In his influential book The Mask of Sanity, Hervey Cleckley presented a series of vignettes which distilled typical features of large numbers of individuals with psychopathy whom he had interviewed (Reference CleckleyCleckley 1941). He described them as charming, callous and superficial, commenting that their lack of conscience or genuine emotion was camouflaged by the ‘mask’ of a healthy, functional individual. People with psychopathy commit a large amount and wide variety of violent and non-violent crimes, and are resistant to attempts at rehabilitation (Reference Reid and GaconoReid 2000). The definition of psychopathy has changed little since Cleckley's time, and the aetiology of the condition remains unknown.
Psychopathy shares general features with personality disorders listed in DSM-IV-TR (American Psychiatric Association 2000), even though it is not included among them. Personality disorders comprise a group of disorders that usually result in impaired interpersonal functioning. Individuals exhibit enduring patterns of cognition, emotion and behaviour that deviate markedly from cultural expectations. The three clusters of personality disorders listed by the DSM-IV-TR (American Psychiatric Association 2000) fall under the headings ‘odd-eccentric’ (Cluster A), ‘emotional-dramatic’ (Cluster B) and ‘anxious-fearful’ (Cluster C), and each contains further subtypes.
Reclassification of psychopathy
Since the publication of DSM-III (American Psychiatric Association 1980), psychopathy is no longer listed as a distinct psychiatric condition. In current diagnostic classifications psychopathy is regarded as being synonymous with antisocial personality disorder in DSM-IV-TR (American Psychiatric Association 2000) and dissocial personality disorder in ICD-10 (World Health Organization 2004). However, although both antisocial and dissocial personality disorders include several traits reflecting psychopathic personality (e.g. lack of guilt/remorse, impulsivity), it is possible to meet the diagnostic criteria for these disorders based on just the behavioural manifestations of antisocial behaviour (e.g. violation of social norms, irresponsibility, criminality). Hence, the emotional dysfunction at the core of Cleckley's notion of ‘the psychopath’ is not essential for antisocial or dissocial personality disorder, even if it is present in some cases. The Psychopathy Checklist (PCL; Reference HareHare 1980) and the later Psychopathy Checklist – Revised (PCL-R; Reference HareHare 1991, Reference Hare2003) were therefore designed to operationalise Cleckley's psychopathy construct to provide a formal diagnostic instrument for the disorder. The PCL-R is considered by many to be the gold standard instrument for this disorder (Reference Morana, Arboleda-Florez and CamaraMorana 2005).
Assessment of psychopathy and personality disorder
The PCL-R consists of 20 items falling broadly into two dimensions: Factor 1 items are predominantly emotional or interpersonal traits such as deception, remorselessness, shallow affect and callousness, whereas Factor 2 items assess behavioural manifestations of the disorder such as criminality, violence and dysfunctional lifestyle (Box 1). Characteristics from both these factors are needed for a diagnosis of psychopathy to be made. Although many consider the first dimension to differentiate psychopathy from other personality disorders (Reference KiehlKiehl 2006), there is considerable overlap between a number of these traits, not only between psychopathy and antisocial/dissocial personality disorder, but also between psychopathy and other DSM-IV Cluster B disorders (Table 1), including borderline, histrionic and narcissistic personality disorders. This supports the view that psychopathy comprises a ‘higher order’ collection of disordered personality traits from many categories (Reference Blackburn, Strack and MillonBlackburn 2005).
