High rates of mortality associated with psychiatric disorders have been extensively reviewed and quantified (Reference Harris and BarracloughHarris 1998; Reference NeelemanNeeleman 2001). The mortality gap between observed and expected deaths among people with severe mental illness (psychosis) has not improved over time, despite increased recognition of the contribution of physical comorbidity (Reference Dembling, Chen and VachonDembling 1999; Reference Saha, Chant and McGrathSaha 2007). A generation of psychiatrists has tried to address deaths from so-called unnatural causes (suicide), but deaths from natural causes (comorbid physical illness) have continued to rise and may account for as much as 80% of premature mortality in people with mental disorders (Reference Harris and BarracloughHarris 1998; Reference Colton and ManderscheidColton 2006; Reference Parks, Svendsen and SingerParks 2006; Reference MitchellMitchell 2009a).
Medical illness affects more than half of people with mental illness, particularly in high-risk groups such as older people, those with intellectual disability, cognitive impairment or substance misuse, those in long-stay institutions and the homeless (Reference Alaja, Seppa and SillanaukeeAlaja 1998; Reference Fisher, Barreira and GellerFisher 2001). A large US study of homeless people with severe mental illness reported that 74% had medical needs, 44% of which were unmet (Reference Desai and RosenheckDesai 2005). In another US study, systematic examination of people with intellectual disability referred to psychiatric services revealed that 75% had undetected medical problems (Reference Ryan and SunadaRyan 1997). Even if medical problems are identified, medical in-patients with mental illness appear to experience more post-operative complications and elevated mortality (Reference Copeland, Zeber and PughCopeland 2008; Reference Khaykin, Ford and PronovostKhaykin 2010). Yet it is the nature as well as the extent of comorbid physical illnesses that is concerning, with an excess of metabolic and cardiovascular problems (Reference Mitchell and MaloneMitchell 2006; Reference Leucht, Burkard and HendersonLeucht 2007; Reference Bresee, Majumdar and PattenBresee 2010). The European METEOR study reported that 70% of patients receiving antipsychotics for schizophrenia had lipid disorders and 40% hypertension (Reference De Hert, Falissard and MauriDe Hert 2008), and a more recent US study found that 90% of Medicaid recipients prescribed second-generation antipsychotics for schizophrenia had at least one major metabolic risk factor (Reference Bell, Farmer and RiesBell 2009).
Mental health professionals have unwittingly increased the risk of medical conditions in their patients by recommending a variety of psychotropic drugs that contribute to cardiovascular disease, diabetes and endocrine disorders (Reference NewcomerNewcomer 2005). In people with unmedicated schizophrenia, metabolic syndrome is relatively uncommon, affecting only about 10% (Reference Chiu, Chen and ChenChiu 2010). Although the mechanisms underlying cardiometabolic complications are not entirely clear, weight gain and cholesterol increases are particular problems with antipsychotics (Reference Rummel-Kluge, Komossa and SchwarzRummel-Kluge 2010). A 3.8 kg gain is typical in drug-naive patients starting antipsychotic treatment (Reference Tarricone, Gozzi and SerrettiTarricone 2010), but 12–15 kg is not uncommon.
Given this background of high rates of medical comorbidity in people with mental ill heath but no clear method to manage the problem, we suggest that it is pertinent to focus on the following questions:
-
1 Do people with severe mental illness in the UK and elsewhere receive the same preventive healthcare and medical care as other people?
