Depression is currently seen as a major problem, with the World Health Organization (2001) predicting that by 2010 depression will be the second most common cause of morbidity worldwide. Forging a greater understanding of the pathophysiology of this destructive affliction is therefore a greater priority than ever. There is an increasing recognition of the heterogeneity of depression, and a consequent realisation that there may be several neurobiological pathways leading to the disorder. This article explores one of these, namely the link between depression and inflammatory processes. We have used references sparingly, but would be happy to provide a full list on request.
This link has its origins in the analogy between sickness behaviour and depression noted and studied by Reference YirmiyaYirmiya (2000) and the macrophage theory of depression developed by Reference MaesMaes (1995). These two lines of enquiry stimulated research that found associations between chronic immune activation and depression, including: medical disorders with an inflammatory pathophysiology; immunotherapy for cancer and hepatitis C; and ageing.
By the same token, it has been reported more recently that other risk factors for depression, including psychosocial stress, psychological trauma, sleep disturbance and pain, all increase inflammatory processes.
Depression in the medically ill
It is estimated that depression in people with medical illnesses is 5–10 times more common than in the general population (Reference YirmiyaYirmiya, 2000). Well-recognised examples are disorders such as cardiovascular disease (see below), rheumatoid arthritis and multiple sclerosis. Studies have specifically reported a higher incidence of depression in illnesses associated with inflammatory processes than in those associated with non-inflammatory processes but equal disability. There is also evidence that in some systemic inflammatory diseases there exists a positive correlation between disease activity and depressive episodes.
Elevated levels of pro-inflammatory cytokines have been proposed as a possible physiological link between depression and a variety of serious medical conditions such as those described, where inflammation plays a key role in the disease process and which are strongly associated with depression. Pre-clinical studies have also suggested that psychological stress induces pro-inflammatory cytokine expression in regions of the brain associated with emotional regulation. Patients with depression have shown exaggerated activation of inflammatory response – specifically IL-6 and NF-κB (see Box 1 for acronyms) – following acute psychological stress.
Box 1 Acronyms used in immunology

| CAM | Cell adhesion molecule |
| CRH | Corticotrophin-releasing hormone |
| IL | Interleukin |
| IFN | Interferon |
| JNK | c-Jun N-terminal kinase |
| NF | Nuclear factor |
| NK | Natural killer |
| MAPK | Mitogen-activated protein kinase |
| sICAM | Soluble intercellular adhesion molecule |
| STAT5 | Signal transducer and transcriptional activator 5 |
| TNF | Tumour necrosis factor |
The evidence for a link between depression and inflammatory processes is further supported by the fact that pro-inflammatory cytokines (notably IL-2, IL-6, IL-1βand TNF-α) are elevated not only in medically ill patients with depression but also in medically healthy but depressed patients. The degree of elevation of certain pro-inflammatory cytokines has also been shown to correlate with the severity of depressive illness. Further evidence has suggested that activation of the systemic inflammatory process (as measured by systemic C-reactive protein) may contribute to the pathophysiology of depression.
Cardiovascular disease and depression
Although recognised for many years, there is a current upsurge in interest in the relationship between depression and cardiovascular disease. To date, over 100 studies have investigated the relationship between major depressive disorder and cardiovascular disease, providing evidence that major depressive disorder has a prevalence of between 20 and 35% in the year following an acute coronary syndrome (myocardial infraction or acute unstable angina) (Reference Rumsfeld and HoRumsfeld & Ho, 2005). There is compelling evidence that major depressive disorder is predictive both of development of cardiovascular disease and of adverse outcomes in those who already have the disease.
Cardiac death
The most worrying feature of all is the relationship between depression and fatal outcome in patients after an acute coronary syndrome. Most studies have found fairly consistent results, with an adjusted risk of death in the 6 months following myocardial infarction around 4 times higher in depressed than non-depressed patients (Reference Frasure-Smith, Lespérance and TalajicFrasure-Smith et al, 1993). Indeed, it is argued that the increased mortality associated with major depressive disorder after an acute coronary syndrome is equal to or greater than any other predictor of risk (Reference Welin, Lappas and WilhelmsenWelin et al, 2000). Again, hospitalisation for depression was shown to be a risk factor for myocardial infarction, after adjustments for lifestyle, lipid profile, coagulation, inflammation, prior cardiovascular events and comorbidity.