TabLE 1 Common traits between Cluster B personality disorders (DSM-IV-TR) and psychopathy (PCL-R)
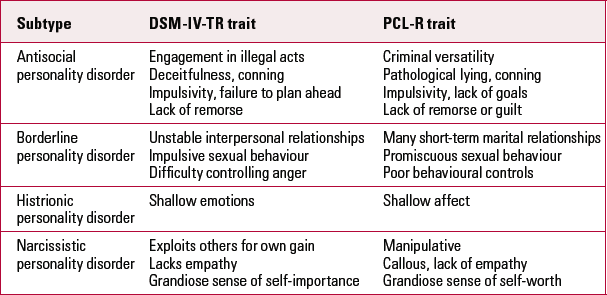
| Subtype | DSM-IV-TR trait | PCL-R trait |
|---|---|---|
| Antisocial personality disorder | Engagement in illegal acts Deceitfulness, conning Impulsivity, failure to plan ahead Lack of remorse |
Criminal versatility Pathological lying, conning Impulsivity, lack of goals Lack of remorse or guilt |
| Borderline personality disorder | Unstable interpersonal relationships Impulsive sexual behaviour Difficulty controlling anger |
Many short-term marital relationships Promiscuous sexual behaviour Poor behavioural controls |
| Histrionic personality disorder | Shallow emotions | Shallow affect |
| Narcissistic personality disorder | Exploits others for own gain Lacks empathy Grandiose sense of self-importance |
Manipulative Callous, lack of empathy Grandiose sense of self-worth |
BOX 1 The 20 items of the Psychopathy Checklist – Revised (PCL-R)
Factor 1
Interpersonal
-
• Glibness – superficial charm
-
• Grandiose sense of self-worth
-
• Pathological lying
-
• Conning – manipulative
Affective
-
• Lack of remorse or guilt
-
• Shallow affect
-
• Callous – lack of empathy
-
• Failure to accept responsibility
Factor 2
Lifestyle
-
• Need for stimulation
-
• Parasitic lifestyle
-
• Lack of realistic, long-term goals
-
• Impulsivity
-
• Irresponsibility
Antisocial
-
• Poor behavioural control
-
• Early behavioural problems
-
• Juvenile delinquency
-
• Revocation of conditional release
-
• Criminal versatility
Additional items ‘Promiscuous sexual behaviour’ and ‘Many short-term marital relationships’ do not load onto these two factors but contribute to an individual's score on this instrument.
Following a brief overview of psychopathy and the Cluster B disorders, this article reviews neurobiological correlates of psychopathy, antisocial and borderline personality disorders, focusing on findings from neuroimaging research.
Psychopathy
Recent epidemiological studies of psychopathy report that it occurs in about 0.6% of the general population (Reference Coid, Yang and UllrichCoid 2009a) and 7.7% of male prisoners (Reference Coid, Yang and UllrichCoid 2009b) in the UK. Psychopathic traits may occur in children, remaining relatively stable throughout adolescence and into adulthood (Reference Frick, Cornell and BarryFrick 2003a; Reference Loney, Taylor and ButlerLoney 2007; Reference Lynam, Caspi and MoffittLynam 2007). Even early in life, psychopathic traits (named ‘callous-unemotional’ traits when present in children) distinguish these youngsters from other children with conduct disorder with regard to the onset and severity of their antisocial behaviour and the risk of associated harm. Children with both conduct disorder and callous-unemotional traits present with more severe behaviour (Reference DolanDolan 2004; Reference Frick, Stickle and DandreauxFrick 2005), have a greater likelihood of their antisocial behaviour persisting into adulthood and manifesting as antisocial personality disorder and psychopathy (Reference Frick, Kimonis and DandreauxFrick 2003b; Reference Lynam, Caspi and MoffittLynam 2007), and have a higher risk of future substance use disorders and other adverse outcomes (Reference Lynam and GudonisLynam 2005).
Studies also suggest that conduct disorder with callous-unemotional traits has a stronger genetic basis than conduct disorder alone (Reference Viding, Blair and MoffittViding 2005), sharing unique risk factors and a significantly greater heritability rate than noncallous-unemotional groups (Reference Frick and DickensFrick 2006). Hence, psychopathy has been viewed as an early-onset developmental disorder, with specific genetic and neurocognitive constraints (Reference Blair, Mitchell and MitchellBlair 2005). Nevertheless, many authors also emphasise the importance of not applying the term ‘psychopath’ to children to avoid stigma, and because decisions about the individual case based on the construct are speculative owing to limitations in the evidence base (Reference Johnstone and CookeJohnstone 2007).