-
2 Is adequate medical monitoring and care provided in psychiatric settings?
-
3 Can guidelines or other interventions improve medical care for people with severe mental illness?
Inequalities in medical treatment and preventive healthcare
Ideally, knowledge that a particular population is vulnerable to excess physical comorbidity should prompt enhanced medical care. Unfortunately, where mental illness is concerned, strong evidence suggests inequitable medical care in most domains, although studies have mostly focused on cardiovascular and diabetes care. One explanation is that inequalities result from the patients’ low attendance or poor adherence to treatment. Yet many people with mental illness, in particular those with depressive disorders, seek help more often and use more general healthcare resources than the rest of the population (Reference Stein, Cox and AfifiStein 2006; Reference Baune, Adrian and JacobiBaune 2007). Furthermore, even when attendance is high, quality of care can be low (Reference Desai, Rosenheck and DrussDesai 2002; Reference Goodwin, Zhang and OstirGoodwin 2004; Reference Jones and CarneyJones 2005; Reference Salsberry, Chipps and KennedySalsberry 2005). Of course, low attendance and poor quality of care is a particularly bad combination (Reference Kaplowitz, Scranton and GagnonKaplowitz 2006). Therefore, predictors of adequate care include measures of both quality and quantity, and deficits in either may result in failing care and poor medical outcomes (Reference Mitchell and LordMitchell 2010).
Our review of studies comparing the quality of medical care received by people with and without mental illness had telling results (Reference Mitchell, Malone and DoebbelingMitchell 2009b). More than 70% of the studies found that patients with psychiatric diagnoses received inferior quality of care in at least one medical area. However, within this dataset there was only a small number of UK-based studies, the vast majority being from North America.
Reference Hippisley-Cox, Parker and CouplandHippisley-Cox and colleagues (2007) used the UK’s QRESEARCH primary care database to identify 127 932 patients with and without mental disorder who had coronary heart disease. There were no differences between groups in the offer of smoking cessation advice or prescription of aspirin, antiplatelet drugs, anticoagulants or beta-blockers. However, patients with schizophrenia were 15% less likely to have a recent prescription for a statin and 7% less likely to have had cholesterol levels recorded; similar results were found for patients with bipolar disorders. Reference Whyte, Penny and PhelanWhyte et al (2007) examined quality of care indicators derived from the General Medical Services contract for UK general practitioners. There was no difference in process measures of care, although patients with mental illness had better than expected HbA1c control. Reference Mangtani, Breeze and KovatsMangtani and colleagues (2005) looked at the take-up of the influenza immunisation programme in the UK shortly after its introduction in 2000. Of their sample of 5572 individuals over the age of 74, 70% of men and 61% of women with depression were vaccinated, compared with 75% of men and 67% women without depression. In a UK case–control study, Reference Roberts, Roalfe and WilsonRoberts et al (2007) found that those with a diagnosis of schizophrenia were about half as likely as comparator groups to have had their blood pressure or smoking status recorded during the 3-year study period.
Inequalities in medical care may extend beyond treatment of active medical conditions to include preventive care. Reference Lord, Malone and MitchellLord and colleagues (2010) reviewed comparisons of preventive care, from 26 studies across Europe and North America, in individuals with and without psychiatric illness. Of their 61 comparisons across 13 healthcare domains, 27 revealed inferior preventive care in those with mental illness, 10 suggested superior preventive care and 24 reported inconclusive findings. Inferior preventive care was most apparent among people with schizophrenia and in relation to osteoporosis screening, blood pressure monitoring, vaccinations, mammography and cholesterol monitoring. One UK-based study examined cross-sectional data of breast screening records for 933 patients with psychiatric illness and 44 195 women without mental health problems aged 50–64 (Reference Werneke, Horn and Maryon-DavisWerneke 2006). The patients were as likely as the reference group to attend breast screening, but patients with a history of multiple detentions under the Mental Health Act were significantly less likely to attend, as were patients with a diagnosis of psychosis.
Medical care and metabolic monitoring in psychiatric settings
Medical care in psychiatric settings has been poorly investigated, with few if any large-scale studies. The focus for early studies was largely the frequency of physical examinations in psychiatric settings. Most mental health clinicians acknowledge the importance of physical examination, but evidence suggests that about 60% of their patients do not receive a physical examination and only 20% receive thorough examination. Responsibility for medical care is often delegated to primary care (Reference McIntyre and RomanoMcIntyre 1977; Reference PattersonPatterson 1978; Reference Bobes, Alegría and Saiz-GonzalezBobes 2011). Physical examination is only one marker of adequate physical healthcare, so this result hints that other areas of care (such as dental) may also be inadequate. However, new evidence suggests that wider deficits exist across a spectrum of relevant medical conditions. Much of this evidence involves audits of physical healthcare monitoring for people recently prescribed atypical antipsychotics. Indeed, guidelines for those not taking antipsychotic medication are currently underdeveloped.