Even mild depressive symptoms are associated with greater risk of cardiac death, and the risk increases with the severity of the depression. Depressive symptoms have also been shown to be a stronger predictor of poor functional improvement than variables such as previous myocardial infarction, diabetes and left ventricular ejection fraction. The most recent data have shown that around 50% of patients with major depressive disorder after acute coronary syndrome have a history of depression apparently unrelated to their cardiovascular disease and about 50% have their first ever episode of major depressive disorder immediately following acute coronary syndrome (Reference Glassman, Bigger and GaffneyGlassman et al, 2006).
Reference De Jonge, van den Brink and SpijkermanDe Jonge et al (2006) report that only patients with an ‘incident’ depression, i.e. no history of depression prior to their acute coronary syndrome, have an impaired cardiovascular prognosis. These findings are similar to those reported by Reference Grace, Abbey and KapralGrace et al (2005). In such incident depression the pathophysiological processes involved in the syndrome itself may be integral to the depression.
Atherosclerosis
One putative link between inflammatory cytokines and cardiovascular disease is the role of inflammation in the development of atherosclerosis. It is now well established that inflammatory phenomena at the site of atherosclerotic plaques are major determinants of the progression and clinical outcome of atherosclerotic disease. This suggests that increased activation of the immune system (including the form seen in depression) predisposes to atherosclerosis.
Moreover, inflammation is a key part of the development and progression of atherosclerosis, which have been linked to both local and systemic release of cytokines (e.g. IL-6), related acute-phase reactants (e.g. C-reactive protein) and the expression and endothelial shedding of cell adhesion molecules (Reference Lespérance, Frasure-Smith and ThérouxLespérance et al, 2004). This systemic pattern of immune activation is common in people with coronary heart disease and is especially active at the time of an acute coronary episode. These markers of inflammation predict not only the development of an acute coronary syndrome but also a worse prognosis following such an event. People with major depressive disorder show patterns of inflammatory activation similar to those seen in cardiovascular disease (Reference Anisman and MeraliAnisman & Merali, 2002). The most consistent pattern of change observed in major depressive disorder is an elevation in the pro-inflammatory cytokines IL-1β, IL-2, IL-6 and TNF-α (Reference Zorrilla, Luborsky and McKayZorrilla et al, 2001).
Changes in endothelial function also appear to accompany major depressive disorder and there is growing evidence that acute-phase proteins and soluble adhesion markers are raised in major depression without cardiac disease. Increases in soluble intercellular adhesion molecules such as sICAM-1 have been linked to deterioration in the blood–brain barrier, thus allowing large molecules such as cytokines access to the brain.
Results from a prospective cohort study demonstrated significant interaction between C-reactive protein and depression, such that moderately increased C-reactive protein in depressed men was predictive of a subsequent first coronary event (Reference Ladwig, Marten-Mittag and LowelLadwig et al, 2005). Another prospective epidemiological study (Reference Empana, Sykes and LucEmpana et al, 2005) found that depression, IL-6 and sICAM-1 were associated with the incidence of cardiac events. Reference Frasure-Smith, Lespérance and IrwinFrasure-Smith et al (2007) assessed men 2 months after acute coronary syndrome and found that depression and C-reactive protein were overlapping prognostic risks.
Taken together, these findings point to an association between inflammation and major depressive disorder (Box 2). Although correlation does not mean causation, such systemic inflammation at the time of and following an acute coronary event might conceivably facilitate the onset of major depressive disorder.