Psychopathy and its relation to antisocial personality disorder
As noted earlier, severe emotional dysfunction (e.g. lack of guilt or victim empathy) is necessary for a diagnosis of psychopathy, but not for antisocial personality disorder. Psychopathy is also distinguished by high levels of both reactive (elicited by frustration) and instrumental (goal-directed) violence (Reference BlairBlair 2001). Further, prevalence rates differ between antisocial personality disorder and psychopathy, suggesting that these diagnoses are not equivalent. In the UK, rates of antisocial personality disorder are estimated at up to 3% in the general population (Reference CoidCoid 2003) and up to 80% among prisoners (Reference HareHare 2003). This compares with less than 1% for psychopathy in the general population (Reference Coid, Yang and UllrichCoid 2009a) and just under 8% among prisoners (Reference Coid, Yang and UllrichCoid 2009b).
Although most adult offenders with psychopathy meet criteria for antisocial personality disorder, only about 30% of those with antisocial personality disorder have psychopathy (Reference Hart and HareHart 1997). This has led to the view that psychopathy should be regarded as a particularly severe subtype of antisocial personality disorder (Reference Dolan and DoyleDolan 2007).
Borderline personality disorder
Borderline personality disorder is characterised by instability in interpersonal relationships, self-image and emotion regulation and marked impulsivity. Individuals with borderline personality disorder are at a greater risk of self-injurious behaviour and suicide compared with the general population (Reference Oumaya, Friedman and PhamOumaya 2008). The incidence of borderline personality disorder in the general population ranges from 0.7 to 2% (Reference Torgersen, Kringlen and CramerTorgersen 2001; Reference CoidCoid 2003) to over 20% in psychiatric settings (American Psychiatric Association 2000) and, in contrast to antisocial personality disorder and psychopathy, is diagnosed more frequently in women – although this may reflect bias and not true gender distribution (Reference Chanen, Velakoulis and CarisonChanen 2008). Self-harming behaviour of individuals with borderline personality disorder contrasts with the outwardly directed aggression characteristic of antisocial personality disorder and psychopathy.
Narcissistic personality disorder
Narcissistic traits include grandiosity, need for admiration and lack of empathy. The estimated rates of narcissistic personality disorder in the general population range from 0.8 to 4% (Reference Torgersen, Kringlen and CramerTorgersen 2001; Reference KayKay 2008), and may decline with increasing age in adulthood (Reference Stinson, Dawson and GoldsteinStinson 2008). Narcissistic personality disorder is commonly comorbid with antisocial personality disorder (e.g. borderline personality disorder and histrionic personality disorder; Reference Stuart, Pfohl and BattagliaStuart 1998). Like borderline personality disorder, it is associated with an elevated risk of suicide (Reference Ronningstam, Weinberger and MaltsbergerRonningstam 2008). Neural correlates of narcissistic personality disorder are unknown, although the neural correlates of particular traits (e.g. lack of empathy) are being investigated in relation to psychopathy (see below).
Histrionic personality disorder
Histrionic traits include excessive emotionality, attention seeking and a seductive demeanour. The general population rate of histrionic personality disorder is around 2%, with no gender difference in prevalence (Reference Grant, Hasin and StinsonGrant 2004). Neural correlates of histrionic personality disorder have not been investigated.
Neuroimaging correlates of psychopathy
A number of neural regions are thought to underlie the core deficits seen in psychopathy, in particular the amygdala and prefrontal cortex. The functional significance of these and other key regions will be outlined here, followed by a brief overview of relevant neuroimaging findings.