Our group reviewed 38 studies, involving 217 539 patients, that examined routine monitoring of patients taking antipsychotics before the implementation of explicit guidelines (Reference Mitchell, Delaffon and VancampfortMitchell 2012). Across all baseline studies, routine monitoring rates were generally low but were highest for blood pressure (67%) and triglycerides (60%). Cholesterol was measured in 47% of patients, glucose in 42% and weight in 44%. Lipids and haemoglobin A1c (HbA1c) were monitored in less than 20%. Rates were similar for patients with schizophrenia in US and UK studies, and for in-patients and out-patients. One UK study was particularly striking: out of 606 in-patients taking antipsychotics, only 19% had weight recorded in their clinical notes and 3.5% had their lipids monitored during their admission (Reference Paton, Esop and YoungPaton 2004).
Guidelines to improve the medical care of patients with severe mental illness
Governments have been slow to acknowledge the problem of medical ill health associated with severe mental illness, and national guidelines are generally devoid of clear mandatory recommendations (Department of Health 1999; Reference Unützer, Schoenbaum and DrussUnützer 2006; Reference Pincus, Page and DrussPincus 2007; US Department of Health and Human Services 1999). Little had been published before 2000, but over the past decade or so publications have abounded. Eighteen sets of guidelines on the medical care of patients with severe mental illness or schizophrenia in the USA, Australia, Brazil, Canada or Europe have been extensively reviewed by Reference De Hert, Vancampfort and CorrellDe Hert and colleagues (2011).
A key publication in the USA (American Diabetes Association 2004) recommends that mental health practitioners carry out regular monitoring of weight, waist circumference, blood pressure, fasting plasma glucose level and fasting lipid profile of patients taking antipsychotics. In the UK, two key guidelines are in place: the revised National Institute for Health and Clinical Excellence (NICE) schizophrenia guidelines (National Collaborating Centre for Mental Health 2010) and the UK Quality and Outcomes Framework (QOF) for primary care (National Institute for Health and Clinical Excellence 2011). The latter provides a financial incentive for general practitioners to provide medical screening for patients with schizophrenia, bipolar affective disorder and other psychoses under NICE indicators NM16–19, focusing on blood pressure, glucose or HbA1c, body mass index and the ratio of total cholesterol to high-density lipoprotein (Reference JamieJamie 2012). It is important to note that the guidelines do not state what comprises adequate testing in clinical practice, although NICE does suggest monitoring physical health at least once a year (National Collaborating Centre for Mental Health 2010).
The guidelines generally address physical investigations, physical history, and examination and treatment advice. In terms of physical investigations, the most common recommendations are for fasting glucose, fasting triglycerides, fasting cholesterol, high- and low-density lipoprotein, and electrocardiogram (Table 1). Regarding recommended physical history and examination, most but not all of the guidelines advise a personal and family history, baseline physical examination, smoking and physical activity history, weight and waist measurement, blood pressure measurement, and diabetes history or examination (Table 2). Finally, regarding interventions, most of the guidelines recommend: advising patients and their families on physical activity and diet; encouraging smoking cessation; switching medication (if required); treatment of diabetes and lipid abnormalities; and referral, if necessary (Table 3). In general, the guidelines do not state who should take responsibility for monitoring (only three mention that it should be those prescribing high-risk medication), the minimum frequency of monitoring, and the importance of auditing ongoing monitoring. None mandates that physical care must take place, leaving monitoring to the discretion of individual clinicians.
