Hyperthermia syndromes are conditions with different aetiologies that present with fever as a final pathway. Some important causes of hyperthermia are listed in Box 1. Presentation with hyperthermia in a psychiatric setting can provoke anxiety, with uncertainties about diagnosis, management and referral.
BOX 1 Differential diagnosis of hyperthermia syndromes
-
• Neuroleptic malignant syndrome
-
• Serotonin syndrome
-
• Malignant (or lethal) catatonia
-
• Anticholinergic toxicity syndrome
-
• Exertional heat stroke
-
• Malignant hyperpyrexia
-
• Parkinsonism–hyperpyrexia syndrome
-
• Sepsis
-
• Encephalitis, meningitis, septicaemia
-
• Thyrotoxicosis (‘thyroid storm’)
-
• Overdoses with sympathomimetics and other drugs
-
• Alcohol or drug withdrawal delirium
This article will primarily focus on neuroleptic malignant syndrome, serotonin syndrome and malignant (or lethal) catatonia.
Neuroleptic malignant syndrome
Background
Neuroleptic malignant syndrome is an uncommon and potentially fatal idiosyncratic reaction associated with the use of dopamine antagonists.
First reported by Reference AydAyd (1956), it was described as syndrome malin des neuroleptiques by Reference Delay, Pichot and LemperiereDelay (1960) (syndrome malin is a non-specific term for fulminant, neurovegetative and hyperthermic states preceding collapse and death), before being named neuroleptic malignant syndrome (Reference Delay, Deniker, Vinken and BruynDelay 1968).
Previous suggestions for renaming the syndrome to focus the attention on hyperthermia have included ‘extrapyramidal symptoms with fever’ (Reference Levinson and SimpsonLevinson 1986), ‘hypodopaminergic hyperpyrexia syndrome’ (Reference KhaldarovKhaldarov 2000) and ‘drug-induced hyperthermic catatonia’ (Reference Adityanjee and AderibigbeAdityanjee 1999).
Epidemiology
Traditionally, the incidence of neuroleptic malignant syndrome is believed to be 0.5–1.0% of all antipsychotic-treated individuals, although more recent estimates are 0.1–0.2% (Reference Caroff and MannCaroff 1993), with a range of 0.02–3.23% for conventional anti-psychotics (Reference Mann, Caroff and KeckMann 2003). Atypical antipsychotics are probably associated with a lower incidence.
The incidence of the syndrome appears to be very similar the world over; for example, 1.41 per 1000 cases in India (Reference Chopra, Prakash and RaguramChopra 1999); 1.23/1000 in China (Reference Deng, Chen and PhillipsDeng 1990); and 1.65/1000 in Mexico (Reference Montoya, Ocampo and Torres-RuizMontoya 2003). The incidence has been diminishing over time (Reference Keck, Pope and McElroyKeck 1991), although older studies with higher incidence were mostly retrospective and had small sample sizes (Reference Gurrera, Simpson and TsuangGurrera 2007).
The mortality rates have ranged from 20 to 38% (e.g. Reference Abbott and LoizouAbbott 1986; Reference Chopra, Prakash and RaguramChopra 1999). Over the past two decades, early recognition and improved management have reduced mortality to <10%.
Risk factors
Neuroleptic malignant syndrome has been reported in all age groups (range 3–64 years; mean age 40 years) (Reference Mann, Caroff and KeckMann 2003). There is a higher risk in younger patients receiving high doses of antipsychotics and it is more common in men, who tend to present with greater agitation in psychosis and therefore receive higher doses of antipsychotics.
Neuroleptic malignant syndrome is not specific to any psychiatric diagnosis and can occur even in the absence of a psychiatric disorder. There is an increased risk in patients with organic mental disorders, intellectual disability, head injury, basal ganglia dysfunction and any cause of decreased central dopamine (e.g. Parkinsonism). The common risk factors are listed in Box 2.
BOX 2 Risk factors for neuroleptic malignant syndrome
Patient characteristics
-
• Younger age
-
• Male
-
• Increased psychomotor activity
-
• Physical exhaustion
-
• Dehydration and electrolyte imbalance
Past and family history
-
• Previous episode of neuroleptic malignant syndrome
-
• Family history of neuroleptic malignant syndrome
Environmental factors
-
• High ambient temperature and humidity
Diagnosis and clinical features
-
• Organic mental disorders (especially dementia with Lewy bodies, basal ganglia dysfunction)
-
• Comorbid substance misuse
-
• Catatonic symptoms
Medication issues
-
• Use of dopamine antagonists for non-psychiatric purposes (e.g. anti-emetic or sedative action)
-
• Antipsychotics
High loading dose; faster rate of loading High potency
Parenteral (intramuscular/intravenous) administration
Depot administration
Sudden withdrawal
Investigations
-
• Low serum iron levels
-
• Raised creatine kinase levels in the setting of acute psychosis
The syndrome has been associated with anti-psychotics used as anti-emetics or sedatives, as well as with other dopamine antagonists such as promethazine and metoclopramide. There have been at least 28 reports of the syndrome in peri-operative settings, half of these related to haloperidol used to treat delirium and agitation (Reference Caroff, Rosenberg and MannCaroff 2001).
There is an increased risk with alcohol and substance use disorders (Reference LevensonLevenson 1985), particularly during alcohol/drug withdrawal, when thermo-regulatory and autonomic mechanisms are already compromised.
Iron plays an important part in the dopaminergic function. A few preliminary studies have found low serum iron in neuroleptic malignant syndrome (Reference Carroll and GoforthCarroll 1995). There is, however, a poor correlation between central and peripheral iron levels, and serum iron is not useful as a diagnostic marker.