Box 2 Cardiovascular disease and depression
-
• Increased mortality is associated with major depressive disorder which has onset after an acute coronary event
-
• Depression is associated with increased risk of coronary death
-
• Inflammatory action and changes in endothelial action in major depressive disorder seem to be similar to those in cardiovascular disease
Immunotherapy and depression
The example of interferon treatment for hepatitis C
It has been noted that administration of alpha interferon commonly causes depressive symptoms as a side-effect, and in 30–45% of patients it causes a diagnosable depressive illness (Reference Raison, Borisov and BroadwellRaison et al, 2006). A higher rate of psychiatric illness exists in patients with hepatitis C than in the general population, but development of neuropsychiatric symptoms on initiation of interferon treatment in those with no psychiatric history has been documented. Alternative mechanisms have been postulated, including interferon's side-effects mimicking the symptoms of depression, and direct neurotoxicity of the hepatitis C virus. However, interferon treatment is used for other diseases, including malignant melanoma, and similar neuropsychiatric side-effects are observed.
Thus, where a pro-inflammatory cytokine is administered to treat a disease it appears to directly cause a depressive illness in a significant proportion of those treated.
Mechanisms underpinning inflammation and depression
A number of mechanisms have been suggested to explain the link between inflammation and depression (Box 3).
Box 3 Potential mechanisms underpinning inflammation and depression
-
• Raised pro-inflammatory cytokine levels in depression act directly on the hypothalamus and amygdala
-
• Depression induces immunosuppression through raised corticotrophin-releasing hormone in the central nervous system and reduced natural killer cell response
-
• Pro-inflammatory cytokines can activate the HPA axis, and persistently high levels result in raised cortisol levels
-
• In treatment with alpha interferon, reduced serotonin synthesis may reduce synaptic serotonin levels
-
• Serotonin transporter activity is increased by certain pro-inflammatory cytokines thus reducing overall serotonergic tone
Direct action of cytokines on the brain
The neuropsychiatric side-effects experienced when cytokines are exogenously administered to treat illnesses such as hepatitis C provide evidence that cytokines can directly affect the brain (Reference Raison, Borisov and BroadwellRaison et al, 2006). The raised pro-inflammatory cytokine profile observed in depression suggests that the raised cytokine levels may be producing depressive symptoms by exerting a direct effect on the brain.
Pro-inflammatory cytokines have been shown to exert multiple behavioural effects, including depressed appetite and weight loss, sleep disturbance, motor retardation, cognitive impairment and anhedonia. It is hypothesised that these are mediated by the direct actions of cytokines on the hypothalamus. Similarly, treatments involving administration of pro-inflammatory cytokines (e.g. IL-2, IFN-α and TNF-α) are also associated with anhedonia and cognitive impairment (Reference Schiepers, Wichers and MaesSchiepers et al, 2005).
More recently, pre-clinical work has identified that following myocardial infarction, rats showed increased apoptosis in the amygdala. Further experiments demonstrated that this increase did not occur if a cytokine synthesis inhibitor was administered after the infarction was induced. This lends support to the hypothesis that changes in the brain due to physical illness are mediated by pro-inflammatory cytokines (Reference Wann, Bah and BoucherWann et al, 2007).
Effects on the immune system
Usually, inhibitory elements such as glucocorticoids and anti-inflammatory cytokines limit the activation of the immune system to levels that are appropriate for clearing the initial antigenic stimulus. However, in certain conditions these inhibitory feedback loops become impaired, allowing chronic immune activation and persistence of sickness symptoms. Depression may correspond to one of these conditions where inhibitory loops are altered and neuroendocrine and immune systems become persistently activated.
Depression is associated with elevated levels of corticotrophin-releasing hormone (CRH) in the central nervous system (CNS). It has been shown that raised levels can decrease natural killer cell activity. This appears to be due to central, as opposed to adrenal, CRH receptor mechanisms. In vitro studies have suggested that central doses of CRH can decrease both cellular and humoral immune responses. This therefore illustrates one possible mechanism by which depression can induce the immuno suppression.
Other findings have shown increased levels of circulating catecholamines and neuropeptide Y in people with depression. This seems to have a strong predictive value regarding decline of natural killer cell response.