TABLE 1 Recommended physical investigations from guidelines on screening for cardiometabolic risk in people with severe mental illness
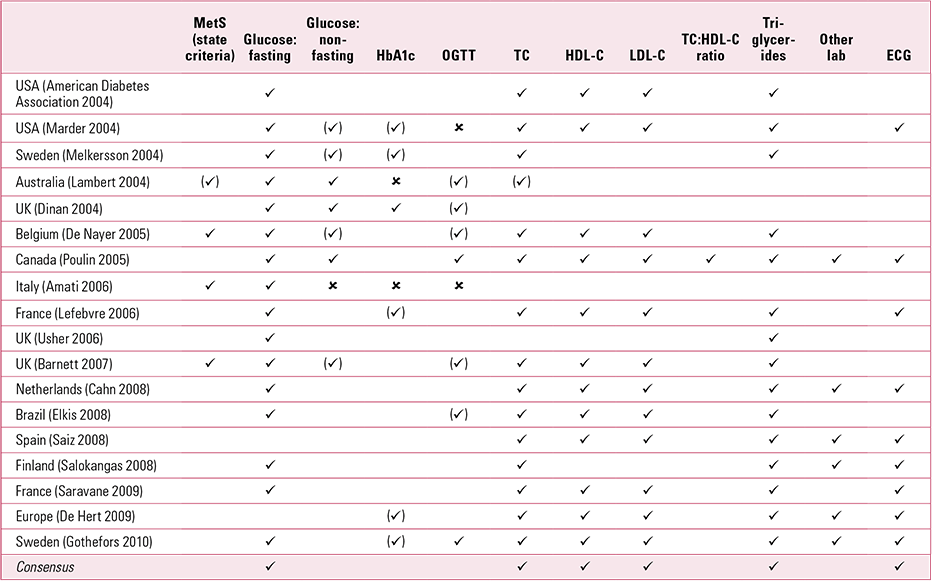
TABLE 2 Recommended physical history and examination from guidelines on screening for cardiometabolic risk in people with severe mental illness
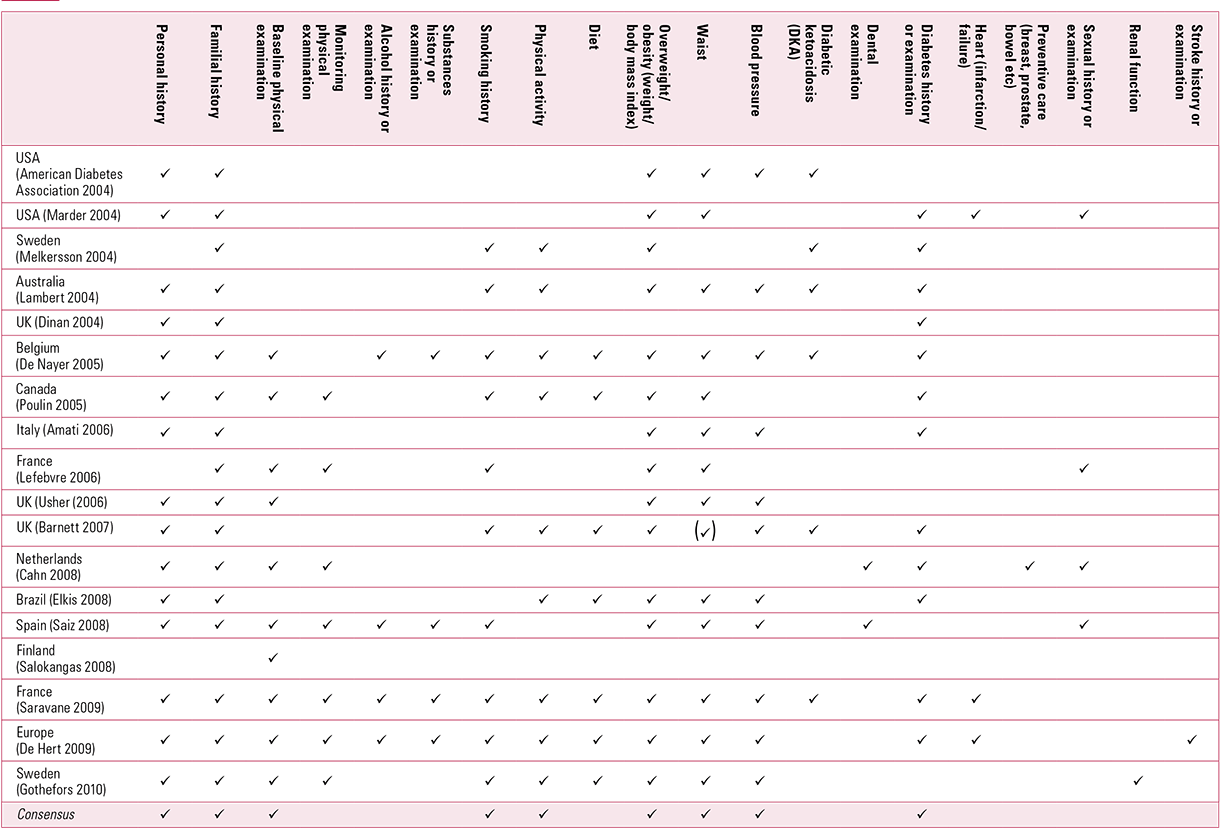
TABLE 3 Recommended treatment advice from guidelines on screening for cardiometabolic risk in people with severe mental illness

Effectiveness of guidelines
Despite the publication of such a large number of guidelines, it is not clear whether any have been successfully implemented. Indeed, the value of enhanced screening or monitoring clinics has itself been poorly studied. To date, there are no randomised controlled trials (RCTs) of physical health monitoring clinics (Reference Tosh, Clifton and MalaTosh 2010), although seven independent studies have looked at monitoring before and after guideline introduction (Reference Mitchell, Delaffon and VancampfortMitchell 2012). A complicating factor is that, given the increased awareness among psychiatrists of metabolic side-effects of antipsychotics, it is possible that any change in practice was not an effect of the guidelines. Nevertheless, our study, which pooled rates of monitoring before and after guideline implementation, suggests that monitoring does appear to have improved for weight (up from 44% of patients to 76%), blood pressure (from 67% to 75%), glucose (from 42% to 56%) and lipids (from 20% to 37%). However, at least a quarter of patients were not being monitored even after guideline introduction. Rates were no better for those recently started on antipsychotics compared with those on existing medication. Thus, from this evidence it seems that, regardless of the published guidelines, medical care involving blood tests (and to a lesser extent clinical tests such as blood pressure and weight monitoring) is often overlooked.
Extensive research shows that in many areas guidelines are difficult to implement and so it should come as no surprise that the improvement following the introduction of guidelines is only modest (Reference PincusPincus 2010). As few as one-third of medical patients receive guideline-concordant, evidence-based care (Reference GrolGrol 2001). In the UK, an evaluation of the many NICE recommendations found variable success in the guideline implementation (Reference Sheldon, Cullum and DawsonSheldon 2004). A number