Amygdala
Emotional dysfunction
Research on the neurobiological basis of emotional dysfunction in psychopathy has focused on the amygdala because of its role in emotion processing (particularly of fearful expressions) and emotional learning. For example, patients with amygdala lesions have deficits in the recognition of fearful expressions (Reference Adolphs, Tranel and HamannAdolphs 1999). Further, in healthy individuals the amygdala is more active during processing of fear and disgust relative to happy and neutral expressions (Reference Costafreda, Brammer and DavidCostafreda 2008). Also, the amygdala is critically involved in stimulus-reinforcer association learning (Reference RollsRolls 2000), which contributes to moral socialisation – for example, by learning to avoid antisocial behaviour because of its association with emotionally negative outcomes. In particular, the psychologist James Blair hypothesised that in healthy individuals distress cues (such as facial and vocal expressions of fear) function as aversive unconditioned stimuli that elicit empathic responses (feeling the distress of others) (Reference BlairBlair 1995, Reference Blair, Mitchell and Mitchell2005). In this view, amygdala dysfunction in the ‘at risk’ child with callous-unemotional traits and in the adult with psychopathy results in insensitivity to distress cues and failure to learn to avoid behaviour that engenders distress in others.
Processing distress cues
Several lines of evidence now support the view that children with callous-unemotional traits and adults with psychopathy have deficits in processing distress cues, in association with abnormalities of the amygdala. For example, a recent meta-analysis has shown that relative to control groups, antisocial populations show significant deficits in recognising fearful, sad and surprised expressions, with a significantly greater deficit in fear recognition relative to other expressions (Reference Marsh and BlairMarsh 2008a). Individuals with psychopathy show reduced potentiation of the eye blink startle reflex by visual threat primes (Reference Patrick, Bradley and LangPatrick 1993), which is related to amygdala function. They also show reduced autonomic responsiveness to distress cues (e.g. a crying face), but not threatening or neutral images (Reference Blair, Jones and ClarkBlair 1997). These selective deficits in autonomic responses to facial distress cues are likely to be related to amygdala dysfunction. For example, adolescents with conduct disorder and callous-unemotional traits show reduced amygdala responses to fearful expressions relative to controls (Reference Marsh, Finger and MitchellMarsh 2008b; Reference Jones, Laurens and HerbaJones 2009). Also, compared with controls, adults with psychopathy show an atypical pattern of greater visual cortical (fusiform gyrus) activity in response to neutral rather than fearful expressions, and also reduced fusiform gyrus activity in response to fearful expressions. This may indicate reduced feedback modulation of fusiform gyrus by the amygdala during fear processing, and contribute to deficits in the recognition of and affective responsiveness to fearful expressions (Reference Deeley, Daly and SurguladzeDeeley 2006).
Reduced amygdala activation was also found in high PCL-R scoring adults during moral decision-making tasks (Reference Glenn, Raine and SchugGlenn 2009).
Structure and function of amygdala
At a structural level, a recent study has shown morphological differences in the amygdala of individuals with psychopathy (Reference Yang, Raine and NarrYang 2009). In addition, volumes were correlated negatively with both total and two-factor PCL-R scores, especially Factor 1. In summary, people with psychopathy show evidence of abnormalities of amygdala structure and function that are related both to clinical features and to the underlying information processing deficits – such as impaired emotion recognition, empathy and associative learning – which may help explain them.
Emotional processing and learning
However, it should also be emphasised that in addition to findings of abnormalities in processing distress cues and the structure and function of the amygdala, there is increasing evidence of abnormalities in distributed brain systems involved in emotional processing and learning. For example, significant differences are seen between healthy individuals and those with psychopathy during fear conditioning. In in fear conditioning paradigms, a previously neutral stimulus is paired with an aversive cue (unconditioned stimulus) so that the neutral stimulus alone will become aversive. While acquiring this association, people with psychopathy show significantly less activity of amygdala, orbitofrontal cortex, anterior cingulate cortex and insula than healthy controls, and additionally fail to show conditioned skin conductance response to presentations of the neutral stimulus (Reference Birbaumer, Veit and LotzeBirbaumer 2005).