More recent research in this field was performed in the context of depression induced by alpha interferon. It was shown that mood and cognitive symptoms were highly responsive to pre-treatment with paroxetine, whereas the neurovegetative syndrome (depressed appetite, sleep disturbance, motor retardation) did not respond to antidepressants (Reference Capuron, Gumnick and MusselmanCapuron et al, 2002a ). This suggests that there may be different pathophysiological mechanisms causing the affective and cognitive symptoms compared with the effects on the immune system.
Cytokines and the HPA axis
Some patients with depression exhibit hypothalamic–pituitary–adrenal (HPA) axis hyperactivity, characterised by hypercortisolaemia. Pro-inflammatory cytokines are activators of the HPA axis, and there is evidence that persistently raised pro-inflammatory cytokine levels counteract the normal negative feedback loop whereby raised corticosteroid levels would reduce HPA axis activity. Raised cortisol levels are also associated with increased anxiety and fear behaviour, and with decreased ability to manage social stress (Reference Burke, Davis and OtteBurke et al, 2005).
A consistent finding is the capacity of cytokines that mediate innate immune responses (e.g. IFN-α, IL-1, IL-6, TNF-α) to increase the release of CRH.
Increased CRH is a reliable finding in major depressive disorder, as evidenced by increased cerebrospinal fluid concentrations of the hormone and increased CRH mRNA and protein in postmortem studies of the hypothalamus of individuals who had had major depression.
It has further been suggested that cytokine-induced activation of the HPA axis may represent a risk factor for depression.
Lastly, inflammatory cytokines have been shown to alter nearly all aspects of glucocorticoid receptor functioning, and the signal transduction pathways by which cytokines affect glucocorticoid receptors include NF-κB, p38-MAPK, JNK and STAT5.
Effect on tryptophan metabolism
Recent research suggests that alpha interferon may exert its effects by altering tryptophan metabolism (Reference Wichers, Koek and RobaeysWichers et al, 2005). Alpha interferon induces indoleamine 2,3-dioxygenase (IDO), an enzyme involved in tryptophan (TRP) metabolism. This is the rate-limiting enzyme in the L-TRP–kynurenine pathway that converts L-TRP (a precursor of tryptophan) to N-formylkynurenine. Thus, induction of IDO results in a diminished central synthesis of 5-HT (serotonin). Decreased 5-HT synthesis has been linked to reduced synaptic serotonin levels, providing a possible mechanism by which alpha interferon produces its neuropsychiatric side-effects.
Further, significant decreases in serum concentrations of tryptophan, the primary precursor of 5-HT, have been reported in patients undergoing IL-12 and/or IFN-α treatment.
Effect on serotonin transport
In the brain, the availability, and hence signalling potential, of released serotonin is regulated solely through the action of the serotonin transporter (SERT). The SERT captures serotonin molecules and transports them back into the nerve terminal, making them available for recycling into new synaptic vesicles. The strength of serotonin activity at serotonin receptors is inversely proportional to the number of functional SERT molecules present at the presynaptic membrane. Selective serotonin reuptake inhibitors (SSRIs) and related antidepressants exploit this by blocking the reuptake of serotonin through SERT, thus increasing serotonin signalling overall.
In cases of established depression there is evidence of a decrease in the availability of SERT, reflecting a loss of serotonergic neurons and a chronic decrease in the amount of serotonin available for transmission, leading to compensatory downregulation of SERT. It has been suggested that antidepressant drugs may reverse this effect. Of relevance to this suggestion are data from pre-clinical studies that indicate that SERT activity is increased by pro-inflammatory cytokines TNF-α, IL-β and IFN-γ.
At the experimental level, the cytokines TNF-α, IL-2 and IFN-α have been found to significantly upregulate the expression and activity of SERT (Reference Mossner, Heils and StoberMossner et al, 1998). These findings have advanced recently with the confirmation that IL-1β and TNF-α acutely regulate neuronal SERT activity via p38-MAPK-linked pathways (Reference Zhu, Carneiro and DostmannZhu et al, 2005).