of factors interfere with successful and widespread implementation (Reference Cabana, Rand and PoweCabana 1999). Frequently reported barriers include a lack of resources or time, inadequate organisational support, clinicians’ reluctance to change, concerns over the quality of the guidelines and lack of responsibility (Reference Francke, Smit and De VeerFrancke 2008; Reference Forsner, Hansson and BrommelsForsner 2010).
Predictors of inferior medical care
Predictors of medical care (metabolic testing) have been examined infrequently. People with severe mental illness are often hesitant to seek medical care because of symptom burden, low confidence, stigma or the attitudes of primary care physician (Reference Berren, Santiago and ZentBerren 1999; Reference Kim, Swanson and SwartzKim 2007). Some refuse the help that is offered (although healthcare professionals should still try to ensure that medical care is adequate). Although patient factors are no doubt important, provider factors are the direct responsibility of healthcare professionals and are amenable to change.
There may be infrastructural difficulties in providing medical care in psychiatric settings (Reference Hewer, Salize and WolfersdorfHewer 2004), and mental health professionals may lack confidence regarding patients’ physical health concerns (Reference Daumit, Crum and GuallasDaumit 2002). The presence of a psychiatric diagnosis can distract clinicians from considering and managing medical illness (Reference Graber, Bergus and DawsonGraber 2000; Reference McDonald, Frakes and ApostolidisMcDonald 2003). In a survey of 250 people with schizophrenia conducted in the USA, 49% felt that doctors took their medical problems less seriously after discovering that they had a psychiatric diagnosis (National Alliance on Mental Illness 2008). In studies of metabolic testing, primary care visits were positively associated with HbA1c and lipid testing (odds ratios OR = 5.01 and 2.21, respectively; Reference Banta, Morrato and LeeBanta 2009), suggesting that shared care or collaborative care may be beneficial (Reference Rubin, Littenberg and RossRubin 2005). In a UK primary care setting, body mass index, blood pressure, cholesterol or HbA1c were monitored in more than 90% of patients under the QOF system. In the USA, patients seen by a fee-for-service psychiatrist were more likely to receive lipid testing (OR = 2.35) and eye examinations (OR = 2.03; Reference Banta, Morrato and LeeBanta 2009).