It is unclear whether a family history of neuroleptic malignant syndrome is a risk factor. The syndrome has been described in a mother and two daughters (all with catatonic schizophrenia), twin brothers with schizophrenia, and two siblings with gangliosidosis type II. A shared vulnerability in central dopamine systems has been hypothesised (Reference Otani, Horiuchi and KondoOtani 1991). A familial occurrence has also been described in malignant catatonia (Reference StauderStauder 1934).
Aetiology
Neuroleptic malignant syndrome is hypothesised to be due to hypodopaminergia caused by a sudden and massive blockade of postsynaptic dopamine receptors (Reference Osman and KhurasaniOsman 1994) or by dopamine receptor down-regulation. A reduced dopamine drive in the hypothalamus and diencephalon leads to hyperthermia, catatonic features and autonomic dysfunction; decreased dopamine in basal ganglia causes extrapyramidal side-effects, and decreased dopamine in mesocortical regions can cause clouding of consciousness (Reference Mann, Caroff and BleierMann 1986).
Alterations in other neurotransmitters (e.g. serotonin (5-HT), glutamate, γ-aminobutyric acid (GABA)), ion channel abnormalities, and dysfunction of frontal neocortical, limbic and brainstem circuitry have been hypothesised.
Antipsychotics interfere with central thermo-regulation and are typically known to reduce body temperature, especially in hyperpyrexia (Reference Hoagland and BishopHoagland 1961; Reference Exton-SmithExton-Smith 1972). Chlorpromazine was an important component of the ‘lytic cocktail’ (pethidine, promethazine and chlorpromazine) previously used in the treatment of eclampsia. Neuroleptic malignant syndrome is an idiosyncratic response to antipsychotics and presents with hyperthermia. The role of dopamine, 5-HT, acetylcholine and noradrenaline is discussed in detail by Reference Mann, Caroff and KeckMann (2003).
Neuroleptic malignant syndrome is more likely to occur during switching, discontinuation or restarting of antipsychotics, when dopaminergic systems are relatively unstable.
Antipsychotics and neuroleptic malignant syndrome
Neuroleptic malignant syndrome is an idiosyncratic reaction which can be seen at therapeutic doses. It can occur even after a single dose of an antipsychotic in a susceptible individual. There is an increased risk and higher mortality with depot antipsychotics (with more reports with fluphenazine decanoate) (Reference Deng, Chen and PhillipsDeng 1990; Reference Chopra, Prakash and RaguramChopra 1999).
A combination of the risk factors listed in Box 2, such as rapidly escalating doses of high-potency parenteral antipsychotics, may be associated with a greater risk of developing the syndrome (Reference Chopra, Prakash and RaguramChopra 1999). Paradoxically, there are reports of neuroleptic malignant syndrome associated with sudden withdrawal of antipsychotics (Reference Dharmarajan, Bullecer and GorichDharmarajan 2001).
Neuroleptic malignant syndrome has been reported with almost all typical antipsychotics.
Atypical antipsychotics
Neuroleptic malignant syndrome with atypical antipsychotics is believed to be less frequent and milder in intensity, with extreme temperatures and elevated creatine kinase less common (Reference Sachdev, Kruk and KneeboneSachdev 1995), although classical full-blown neuroleptic malignant syndrome can indeed occur (Reference Hasan and BuckleyHasan 1998; Reference Yacoub and FrancisYacoub 2006).
A recent review found 88 reports of the syndrome with atypical antipsychotics, including clozapine, olanzapine, risperidone, aripiprazole, ziprasidone and quetiapine (Reference Zarrouf and BhanotZarrouf 2007).
Although some reports suggest equal incidence with typical and atypical drugs, there may be a publication bias with a ‘rush’ to report cases involving atypicals.
Neuroleptic malignant syndrome with clozapine tends to present with less rigidity and tremor (Reference Caroff, Mann and CampbellCaroff 2000).
Dopamine
That the relationship between reduced dopamine levels and neuroleptic malignant syndrome is causal is supported by the occurrence of the syndrome with dopamine antagonists, both antipsychotic and non-antipsychotic (e.g. metoclopramide), as well as with withdrawal from dopamine agonists (e.g. L-dopa, bromocriptine) (Reference LevensonLevenson 1985; Reference Nehru and AhujaNehru 2002). There are reports of decreased cerebrospinal fluid homovallinic acid (a dopamine metabolite) in neuroleptic malignant syndrome (Reference Ueda, Hamamoto and NagayamaUeda 2001).
Single photon emission computed tomography brain perfusion studies provide some support for hypodopaminergia, suggesting that basal ganglia disturbance is related to the development of neuroleptic malignant syndrome. During the acute phase, there was no binding of tracer 123I-iodobenzamide to D2-receptors and the D2-receptor binding correlated inversely with extrapyramidal side-effects (rigidity and akinesia) (Reference Jauss, Krack and FranzJauss 1996).
Clinical features
Neuroleptic malignant syndrome presents with hyperthermia, associated autonomic dysfunction (e.g. tachycardia, fluctuating blood pressure, diaphoresis, dyspnoea), extrapyramidal side-effects (e.g. generalised rigidity, dystonia, tremors) and altered consciousness.
Neuroleptic malignant syndrome typically occurs about 2 weeks after antipsychotic exposure, although it is important to remember that it can occur at any time. It worsens over 24–72 h and usually subsides within 14 days. It tends to remit once antipsychotic treatment has been withdrawn. Not surprisingly, it can last longer (up to 10–21 days) in individuals receiving depot antipsychotics.
Some of the early signs include mental status changes (particularly clouding of consciousness), catatonic symptoms, tachycardia, diaphoresis, incontinence, low-grade fever, rigidity, tremor or other extrapyramidal side-effects unresponsive to anti-Parkinsonian drugs, and an increase in creatine kinase. These non-specific signs may or may not be followed by neuroleptic malignant syndrome (Reference Caroff and MannCaroff 1993). Hyperthermia (>42°C) appears to be a late sign; fever occurs in 98% of cases, with a temperature >38°C in 87% and >40°C in 40% of patients (Reference Caroff and MannCaroff 1988).