Another study employing an affective memory task reported reduced affect-related activity in the amygdala/hippocampal formation, para-hippocampal gyrus, ventral striatum and in the anterior and posterior cingulate gyri in criminals with psychopathy compared with controls (Reference Kiehl, Smith and HareKiehl 2001). The criminals also showed evidence of overactivation in bilateral frontotemporal cortices when processing affective stimuli.
Hence, individuals with psychopathy show differences in distributed brain systems involved in emotion processing and memory in two kinds of emotional learning task. Widespread neurobiological differences in responding to and learning about emotive stimuli may contribute to the deficient affective experience and fearlessness. These differences may be in addition to more specific deficits in processing and learning from distress cues that may account for impairments in empathy and the use of instrumental aggression.
Prefrontal cortex
The importance of the frontal lobes to social behaviour was first recognised in the 19th century following the case of Phineas Gage, in whom frontal lobe damage resulted in profound personality change marked by inappropriate social behaviour (Reference Harlow, Harlow and MillerHarlow 1869). A ‘frontal lobe’ syndrome was subsequently described based on clinical observation of the behaviour of patients with frontal lobe lesions (Reference LishmanLishman 1998). Characteristic features included apathy, emotional lability, lack of social awareness, unconcern for social rules, impulsiveness and frustrative aggression (more commonly known as reactive aggression). This has led to the suggestion that related traits in psychopathy and other personality disorders may also result from frontal lobe abnormalities (Reference DamasioDamasio 2000).
Subsequent advances in neuroimaging have allowed the neuropsychological, symptom and trait associations of frontal regions in clinical populations to be defined with increasing precision. Key regions include the prefrontal cortex (frontal regions anterior to motor cortices) and its subdivisions (anterior portions of dorsolateral and medial cortices, and the orbital cortex). The ventromedial prefrontal cortex is an additional term used to refer to the orbitofrontal and/or the ventral portion of the medial wall of the frontal lobe (Reference Stuss and LevineStuss 2002). Lesion-deficit studies demonstrate that damage to the ventromedial prefrontal cortex in particular is associated with an increased risk of reactive aggression (Reference Blair and CipolottiBlair 2000). Furthermore, a recent study showed ventromedial prefrontal cortex damage to be associated with deficits in moral judgement. Vignettes detailing failed attempts at harming others were judged as more morally permissible by patients with ventromedial prefrontal cortex lesions compared with controls without such lesions (Reference Young, Bechara and TranelYoung 2010).
Some studies have examined prefrontal or frontal cortex as a whole rather than its subdivisions. For example, two studies reported reduced prefrontal (Reference Yang, Raine and LenczYang 2005) or frontal (Reference Muller, Ganssbauer and SommerMuller 2008) grey matter volume in adults with psychopathy. Also, higher total as well as subfactor psychopathy scores (arrogant/deceptive, affective and impulsive/unstable) are associated with low prefrontal grey volume (Reference Yang, Raine and LenczYang 2005). Further, cortical blood perfusion measured with single photon emission computed tomography (SPECT) was found to be inversely related to PCL subscores in the frontal and temporal lobes of violent offenders (Factor 1, ‘disturbed interpersonal attitudes’) (Reference Soderstrom, Hultin and TullbergSoderstrom 2002). Hence, there is evidence of reduced prefrontal volume and abnormal frontal and temporal blood flow in individuals with psychopathy relative to controls.
However, other studies have revealed more specific associations between psychopathic traits and subregions of the prefrontal cortex. In particular, the association between ventromedial prefrontal cortex damage and reactive aggression in patients with brain injury is mirrored by impairments of ventromedial prefrontal cortex structure and function in individuals with psychopathy, among other abnormalities. For example, one study revealed reduced grey matter volume in the orbitofrontal cortex, frontopolar cortex and postcentral gyri in adult males with antisocial personality disorder relative to controls, with individuals with psychopathy showing the smallest volumes in these areas (Reference Tiihonen, Rossi and LaaksoTiihonen 2008).