Another factor is clarity regarding medical responsibility during periods of both active mental health treatment and monitoring (relapse prevention). In an Australian study, 69% of mental healthcare staff were unsure about who should follow up abnormal cardiometabolic screening results (Reference Organ, Nicholson and CastleOrgan 2010). There have been numerous recommendations to remedy this problem (Reference Horvitz-Lennon, Kilbourne and PincusHorvitz-Lennon 2006; Reference Copeland, Zeber and PughCopeland 2008; Reference Lambert and NewcomerLambert 2009). During acute and continuation care, basic medical checks should be the responsibility of the main mental health professional (unless this care is already delegated). If they were the responsibility of primary care or medical specialists, it is unlikely that problems would be detected. If a medical problem is detected, it should be treated by an appropriate practitioner who is skilled and confident to do so.
One method that may help to improve screening and treatment in mental health settings is the creation of physical health (or weight management) clinics, usually run by mental health professionals with variable input from primary care or hospital specialists (Reference Holt, Pendlebury and WildgustHolt 2010; Reference MillarMillar 2010). These should offer patients easily accessible basic health checks and metabolic monitoring. At present, many healthcare professionals say that they have no access to basic medical equipment such as scales (15%), a height rod (88%) or a tape measure (66%; Reference Verdoux, Boulon and CougnardVerdoux 2008) and, in our opinion, even those with the equipment may not be confident to conduct health checks.
Improving medical care
It is important to note that effective monitoring of metabolic disturbances is not sufficient on its own; appropriate treatment is also required. Data from the US National Ambulatory Medical Care Survey from 1992 and 1996 found psychiatrists offered smoking-cessation advice to individuals with mental ill health on only 12% of visits (Reference Himelhoch and DaumitHimelhoch 2003). Active help to stop smoking is rare in mental health settings (Reference Price, Ambrosetti and SidaniPrice 2007). Individuals with mental health problems who wish to stop smoking can be helped by buproprion as well as other strategies (Reference Tsoi, Porwal and WebsterTsoi 2010).
Physical health problems are often unrecognised or inadequately treated in people with mental illness (Reference Taylor, Young and MohamedTaylor 2005). For example, in a sample of in-patients with schizophrenia, 84% of those found to be hypertensive on screening were not recognised as hypertensive on admission (Reference Bernardo, Carlas and BanegasBernardo 2009). In the US-based Clinical Anti-psychotic Trials of Intervention Effectiveness (CATIE) study, about a third of patients met National Cholesterol Education Program (NCEP) criteria for metabolic syndrome at baseline, but 88% of patients with dyslipidemia were untreated, as were 62% with hypertension and 38% with diabetes (Reference McEvoy, Meyer and GoffMcEvoy 2005; Reference Nasrallah, Meyer and GoffNasrallah 2006). In another US study, 62% of patients treated with second-generation antipsychotics who had elevated low-density lipoprotein levels did not receive medical treatment, despite the fact that they were in-patients (Reference Correll, Harris and Pantaleon MoyaCorrell 2007). A Spanish study reported that, among in-patients with schizophrenia, only 60% of those with diabetes, 28% of those with hypertension and 14% of those with dyslipidaemia were receiving active medical treatment on admission (Reference Bernardo, Carlas and BanegasBernardo 2009).