Effect of inflammation on neurogenesis
It was confirmed in the late 1990s that neurogenesis occurs in the adult human brain, in the dentate gyrus of the hippocampus and in the subventricular zone. It is thought that neurogenesis in the dentate gyrus is linked to hippocampal functions, particularly learning. There is evidence that hippocampal volume is reduced in depression and that hippocampal functions, including recall, are impaired in major depression. Pre-clinical studies suggest that IL-1β can impair neurogenesis. The hippocampus contains a high concentration of receptors for IL-1β, and studies suggest that IFN-α can impair neurogenesis, acting through an IL-1β-mediated mechanism (Reference Kaneko, Kudo and MabuchiKaneko et al, 2006). There is also evidence that increased TNF-α can impair hippocampal neurogenesis. Reference Monje, Toda and PalmerMonje et al (2003) reported that neuro-inflammation inhibits neurogenesis and that inflammatory blockage with a non-steroidal anti-inflammatory drug restores neurogenesis. In exploring a potential mechanism underlying the depression induced by alpha interferon treatment, Reference Kaneko, Kudo and MabuchiKaneko et al (2006) found that the drug suppressed neurogenesis in the dentate and that IL1-β played an essential role in that suppression.
How do cytokines reach the brain?
Cytokines are large molecules that are generally unable to penetrate the blood–brain barrier. Several mechanisms have been proposed by which elevated cytokine levels can act on the brain (Box 4).
Box 4 How do cytokines reach the brain?
-
• Cytokines act at sites where the blood–brain barrier is weak or non-existent
-
• Active transport into the brain
-
• Peripheral cytokines trigger the central production or release of cytokines
-
• Peripheral cytokines act on the brain via neural pathways
Entry at compromised sites in the blood–brain barrier
There are points in the CNS where the blood–brain barrier is more permeable than others. Experiments have suggested that peripheral cytokines act on the organum vasculosum of the lamina terminalis. The assumption is that cytokines diffuse through fenestrated capillaries and exert their effect via secondary mediators. There are, however, several problems with this hypothesis. First, peripheral immune challenges are associated with a delayed rise in brain cytokines. Other work has shown that the main source of IL-1β in the brain appears to be microglial cells, and perivascular and meningeal macrophages. Later work has suggested that microglial cells can produce IL-1 without specific activation in response to peripheral cytokines.
Active transport into the brain
It has been suggested for several years that an active transport mechanism exists to transport pro-inflammatory cytokines across the blood–brain barrier. There has recently been a small but growing body of evidence to support this. Murine models of the decreased ability to produce fever in response to infection indicated that IL-1β crosses the blood–brain barrier by active transport, and that this ability decreases with age. More recent work has suggested that this is more widespread, and that endothelial proteins at the blood–brain barrier may respond to stimuli at one side of the barrier to release compounds (including cytokines) into the cerebral circulation.
Peripheral trigger of central production
Pre-clinical studies show how systemic infusion of pro-inflammatory cytokines can produce sickness behaviour. Reference Kent, Bret-Dibat and KelleyKent et al's (1996) work also showed that the timescale of central and peripheral effects of IL-1 differed, suggesting two processes at work. It has been noted that significantly lower doses of IL-1 are needed to produce effects in the CNS than in the periphery. This ratio can be 100 or 1000 to 1. It has been hypothesised that peripheral cytokines trigger perivascular macrophage-like cells in the circumventricular organs to produce pro-inflammatory cytokines. These cytokines in turn would act directly or indirectly on neurons projecting to other brain structures.
Further evidence for the brain possessing its own cytokine system is the high degree of cytokine regulation in the brain. There is evidence that both neurons in the healthy mouse brain and endothelial cells in the rat brain can produce IL-1. Following brain injury, IL-1 is expressed by microglia or astrocytes, and this has also been proposed as a mechanism in neurodegenerative and psychiatric illness (Reference Rothwell and LuheshiRothwell & Luheshi, 2000). There is a suggestion that stress can directly induce brain IL-1 production. Cells that respond to IL-1 in the case of raised peripheral cytokine levels also express specific IL-1 receptor antagonist. There is also evidence from in vivo pre-clinical trials to suggest a negative feedback mechanism between IL-1 and TNF-α (pro-inflammatory cytokines) and IL-10 (an anti-inflammatory cytokine). The effects of other anti-inflammatory cytokines seem to be more complex.