A recent study of children with callous-unemotional traits and conduct disorder reported increased grey matter concentration in medial orbitofrontal cortex and anterior cingulate cortex (Reference De Brito, Mechelli and WilkeDe Brito 2009). Given evidence that callous-unemotional traits in childhood may contribute to the development of adult psychopathy (Reference Lynam, Caspi and MoffittLynam 2007), these findings may indicate an early developmental origin of prefrontal abnormalities in individuals at risk of adult psychopathy.
Neuropsychological deficits
These findings raise the question of how abnormalities of prefrontal cortical structure and function contribute to emotional dysfunction and/or antisocial behaviour. Neuropsychological studies attempt to identify impairments in information processing linked to abnormal brain structure and function that contribute to clinical phenotypes. Here, we consider two models of neuropsychological deficits in psychopathy linked to ventromedial prefrontal cortex dysfunction, tested by reversal learning and gambling tasks respectively. In individuals with psychopathy, both tasks may show differences in affective learning associated with ventromedial prefrontal cortex dysfunction that contributes to insensitivity to risk and punishment and recidivism.
Reversal learning
On tests of reversal learning, individuals are required to alter a dominant rewarded response when task contingency is reversed (a previously rewarded response is now punished). Impaired reversal learning performance by individuals with ventromedial prefrontal cortex damage highlights involvement of this region in this task (Reference Fellows and FarahFellows 2003). Individuals with psychopathy show greater response perseveration and during functional magnetic resonance imaging (fMRI) display aberrant activation of ventromedial prefrontal cortex during the reversal phase (Reference Budhani, Marsh and PineBudhani 2007; Reference Finger, Marsh and MitchellFinger 2008). Increased ventromedial prefrontal cortex activity to punished error may underpin an inability to respond appropriately to aversive reinforcement in order to regulate behaviour (Reference Rolls, Hornak and WadeRolls 1994).
Gambling tasks
Ventromedial prefrontal cortex dysfunction in psychopathy is also suggested by response patterns on the Iowa Gambling Task that resemble those of patients with orbitofrontal cortex damage (Reference Bechara, Damasio and DamasioBechara 1994; Reference Mitchell, Colledge and LeonardMitchell 2002; Reference van Honk, Hermans and Putmanvan Honk 2002). This task requires individuals to choose cards from four decks of playing cards in order to receive rewards (financial gain) and avoid punishment (financial loss). The four decks are unequally weighted for losses and gains, with two decks consistently producing both high rewards and high losses and the other two small rewards and small losses. Over time, healthy individuals choose the latter two packs to produce a net gain. By contrast, individuals with ventromedial prefrontal cortex lesions show no bias towards these safer packs, demonstrating this region's importance in this type of decision-making (Reference Bechara, Damasio and DamasioBechara 1994). Studies have shown that individuals with subclinical psychopathy (Reference van Honk, Hermans and Putmanvan Honk 2002), children with callous-unemotional traits (Reference BlairBlair 2001) and adults with psychopathy (Reference Mitchell, Colledge and LeonardMitchell 2002) perform similarly to patients with ventromedial prefrontal cortex lesions on gambling tasks.
Frustration/aggression
Ventromedial prefrontal cortex dysfunction is also associated with an increased risk of reactive aggression in both individuals with brain injury and individuals with psychopathy (Reference Blair and CipolottiBlair 2000). Possible explanations of this association include increased frustration due to perseveration of unsuccessful responses or recurrent social conflict because of a more general inability to suppress impulses and desires (including aggressive impulses when challenged or threatened).
Disconnectivity between prefrontal cortex and limbic regions in psychopathy
Recent evidence suggests that abnormalities in the structure and function of prefrontal cortex and limbic regions in children with callous-unemotional traits and/or individuals with psychopathy should not be viewed in isolation from one another. For example, an fMRI study reported reduced connectivity between the amygdala and prefrontal areas in adolescents with conduct disorder compared with controls, when watching displays of deliberately inflicted pain (Reference Decety, Michalska and AkitsukiDecety 2009).