The only area, other than smoking cessation, in which medical management has been comprehensively examined in psychiatric settings is weight control (Reference Lowe and LubosLowe 2008). One review of ten RCTs showed that non-pharmacological interventions such as cognitive–behavioural strategies, nutritional counselling and exercise programmes, delivered on an individual or group basis, were modestly effective in reducing or attenuating antipsychotic-induced weight gain (Reference Álvarez-Jimenez, Hetrick and González-BlanchÁlvarez-Jiménez 2008). Mean weight losses across studies are about 4 kg for 6-month interventions, with potentially positive effects on insulin regulation and HbA1c (Reference Gabriele, Dubbert and ReevesGabriele 2009). One additional RCT of note, the US-based Primary Care Access, Referral and Evaluation (PCARE) study examined a package of medical care in community mental health settings: 407 patients with severe mental illness were randomly assigned to either the medical care management intervention or usual care (Reference Druss, von Esenwein and ComptonDruss 2010a). At a 12-month follow-up evaluation, the intervention group had received an average of 59% of recommended preventive services, compared with 22% in the usual care group. They also received a significantly higher proportion of evidence-based services for cardiometabolic conditions (35% v. 28%) and were more likely to have a primary care provider (71% v. 52%) and receive a physical examination. Reference Druss, Zhao and von EsenweinDruss and colleagues (2010b) have also trialled patient-led peer support over a six-session programme. After 6 months, there was a significantly elevated rate of visits to primary care (68.4% v. 51.9%) in those receiving peer support.
Conclusions
Patients with mental ill health, particularly those taking antipsychotic medication, are a vulnerable group with high rates of comorbid medical illness. Yet they often receive relatively low levels of medical (or preventive) care in medical settings, as well as suboptimal levels of physical examination and medical monitoring in psychiatric settings. Reasons for these inequalities in care are complicated and are probably related to both patient and provider factors. These issues are not easy to resolve but provider factors remain a priority and should be examined at organisational level. Closer integration of primary care and mental health services and peer-based support may help, but it must not obscure responsibility for testing at key periods, such as on admission or before starting antipsychotic medication.
Basic psychiatric care may need to be supplemented by physical health clinics (for those receiving mental healthcare), weight management clinics or metabolic clinics and a system of audit to ensure that testing and appropriate management of identified abnormalities takes place. Despite the availability of numerous guidelines, implementation has been patchy and many demand a culture shift towards joint mental and physical healthcare. To support implementation, educational campaigns and quality assurance initiatives are recommended, as well as more research into the most effective strategies to improve cardiometabolic monitoring, medical management and preventive healthcare in the vulnerable population of mentally ill patients.
MCQs
Select the single best option for each question stem
-
1 Regarding comorbid medical conditions in mental illness:
-
a the publication of guidelines for monitoring physical health has invariably been followed by improvement in standards of clinical practice
-
b the prevalence of comorbid medical conditions in people with schizophrenia is less than 25%
-
c atypical antipsychotic medication has negligible contribution to cardiometabolic risk
-
d drug-naive patients with first-episode schizophrenia have about a 10% prevalence rate for metabolic syndrome
-
e the elevated mortality rates among people with mental disorder are entirely attributable to suicide.
-
-
2 According to NICE, the professional or team responsible for monitoring metabolic risk factors in schizophrenia is:
-
a the primary care trust/general practitioner
-
b the metabolic monitoring clinic
-
c the cardiologist
-
d the psychiatrist/mental health teams
-
e unclear.
-
-
3 The NICE guidelines on schizophrenia recommend monitoring physical health:
-
a once a month
-
b 3 monthly
-
c 6 monthly
-
d once a year
-
e at every clinic review.
-
-
4 All of the following are true except:
-
a women with a history of multiple mental health detentions are less likely to attend breast screening than the general population
-
b patients with schizophrenia have inferior preventive care for osteoporosis screening compared with the general population
-
c people with a mental disorder and coronary heart disease are less likely to be offered smoking cessation advice than mentally healthy people with such disease
-
d people with mental disorder are as likely to be offered antiplatelet drugs as those without
-
e diabetes care is disproportionately low among those with mental illness.
-
-
5 Under the UK Quality and Outcomes Framework for primary care, general practitioners are incentivised to provide medical screening for those with schizophrenia, psychoses and bipolar disorder. This includes all of the following except:
-
a blood pressure
-
b breast screening
-
c glucose
-
d body mass index
-
e cholesterol:high-density lipoprotein ratio.
-
MCQ answers

| 1 | d | 2 | e | 3 | d | 4 | c | 5 | b |






eLetters
No eLetters have been published for this article.