Antipsychotic-induced catatonia can be seen as an intermediate stage in progression from simple extrapyramidal side-effects to neuroleptic malignant syndrome (Reference Woodbury and WoodburyWoodbury 1992). However, it appears that antipsychotic-induced catatonia and extrapyramidal side-effects represent earlier stages, both of which can progress to neuroleptic malignant syndrome. Catatonic symptoms can be under-diagnosed in neuroleptic malignant syndrome, although they occur in a significant number of patients (Reference Troller and SachdevTroller 1999; Reference Koch, Chandragiri and RizviKoch 2000).
Neuroleptic malignant syndrome with incomplete features (forme fruste) has been described as atypical neuroleptic malignant syndrome, mild neuroleptic malignant syndrome, neuroleptic malignant syndrome without fever, and neuroleptic malignant syndrome spectrum. Some reviews have used very broad definitions of the syndrome (e.g. Reference KellamKellam 1987), whereas Reference Adityanjee and SinghAdityanjee (1988) emphasised the need for a narrower definition. He suggested that neuroleptic malignant syndrome should not be diagnosed in the absence of even one of the four essential components: altered sensorium, muscular rigidity, hyperpyrexia of unknown origin (with temperature >39°C) and autonomic dysfunction (Reference Adityanjee and SinghAdityanjee 1988). Raised creatine kinase alone is insufficient for a diagnosis of neuroleptic malignant syndrome.
Diagnosis
There are many sets of diagnostic criteria for neuroleptic malignant syndrome. The DSM–IV–TR criteria (American Psychiatric Association 2000) require presence of hyperthermia (>38°C) and severe muscle rigidity following use of anti-psychotics, with two of the following features: diaphoresis, dysphagia, tremor, incontinence, changes in consciousness level, mutism, tachycardia, increased/labile blood pressure, raised creatine kinase and leucocytosis. It is important to exclude a neurological/medical cause.
The severity and progress of neuroleptic malignant syndrome can be measured using an objective scale such as the Hynes–Vickar Scale (Reference Hynes and VickarHynes 1996) or the Francis–Yacoub NMS Rating Scale (Reference Yacoub and FrancisYacoub 2006).
Serotonin syndrome
Background
Serotonin syndrome is characterised by increased 5-HT activity in the central nervous system (CNS), presenting with the classic clinical triad of mental status changes, autonomic hyperactivity and neuromuscular abnormalities. The mental status changes can range from anxiety and agitation to extreme confusion, and autonomic hyperactivity is manifested by increased heart rate, tremor, flushing, hyperthermia and excessive sweating. The neuromuscular abnormalities can include generalised hyperreflexia, clonus (e.g. ankle clonus, ocular clonus), myoclonus and rigidity (Reference Isbister, Buckley and WhyteIsbister 2007).
The first case was described by Reference MitchellMitchell (1955) as ‘toxic encephalitis’ due to the co-prescription of meperidine (pethidine) and iproniazid. Later, Reference Oates and SjoerdsmaOates & Sjoerdsma (1960) described ‘indoleamine syndrome’ following the co-prescription of L-tryptophan and a monoamine oxidase inhibitor (MAOI).
Hyperthermia appeared as a clinical feature in later reports and the condition was renamed serotonin syndrome (Reference Insel, Roy and CohenInsel 1982). The number of reported cases rapidly increased from 38 (Reference SternbachSternbach 1991) to 168 (Reference Mann, Caroff and KeckMann 2003).
Epidemiology
The exact incidence is difficult to assess; however, it is seen in all age groups. It occurs in 15% of those who overdose on selective serotonin reuptake inhibitors (Reference Isbister, Buckley and WhyteIsbister 2007). Estimated rates per 1000 patient-months of treatment are 0.5 for fluoxetine and moclobemide, 0.6 for sertraline, and 0.9 for paroxetine and venlafaxine. Despite this significant incidence, psychiatrists are probably more aware of neuroleptic malignant syndrome than serotonin syndrome as a cause of hyperthermia. Certainly, about 85% of general practitioners are unaware of serotonin syndrome (Reference Mackay, Dunn and MannMackay 1999).
Risk factors
The most common risk factor is co-prescription of serotonergic drugs, especially with MAOIs. Although some individuals appear more sensitive, it is not clear whether this is due to serotonin receptor polymorphism(s), pharmacokinetic mechanisms or other factors (Reference Isbister, Buckley and WhyteIsbister 2007).
Aetiology
Serotonin syndrome occurs because of excessive CNS 5-HT levels, which can result from several mechanisms (Table 1). In most cases, it arises when two serotonergic drugs are administered simultaneously, although serotonin syndrome can occur with the initiation of a single drug or increasing the dose in a ‘sensitive’ individual. It is much more common following overdoses, and there are some reports with therapeutic doses. Therefore, it has been suggested that serotonin toxicity (Reference Isbister, Buckley and WhyteIsbister 2007) is a better term than serotonin syndrome.
Stimulation of 5-HT2A and possibly postsynaptic 5-HT1A receptors has been implicated in causing serotonin syndrome, and there is some evidence that hyperthermia and rigidity may be mediated receptors. by 5-HT2A
Clinical features
Serotonin toxicity can be mild, but may progress to become life threatening. The initial symptoms include akathisia, agitation, tremor, tachycardia, autonomic instability (usually hypertension), increased bowel sounds, diarrhoea, mydriasis and altered mental status.
Clonus can be induced by the examining doctor and in more severe cases is sustained. Ocular clonus can present as slow, continuous, horizontal eye movements. Clonus can be followed by muscular hypertonicity and potentially fatal hyperthermia. Both hyperreflexia and clonus are more severe in the lower extremities and their presence should prompt a consideration of serotonin syndrome (Reference Dunkley, Isbister and SibbrittDunkley 2003).