Action via neural pathways
There is evidence linking the vagus nerve to cytokine effects (Reference Bret-Dibat, Bluthe and KentBret-Dibat et al, 1995). There is also evidence that vagotomy can block central cytokine-related immune responses. However, further work suggests that vagotomy can block effects of intraperitoneal IL-1β, although not if it is administered systemically or directly into cerebral ventricles. This provides evidence for several afferent pathways from the periphery to the brain, and suggests that vagal transmission cannot entirely explain the relationship between peripheral and central cytokine effects. There is also evidence that peripheral cytokines act via neural transmission to sensitise brain target areas to them (Reference Dantzer, Konsman and BlutheDantzer et al, 2000). This may explain the lower levels of circulating cytokines needed to produce CNS effects.
It has been noted that intraperitoneal infusion of IL-1 in rats can activate the HPA axis, elevating circulating glucocorticoid levels. Additionally, IL-1 infusion has been shown to activate noradrenaline metabolism in the brain. This provides another mechanism by which raised levels of circulating pro-inflammatory cytokines can affect brain function.
The anti-inflammatory activity of antidepressants
There is a growing body of evidence to suggest that commonly used antidepressant drugs also show anti-inflammatory properties. Pre-clinical studies have shown anti-inflammatory actions in desipramine and fluoxetine, venlafaxine, bupropion, amitriptyline and duloxetine. One in vivo animal study suggests that antidepressants (reboxetine, desipramine, fluoxetine and clomipramine) are able to suppress production of IFN-γ independent of their effects on monoamine blockade. Other studies have suggested similar effects regarding other pro-inflammatory cytokines, including TNF-α. This strongly suggests that antidepressants can act to normalise the mechanisms responsible for increased cytokine production.
A second hypothesis is that in depression it is the interplay between the HPA axis and the cytokine system that is disturbed. It is suggested that elevated HPA axis activity in acute depression suppresses cytokine regulatory mechanisms, and that successful antidepressant treatment normalises this relationship (Reference Himmerich, Binder and KunzalHimmerich et al, 2006).
There is also evidence that non-pharmaceutical treatments for depression exert an effect on the immune system. One such is exercise, which has been shown to have an anti-cytokine effect (Reference Kohut, McCann and RussellKohut et al, 2005). Omega-3 fatty acids can lower levels of pro-inflammatory cytokines (IL-1α, IL-1β, IL-6 and TNF-α) and raise levels of IL-10 (an anti-inflammatory cytokine), and there is also some evidence for their efficacy at treating depression. St John's Wort also shares antidepressant and anti-inflammatory properties.
The ‘antidepressant’ effect of anti-cytokine treatments
There exists a long-recognised association between adverse emotional states and rheumatoid arthritis, and research suggests a significantly raised prevalence of depression in a population of patients with the disease (Reference Patten, Williams and WangPatten et al, 2006). Etanercept is an anti-TNF-α treatment used in the management of rheumatoid arthritis and psoriasis. The drug has been shown to relieve both the symptoms of psoriasis and the depression and fatigue associated with it (Reference Tyring, Gottlieb and PappTyring et al, 2006). This is in contrast to a study that alternative treatment involving psoralen administration and exposure to ultraviolet light produced a marked improvement in the clinical severity of psoriasis, but no significant effect on symptoms of depression or anxiety.
Antidepressants have been found to reverse major depression induced by cytokine therapy (Reference Capuron, Hauser and Hinze-SelchCapuron et al, 2002b ). One small study showed that the antidepressants exhibit a more rapid onset of action if augmented with aspirin (Reference Mendlewicz, Kriwin and OswaldMendlewicz et al, 2006).
