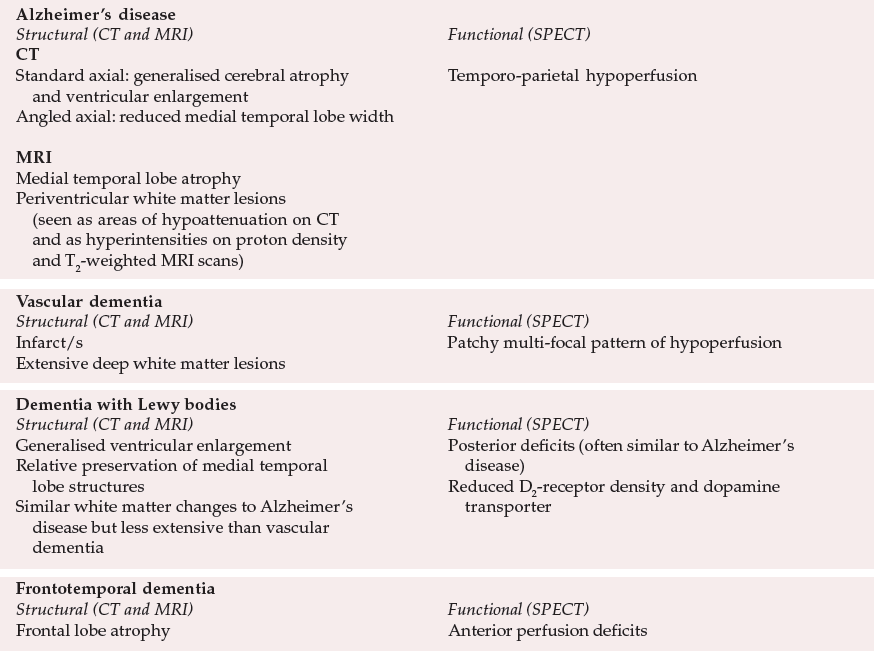
| Alzheimer's disease | |
| Structural (CT and MRI) | Functional (SPECT) |
| CT | |
| Standard axial: generalised cerebral atrophy and ventricular enlargement | Temporo-parietal hypoperfusion |
| Angled axial: reduced medial temporal lobe width | |
| MRI | |
| Medial temporal lobe atrophy | |
| Periventricular white matter lesions (seen as areas of hypoattenuation on CT and as hyperintensities on proton density and T2-weighted MRI scans) | |
| Vascular dementia | |
| Structural (CT and MRI) | Functional (SPECT) |
| Infarct/s | Patchy multi-focal pattern of hypoperfusion |
| Extensive deep white matter lesions | |
| Dementia with Lewy bodies | |
| Structural (CT and MRI) | Functional (SPECT) |
| Generalised ventricular enlargement | Posterior deficits (often similar to Alzheimer's disease) |
| Relative preservation of medial temporal disease lobe structures | Reduced D2-receptor density and dopamine transporter |
| Similar white matter changes to Alzheimer's disease but less extensive than vascular dementia | |
| Frontotemporal dementia | |
| Structural (CT and MRI) | Functional (SPECT) |
| Frontal lobe atrophy | Anterior perfusion deficits |
MCQ answers
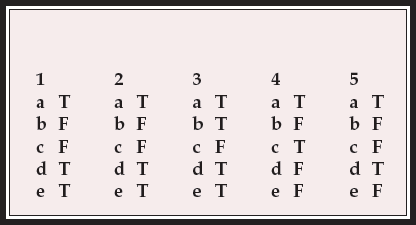
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | T | a | T | a | T | a | T | a | T |
| b | F | b | F | b | T | b | F | b | F |
| c | F | c | F | c | F | c | T | c | F |
| d | T | d | T | d | T | d | F | d | T |
| e | T | e | T | e | T | e | F | e | F |

Fig. 1. (a) CT protocol used to acquire angled axial scans. (b, c) Angled axial scan showing medial temporal lobe width (arrows) from a patient with a clinical diagnosis of Alzheimer's disease (b) and a normal subject (c).

Fig. 2. Coronal T1-weighted MRI scan showing temporal lobes from a subject with Alzheimer's disease. Note: extensive bilateral atrophy of the hippocampus (H) and parahippocampal gyri (PHG) in addition to enlargement of the temporal horn of the lateral ventricles (TH).

Fig. 3. CT and MRI scans from a subject showing basal ganglia infarct (thick arrow) and white matter changes (thin arrows). (a) Axial CT scan. (b) Axial proton density MRI scan. (c) Axial T2-weighted MRI scan. Note: periventricular white matter changes appear hyperintense (PVHyper) on proton density and T2-weighted MRI scans and hypoattenuated (PVHypo) on CT scans. Foci of deep white matter hyperintensities (DWMH) can also be seen (more clearly) on MRI scans.







eLetters
No eLetters have been published for this article.