There are similarities with other hyperthermia syndromes; however, hyperreflexia and clonus (ankle and ocular) appear to be specific features of serotonin syndrome (Table 2).
It is important to recognise the earlier stages of serotonin syndrome, as continuing or increasing the offending drug(s) can worsen the syndrome (Reference Boyer and ShannonBoyer 2005). A description of the syndrome in incremental stages can be helpful in early identification (Reference Isbister, Buckley and WhyteIsbister 2007). Hyperthermia is present in the more severe form.
Diagnosis
As the diagnosis of serotonin syndrome is largely clinical, it is helpful to use defined diagnostic criteria. Sternbach's criteria specify ingestion of a serotonergic agent with the presence of three of the following signs: mental status changes, restlessness, myoclonus, hyperreflexia, diaphoresis, shivering, tremor, diarrhoea, incoordination and fever (Reference SternbachSternbach 1991). It is important to exclude other causes, including new antipsychotic use or increased dose of an existing antipsychotic.
Hunter criteria have higher specificity (97%) and sensitivity (84%) than Sternbach's criteria (Reference Dunkley, Isbister and SibbrittDunkley 2003). Following ingestion or overdose of a serotonergic agent, any one of the following must be present (adapted from Reference Isbister, Buckley and WhyteIsbister 2007):
Malignant (or lethal) catatonia
Malignant catatonia was first described by Calmeil in 1832, later named ‘Bell's mania’ in 1849, and then termed ‘lethal catatonia’ by Stauder in 1934 (cited in Reference KellamKellam 1987). There is usually a prodrome of about 2–8 weeks, characterised by non-specific or frank psychotic symptoms. It then presents with multiple, severe catatonic symptoms (Box 3), including intense motor excitement lasting several hours or days. Clouding of consciousness is a prominent feature. Autonomic dysfunction (e.g. tachycardia, diaphoresis, fluctuating blood pressure) is often associated with other catatonic signs (e.g. mutism, alternating stupor/excitement, refusal of food and fluids) and extreme hyperthermia. In later stages, excitement can be followed by stupor, coma and eventually death.
BOX 3 Catatonic signs and symptoms seen in malignant catatonia1
Ambitendency: motor indecisiveness and hesitancy.
Automatic obedience: exaggerated cooperation with examiner's request. Excessive cooperation despite requests to the contrary is called mitmachen, whereas the more severe forms are called mitgehen. Repeated inviting gestures (e.g. extending one's hands) despite instructions are called gegengreifen. It can include forced grasping of examiner's hand when it is offered.
Echophenomena: mimicking of another's movements, gestures, expressions, postures (echopraxia) or speech (echolalia).
Excitement: severe, non-goal-directed constant motor hyperactivity that can include aggression and combativeness.
Gegenhalten: resistance to passive movement proportional to the strength of the stimulus. This is often associated with rigidity (it is important to exclude extrapyramidal rigidity).
Grimacing: odd facial expressions maintained.
Mannerisms: repetitive, goal-directed but semi-purposive movements (e.g. saluting).
Mutism: markedly decreased or absent verbal responsiveness.
Negativism: resistance to instructions/attempts to move/examine or contrary behaviour (doing opposite of the instruction/attempt).
Perseveration: repetition of the same topic or movement when no longer relevant (e.g. what is your name: Rob; where do you live: Rob; what date is it today: Rob).
Posturing: spontaneously maintained posture for long periods; the most severe form is called catalepsy; psychological pillow is posturing of head above the bed without any support. This is often associated with waxy flexibility which allows re-posturing by examiner, with initial resistance before repositioning (wax-like flexibility).
Staring: fixed and/or avoidant gaze, decreased blinking.
Stereotypies: repetitive, non-goal-directed movements (e.g. repetitive tapping of forehead).
Stupor: severe decrease in activity, immobility and/or minimal responsiveness to stimuli, often co-existing with mutism.
Verbigeration: meaningless repetition of phrases/sentences; a severe form is called logorrhoea.
Withdrawal: refusal to eat, drink and/or make eye contact.
1. Excitement, withdrawal or a combination of both can be present.
The most likely pathophysiology of malignant catatonia involves decreased dopamine, particularly in the basal ganglia and hypothalamus (Reference Mann, Caroff and KeckMann 2003). Post-mortem studies have shown small but discrete haemorrhages in the hypothalamus and pituitary (Reference Billig and FreemanBillig 1944).
Catatonic symptoms can occur in several other neurological, medical and psychiatric disorders (Reference GelenbergGelenberg 1976; Reference AhujaAhuja 2000; Reference Fink and TaylorFink 2003; Reference Mann, Caroff and KeckMann 2003). Mood disorders, rather than schizophrenia, are the most common psychiatric cause (Reference Abrams and TaylorAbrams 1976).
It has been suggested that malignant catatonia and neuroleptic malignant syndrome are virtually indistinguishable, except for exposure to dopamine antagonists in the latter (Reference Ahuja and NehruAhuja 1990) (Table 2). Catatonia appears to be the harbinger of (and a risk factor for) neuroleptic malignant syndrome (Reference WhiteWhite 1992) and may constitute antipsychotic-induced malignant catatonia (Reference Mann, Caroff and BleierMann 1986, Reference Mann, Caroff and Keck2003). Episodes of malignant catatonia and neuroleptic malignant syndrome have been described in the same patient (Reference WhiteWhite 1992).
The severity of catatonia can be recorded and monitored by using the Catatonia Rating Scale (Reference Bush, Fink and PetridesBush 1996). The key aspects of catatonia were reviewed in Advances by Reference RajagopalRajagopal (2007).
Although mortality due to malignant catatonia has decreased recently, it is still reported to be around 9% (Reference Mann, Caroff and KeckMann 2003).