Conclusions
Regardless of whether activation of the cytokine pathway is the core pathological process or a consequence that drives some symptoms, it is not unreasonable to examine the potential for reversing the process as an effective treatment for depression. Although there is little evidence that anti-inflammatories are effective as monotherapy for depression, there is evidence that etanercept (an anti-TNF drug) has a hedonic effect. Pre-clinical work implies that other anti-cytokine treatments may reduce the effects of psychosocial stressors, but the evidence is not conclusive (Reference Raison, Capuron and MillerRaison et al, 2005).
There is much stronger evidence that existing antidepressant drugs also possess anti-inflammatory properties. Both clinical and pre-clinical studies have shown that antidepressants can reduce levels of pro-inflammatory cytokines.
Understanding the relationship between depression and somatic disease can be useful in patient care. Simply realising that depression is far more likely to occur if a patient is physically unwell, especially in the context of certain diseases, can aid early identification and management. It has been proposed that depression affects prognosis both directly (as described for cardiac disease) and indirectly in terms of participation in treatment and rehabilitation programmes (Reference Lauzon, Beck and HuynhLauzon et al, 2003).
From the above there is strong evidence that there exists a link between inflammation and depression. At present there is insufficient evidence to determine which is the true causative process: whether increased pro-inflammatory cytokine levels are the core pathological change in depression or whether it is an effect of another core process. The role of inflammation in various pathologies, from cardiovascular disease to cancer, is being better understood with time, and the role of inflammation in depression may explain the striking comorbidity between depression and these illnesses. This research also provides new opportunities to understand the pathophysiology of depression and to expand our paradigms for investigating new treatments.
Declaration of interest
None.
MCQs
-
1 The following have been found consistently in depression:
-
a raised pro-inflammatory cytokines
-
b raised alanine aminotransferase
-
c high rheumatoid factor
-
d raised cholesterol
-
e raised troponin 1.
-
-
2 Regarding cardiovascular disease and depression:
-
a depression has a prevalence of >50% in the year following acute coronary syndrome
-
b there is no consistent association between depression and mortality after acute coronary syndrome
-
c depression as a risk factor for acute coronary syndrome disappears when lifestyle factors, lipid profile and similar variables are factored in
-
d there is little association between the inflammatory profile of acute coronary syndrome and depression
-
e after acute coronary syndrome incident depression is more strongly associated with mortality than is depression that has a previous history.
-
-
3 As regards immunotherapy and depression:
-
a treatment with alpha interferon produces high levels of neuropsychiatric side-effects
-
b over 50% of patients treated with alpha interferon become depressed
-
c interferon treatment for diseases other than hepatitis C does not result in neuropsychiatric side-effects
-
d people with hepatitis C who do not receive alpha interferon do not exhibit psychiatric symptoms
-
e there is little evidence that alpha interferon causes depression.
-
-
4 Inflammation and the brain:
-
a depression is associated with lower CRH in the CNS
-
b increased CRH in the CNS leads to decreased levels of natural killer cells
-
c neurovegetative symptoms induced by alpha interferon are responsive to antidepressants
-
d TNF-α decreases the release of CRH
-
e pro-inflammatory cytokines have limited effects on glucocorticoid receptor function.
-
-
5 Possible mechanisms linking depression and inflammation:
-
a alpha interferon inhibits IDO
-
b increase in tryptophan has been associated with alpha interferon treatment
-
c the serotonin transporter is activated by pro-inflammatory cytokines
-
d the serotonin transporter is downregulated by IL-6
-
e the principal protein kinase system relating the serotonin system with inflammation is JNK.
-
MCQ answers
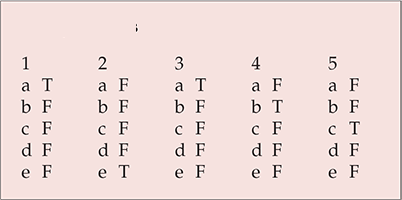
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | T | a | F | a | T | a | F | a | F |
| b | F | b | F | b | F | b | T | b | F |
| c | F | c | F | c | F | c | F | c | T |
| d | F | d | F | d | F | d | F | d | F |
| e | F | e | T | e | F | e | F | e | F |



eLetters
No eLetters have been published for this article.