Other hyperthermia syndromes
Exertional heat stroke
Hyperthermia due to exertional heat stroke is uncommon in the UK (annually, ∼80 cases per million population); however, the heat wave in 2003 led to 11 435 deaths in France (especially in the elderly in residential care) and another 11 000 deaths in Finland. This brings to the fore the importance of considering exertional heat stroke as a differential diagnosis.
Parkinsonism–hyperpyrexia syndrome
This has also been called neuroleptic malignant-like syndrome, as its pathophysiology and clinical presentation are similar to those of neuroleptic malignant syndrome. First described in 1981, it has also been called dopa-withdrawal malignant syndrome and acute dopamine depletion syndrome (Reference Nehru and AhujaNehru 2002).
The triggers of Parkinsonism–hyperpyrexia syndrome in Parkinsonism are very similar to those seen in neuroleptic malignant syndrome, for example dopamine withdrawal, intercurrent infection(s), dehydration, hot weather and decreased dopamine in ‘off’ periods of ‘on–off’ effects (Reference Mizuno, Takubo and MizutaMizuno 2003).
Malignant hyperpyrexia
First described in 1960, malignant hyperpyrexia is a hypersensitive reaction in genetically predisposed individuals when exposed to certain anaesthetics (such as halothane and suxamethonium) (Reference Denborough and LovellDenborough 1960). Reference Lazarus and RosenbergLazarus & Rosenberg (1991) report a case of malignant hyperpyrexia in a patient receiving electroconvulsive therapy.
The pattern of inheritance is autosomal dominant with variable penetrance, with several loci including 19q13. It is usually caused by mutations in the ryanodine receptor 1 (RYR1) gene, which encodes the key channel that mediates calcium release in skeletal muscles during excitation–contraction coupling (Reference Mizuno, Takubo and MizutaMizuno 2003). Malignant hyperpyrexia is characterised by increased reuptake of calcium, necessary for termination of muscle contraction, by the sarcoplasmic reticulum. This leads to sustained muscle contraction and subsequent hyperthermia.
Diagnostic evaluation and investigations of hyperthermia syndromes
It is important to take seriously the occurrence of fever in any patient on psychotropic medication, as early detection can help prevent progression to hyperthermia. It is also essential to rule out other causes of hyperthermia (Box 1). A detailed history including medication, physical examination (with emphasis on the CNS) and comprehensive laboratory evaluation are helpful.
Creatine kinase
Creatine kinase is markedly raised in 97% of cases of neuroleptic malignant syndrome (Reference LevensonLevenson 1985; Reference Caroff and MannCaroff 1993), and levels of 15 000–45 000 mU/ml are not unusual. Excess creatine kinase in neuroleptic malignant syndrome originates largely from striated muscles (creatine kinase MM isoenzyme).
There are reports of raised creatine kinase with clozapine in the absence of other features of neuroleptic malignant syndrome, but the syndrome is also reported without significant increases in creatine kinase. Increased creatine kinase is not an essential criterion for the diagnosis of neuroleptic malignant syndrome and appears to be a non-specific epiphenomenon rather than a core feature (Adityanjee 1991). However, if creatine kinase is high, regular (daily) monitoring is helpful in estimating progress of muscle breakdown.
Raised creatine kinase is also reported in 67% of malignant catatonia cases (Reference Mann and CaroffMann 1987) as well as in serotonin syndrome. There are reports of transient creatine kinase increases with acute psychosis in up to 70% of in-patients (Reference Meltzer, Ross-Santon and SchelssingerMeltzer 1980) (Box 4). High creatine kinase in acute psychosis may constitute a risk factor for neuroleptic malignant syndrome.
BOX 4 Causes of raised creatine kinase
-
• Neuroleptic malignant syndrome
-
• Any cause of hyperthermia such as serotonin syndrome, malignant catatonia
-
• Any cause of muscle damage causing rhabdomyolysis:
-
• intramuscular injections
rigidity
agitation
acute psychosis
ischaemia
use of restraints
isotonic muscle exercise
dystonia with antipsychotics
-
• Muscular dystrophies and disorders causing rhabdomyolysis
-
• Myocardial injury, including myocardial infarction (modest rise in creatine kinase MB fraction)
Other laboratory investigations
Leucocytosis is seen in 79–98% of neuroleptic malignant syndrome cases (Reference LevensonLevenson 1985; Reference Caroff and MannCaroff 1993). There can be increased serum aldolase, lactate dehydrogenase and/or transaminases; hypoxia; metabolic acidosis; hyperglycaemia; hypo- or hypernatraemia; raised prothrombin time; and reduced platelets (with disseminated intravascular coagulation). Similar changes can be seen in malignant catatonia (Reference Mann, Caroff and BleierMann 1986) and serotonin syndrome.
Appearance of myoglobinuria is an indicator of risk of acute renal failure. Non-specific electroencephalogram abnormalities can be seen in nearly half the cases (Reference Mann, Caroff and KeckMann 2003).
Muscle biopsy
The toxic myopathy in neuroleptic malignant syndrome and malignant hyperpyrexia can appear similar and a peripheral mechanism for hyperthermia in neuroleptic malignant syndrome has been suggested.
In vitro muscle contracture testing can be used to differentiate between neuroleptic malignant syndrome and malignant hyperpyrexia (Reference Caroff, Rosenberg and GerberCaroff 1983). In the halothane–caffeine contracture test, the malignant hyperpyrexia muscle in vitro responds to halothane with a contracture, whereas a normal muscle responds to caffeine. The false-positive rate with this test is 10–20%, but the false-negative is close to 0%. In the ryanodine contracture test, the plant alkaloid ryanodine binds specifically and strongly to the calcium release channels of the muscle's sarcoplasmic reticulum, the proposed site of malignant hyperpyrexia defect. In malignant hyperpyrexia, the defined contracture levels are reached significantly sooner than in neuroleptic malignant syndrome or controls.
Outcome of hyperthermia syndromes
People with mild cases of hyperthermia syndromes, particularly serotonin syndrome, usually make a good recovery on withdrawal of the offending agent(s). Serious complications can occur, such as rhabdomyolysis with raised creatine kinase, myoglobinuria and metabolic acidosis. In neuroleptic malignant syndrome, death can result from cardiac or respiratory arrest occurring suddenly or following cardiac failure, myocardial infarction, cardiac arrhythmias, aspiration pneumonia, pulmonary emboli, myoglobinuric renal failure or disseminated intravascular coagulation (Reference Mann, Caroff and KeckMann 2003). Hyperthermia of >39.5°C correlates highly with mortality (Reference Reulbach and BleichReulbach 2005). Appearance of disseminated intravascular coagulation or acute myoglobinuric renal failure is an ominous sign.
Persistent amnestic syndrome has been described with hyperthermia. There are reports of extrapyramidal or cerebellar disorders that may persist for weeks, months or indefinitely (Reference Rosebush and StewartRosebush 1989a).
Treatment issues
The importance of prevention (by reducing modifiable risk factors), early recognition (by considering the possibility of hyperthermia syndromes at the onset of fever), exclusion of other causes (particularly sepsis) and correct diagnosis cannot be overemphasised.
Stopping the offending drug
The most important first step is to discontinue the offending drug(s) in both neuroleptic malignant syndrome and serotonin syndrome. It is important not to prescribe any antipsychotic in malignant catatonia and neuroleptic malignant syndrome, as it is likely to worsen the presentation.
Supportive measures
Treatment is best provided in emergency medicine and supportive measures are absolutely vital. Initial treatment must focus on patency of the airway, breathing and maintenance of circulation. Specific pharmacological measures should be considered later.
The supportive measures may include control of hyperthermia, sedation, intubation, ventilatory support, neuromuscular paralysis, control of autonomic instability and treatment of any infections. Management of fluid and electrolyte balance is important, as dehydration is one of the common complications (Reference Levinson and SimpsonLevinson 1986). Disseminated intravascular coagulation, if present, needs to be treated urgently.
The use of antipyretics in an established hyperthermia syndrome is not usually helpful as drugs such as paracetamol require ‘functioning’ hypothalamic thermoregulatory mechanisms (Reference McGuganMcGugan 2001).
At least one review (Reference McGuganMcGugan 2001) suggests that lowering the body temperature to <38.9°C within 30 min of presentation improves survival in hyperpyrexia. Rapid cooling with the help of tepid water spray (to encourage evaporative cooling) and ice packs to axilla, neck and groin are helpful.
Prompt treatment is essential, as morbidity and mortality increase with the duration of hyperthermia. Physical restraint is ill advised given the risk of lactic acidosis, myoglobinuria and worsening of hyperthermia.
Renal dialysis is helpful in patients with acute renal failure secondary to myoglobinuria. It is, however, ineffective in removing antipsychotics and antidepressants, most of which are protein and lipid bound.
Dopamine agonists
Dopamine agonists are helpful in treatment of disseminated intravascular coagulation, although a response in malignant catatonia is uncommon.
Bromocriptine can be given either orally or through a nasogastric tube (2.5–7.5 mg, three or four times a day; maximum dose 60 mg/24 h). An early start is really important as the onset of action is at least 4 h. Other dopaminergic drugs such as levodopa (oral/intravenous), apomorphine and amantadine (also a glutamate antagonist) have also been used beneficially.
Dopaminergic agents have the potential of worsening the underlying psychosis.
It is important to differentiate serotonin syndrome from neuroleptic malignant syndrome in patients who receive both antipsychotics and serotonergic agents. Unless a confident diagnosis of either syndrome is made, patients should not receive bromocriptine, which is helpful for neuroleptic malignant syndrome but can exacerbate serotonin syndrome. Bromocriptine should not be used if an MAOI has been used prior to the onset of symptoms.
Dantrolene
Dantrolene is helpful in treatment of malignant hyperpyrexia and its use has been extended to neuroleptic malignant syndrome. A peripheral muscular relaxant, it acts by inhibiting ionised calcium release from sarcoplasmic reticulum, resulting in direct muscular relaxation and reduction in fever and rigidity. It can be administered parenterally (50–75 mg intravenously immediately, and then every 6 h; maximum dose 10 mg/kg every 24h).
Response to dantrolene is better in malignant hyperpyrexia than in neuroleptic malignant syndrome. Although it can be a helpful adjunct to bromocriptine, dantrolene is not recommended as a solitary treatment for neuroleptic malignant syndrome (Reference Reulbach, Dütsch and BiermannReulbach 2007).
5-HT antagonists
Cyproheptadine, a 5-HT and histamine H1 antagonist with antimuscarinic properties, is a useful treatment for serotonin syndrome.
Most cases of serotonin syndrome typically resolve within 24 h after the offending drug(s) have been stopped, although administration of oral cyproheptadine (4–12 mg initial dose; maximum dose 32 mg/24 h in four divided doses) is helpful. Rapid reversal of mydriasis by cyproheptadine can suggest a diagnosis of serotonin syndrome and a good eventual response (Reference McDanielMcDaniel 2001). Cyproheptadine is contraindicated if overdose of another anticholinergic drug is suspected.
Other 5-HT antagonists (e.g. chlorpromazine, propranolol) have been used in serotonin syndrome. Chlorpromazine should not be given if neuroleptic malignant syndrome is suspected.
Benzodiazepines
Benzodiazepines (lorazepam 1–8 mg/24 h oral or parenteral; or diazepam) are helpful in neuroleptic malignant syndrome, malignant catatonia and serotonin syndrome. They act by GABA-mimetic activity and it has been suggested that they also indirectly increase dopaminergic function in the basal ganglia (Reference KhaldarovKhaldarov 2000).
In malignant catatonia, higher doses of lorazepam (up to 8–24 mg/24 h) have been recommended (Reference Fink and TaylorFink 2003). Benzodiazepines are helpful in controlling agitation, particularly as antipsychotics are unsafe in neuroleptic malignant syndrome and malignant catatonia.
Anticholinergics
Anticholinergics should be helpful in preventing neuroleptic malignant syndrome by reducing the extrapyramidal side-effects of antipsychotics (poorly controlled extrapyramidal side-effects are a risk factor for the syndrome; Reference Levinson and SimpsonLevinson 1986). However, by inhibiting sweating, they increase the likelihood of neuroleptic malignant syndrome and worsen it once already present. Anticholinergics have no role in the treatment of neuroleptic malignant syndrome, exertional heat stroke, malignant catatonia, malignant hyperpyrexia or serotonin syndrome.
Electroconvulsive therapy
Electroconvulsive therapy can be indicated in the treatment of malignant catatonia and neuroleptic malignant syndrome. It was helpful in 74% of severe neuroleptic malignant syndrome cases, in particular for individuals with psychotic depression or catatonia, with onset of response occurring after four sessions of therapy (Reference Troller and SachdevTroller 1999). Electroconvulsive therapy is an effective and safe mode of treatment, although Troller & Sachdev advise caution regarding increased risk of cardiovascular complications.
Re-challenge in neuroleptic malignant syndrome
Re-emergence of neuroleptic malignant syndrome occurs on re-challenge with antipsychotics in more than a third of patients. Re-exposure should be carried out very carefully and a wait of at least 2 weeks after full resolution of symptoms is recommended (Reference Rosebush, Stewart and GelenbergRosebush 1989b).
Conclusions
The emergence of fever in psychiatric patients is diagnostically and therapeutically challenging. Early consideration of treatment-related hyperthermia syndromes is vital. The keys to successful treatment and good outcome are rapid withdrawal of the offending drug and supportive therapies. New classes of psychotropic medication appear to be altering the epidemiology of hyperthermia syndromes. Psychiatrists need to be aware of this and of their role in alerting colleagues in primary care to the significance of serotonin syndrome.
MCQs
-
1 As regards neuroleptic malignant syndrome:
-
a non-antipsychotic dopamine antagonists do not cause neuroleptic malignant syndrome
-
b it is an idiosyncratic reaction that can occur at therapeutic doses of antipsychotics
-
c it does not result from the use of clozapine
-
d extremely elevated levels of creatine kinase are diagnostic
-
e muscle biopsy does not differentiate between neuroleptic malignant syndrome and malignant hyperpyrexia.
-
-
2 As regards serotonin syndrome:
-
a monoamine oxidase inhibitors are unlikely to be the cause
-
b it is an idiosyncratic reaction that generally occurs at therapeutic doses of serotonergic agents
-
c hyperthermia is usually a late feature
-
d the classic triad consists of autonomic hyper-activity, mental status changes and skin haematomas
-
e the drug of choice for treatment is bromocriptine.
-
-
3 As regards catatonic syndromes:
-
a catatonia with hyperthermia came to be recognised only in ‘the antipsychotic era’
-
b catatonic symptoms can be a harbinger of neuroleptic malignant syndrome
-
c benzodiazepines should be avoided because of the risk of respiratory failure
-
d the most common psychiatric cause is schizophrenia
-
e the treatment of choice is a low dose of atypical antipsychotics.
-
-
4 As regards hyperthermia syndromes:
-
a there is widespread awareness among general practitioners regarding diagnosis and treatment of neuroleptic malignant syndrome and serotonin syndrome
-
b hyperreflexia and spontaneous clonus are characteristic features of severe neuroleptic malignant syndrome
-
c unlike Parkinsonism–hyperpyrexia syndrome, neuroleptic malignant syndrome is characterised by central hypodopaminergia
-
d malignant hyperpyrexia is an autosomal dominant condition
-
e recovery is much quicker in neuroleptic malignant syndrome than in serotonin syndrome.
-
-
5 As regards hyperthermia syndromes:
-
a dantrolene is the drug of choice for treatment of hyperthermia syndromes
-
b the focus should always be on supportive treatments first
-
c the use of antipyretics is crucial
-
d electroconvulsive therapy is contraindicated in the treatment of neuroleptic malignant syndrome
-
e chlorpromazine is contraindicated in both neuroleptic malignant syndrome and serotonin syndrome.
-
MCQ answers
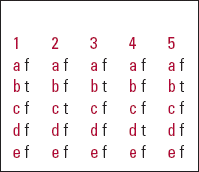
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | f | a | f | a | f | a | f | a | f |
| b | t | b | f | b | t | b | f | b | t |
| c | f | c | t | c | f | c | f | c | f |
| d | f | d | f | d | f | d | t | d | f |
| e | f | e | f | e | f | e | f | e | f |
TABLE 1 Examples of drugs involved in serotonin syndrome
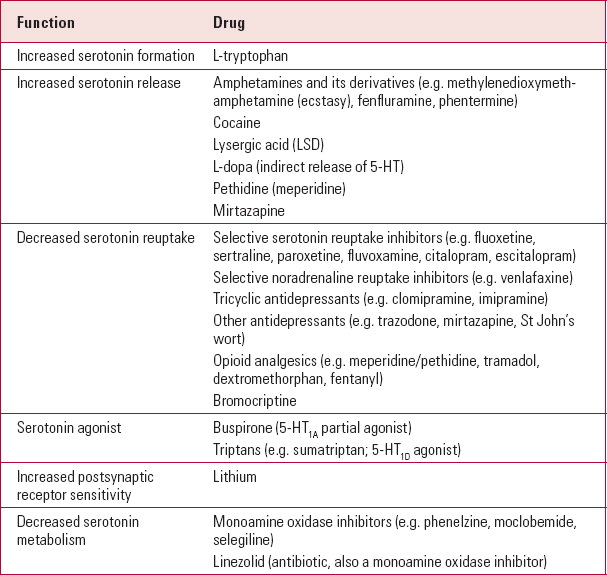
| Function | Drug |
|---|---|
| Increased serotonin formation | L-tryptophan |
| Increased serotonin release | Amphetamines and its derivatives (e.g. methylenedioxymeth- amphetamine (ecstasy), fenfluramine, phentermine) |
| Cocaine | |
| Lysergic acid (LSD) | |
| L-dopa (indirect release of 5-HT) | |
| Pethidine (meperidine) | |
| Mirtazapine | |
| Decreased serotonin reuptake | Selective serotonin reuptake inhibitors (e.g. fluoxetine, sertraline, paroxetine, fluvoxamine, citalopram, escitalopram) |
| Selective noradrenaline reuptake inhibitors (e.g. venlafaxine) | |
| Tricyclic antidepressants (e.g. clomipramine, imipramine) | |
| Other antidepressants (e.g. trazodone, mirtazapine, St John's wort) | |
| Opioid analgesics (e.g. meperidine/pethidine, tramadol, dextromethorphan, fentanyl) | |
| Bromocriptine | |
| Serotonin agonist | Buspirone (5-HT1A partial agonist) |
| Triptans (e.g. sumatriptan; 5-HT1D agonist) | |
| Increased postsynaptic receptor sensitivity | Lithium |
| Decreased serotonin metabolism | Monoamine oxidase inhibitors (e.g. phenelzine, moclobemide, selegiline) |
| Linezolid (antibiotic, also a monoamine oxidase inhibitor) |
TABLE 2 Comparison of some hyperthermia syndromes
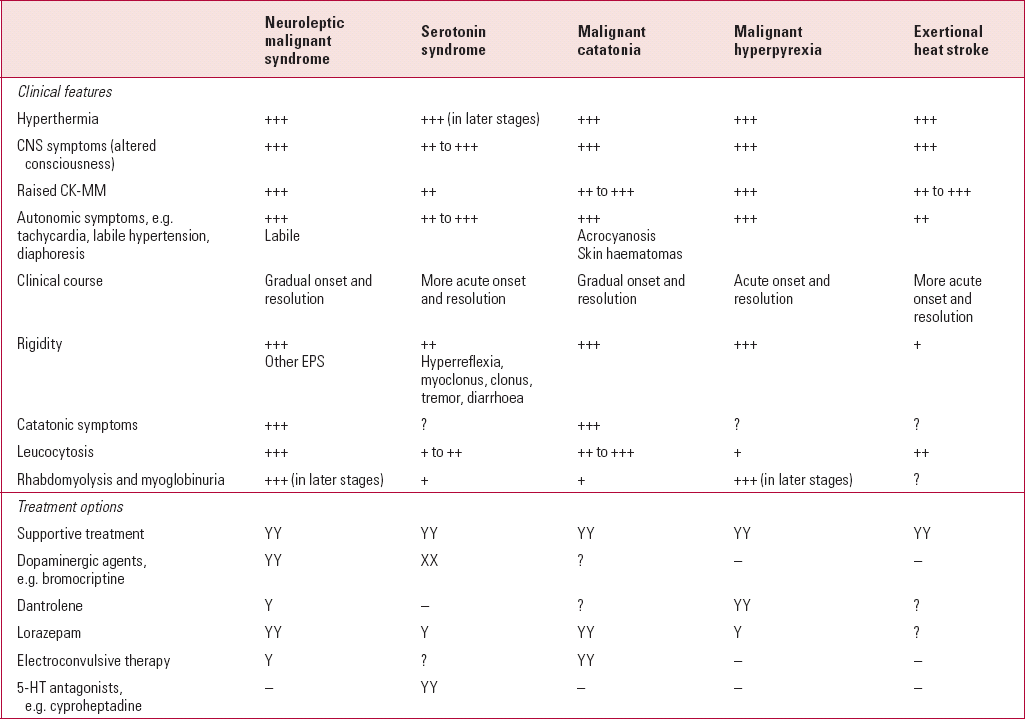
| Neuroleptic malignant syndrome | Serotonin syndrome | Malignant catatonia | Malignant hyperpyrexia | Exertional heat stroke | |
|---|---|---|---|---|---|
| Clinical features | |||||
| Hyperthermia | +++ | +++ (in later stages) | +++ | +++ | +++ |
| CNS symptoms (altered consciousness) | +++ | ++ to +++ | +++ | +++ | +++ |
| Raised CK-MM | +++ | ++ | ++ to +++ | +++ | ++ to +++ |
| Autonomic symptoms, e.g. tachycardia, labile hypertension, diaphoresis | +++ Labile |
++ to +++ | +++ Acrocyanosis Skin haematomas |
+++ | ++ |
| Clinical course | Gradual onset and resolution | More acute onset and resolution | Gradual onset and resolution | Acute onset and resolution | More acute onset and resolution |
| Rigidity | +++ Other EPS |
++ Hyperreflexia, myoclonus, clonus, tremor, diarrhoea |
+++ | +++ | + |
| Catatonic symptoms | +++ | ? | +++ | ? | ? |
| Leucocytosis | +++ | + to ++ | ++ to +++ | + | ++ |
| Rhabdomyolysis and myoglobinuria | +++ (in later stages) | + | + | +++ (in later stages) | ? |
| Treatment options | |||||
| Supportive treatment | YY | YY | YY | YY | YY |
| Dopaminergic agents, e.g. bromocriptine | YY | XX | ? | – | – |
| Dantrolene | Y | – | ? | YY | ? |
| Lorazepam | YY | Y | YY | Y | ? |
| Electroconvulsive therapy | Y | ? | YY | – | – |
| 5-HT antagonists, e.g. cyproheptadine | – | YY | – | – | – |





eLetters
No eLetters have been published for this article.