A study employing diffusion tensor imaging (DTI) found that individuals with psychopathy showed reduced fractional anisotropy – a measure of tract microstructural integrity – of the uncinate fasciculus (Reference Craig, Catani and DeeleyCraig 2009). This white matter pathway connects limbic and ventral frontal brain regions. This reduction was inversely correlated with PCL Factor 2 scores (antisocial behaviour). These findings imply that reduced communication between limbic (emotional processing) and frontal (executive) regions may contribute to the behavioural problems seen in populations with antisocial personality disorder.
Individuals with psychopathy show evidence of structural and functional abnormalities of ventromedial prefrontal cortex, amygdala and other components of distributed networks involved in recognising and responding to distress cues, more general emotional processing and learning, behavioural regulation and decision-making.
Neuroimaging correlates of personality disorders
Few studies of Cluster B personality disorders relate to antisocial and borderline personality disorder. Here these data will be outlined.
Antisocial personality disorder
Similarly to psychopathy, people with antisocial personality disorder show structural and functional abnormalities of prefrontal cortex. Prefrontal grey matter volume has been found to be reduced in adults (Reference Raine, Lencz and BihrleRaine 2000) and children (Reference Huebner, Vloet and MarxHuebner 2008) with antisocial personality compared with healthy controls. Medial frontal cortical thinning has been found in antisocial personality disorder (Reference Narayan, Narr and KumariNarayan 2007). Reduced anterior cingulate cortex activation is also seen in children with the disorder while viewing negative affective stimuli (Reference Sterzer, Stadler and KrebsSterzer 2005; Reference Stadler, Sterzer and SchmeckStadler 2007). Positron emission tomography (PET) studies have reported reduced metabolism in prefrontal areas in violent individuals (Reference Goyer, Andreason and SempleGoyer 1994; Reference Volkow, Tancredi and GrantVolkow 1995).
Reduced volume of temporal regions is also seen in adults (Reference Barkataki, Kumari and DasBarkataki 2006) and children (see Reference Sterzer and StadlerSterzer 2009) with antisocial personality disorder, including reduced amygdala volume (Reference Sterzer, Stadler and PoustkaSterzer 2007) and additional hippocampal reduction, with both volumes inversely related to severity of conduct disorder (Reference Huebner, Vloet and MarxHuebner 2008). Similarly, reduced amygdala function has been reported in children with conduct disorder to negative emotional visual stimuli (Reference Sterzer, Stadler and KrebsSterzer 2005), although increased activation was also found (Reference Herpertz, Huebner and MarxHerpertz 2008).
Similarly, enhanced activation of the amygdala plus striatal and temporal areas were found within a sample of boys with conduct disorder while viewing scenes showing others experiencing pain (Reference Decety, Michalska and AkitsukiDecety 2009). Increased signals in these areas were not shown in the control sample, despite both groups displaying similar activation increases within the pain matrix comprising the anterior insula, medial cingulate cortex, somatosensory cortex and periaqueductal grey. In addition, the authors found reduced functional connectivity between amygdala and prefrontal regions in the conduct disorder group (Reference Decety, Michalska and AkitsukiDecety 2009). However, comparisons of findings across ages should be made with caution.
Existing neuroimaging data indicate that prefrontal and temporal regions show reduced grey matter in antisocial personality disorder, and this may relate to functional deficits additionally seen in these regions. However, studies are not consistent in their methods of allocating or defining clinical groups, making it difficult to draw reliable conclusions from studies of antisocial personality disorder and psychopathy, and when comparing these groups. For example, some studies do not administer the PCL or equivalent measure (e.g. Reference Barkataki, Kumari and DasBarkataki 2006) – hence these studies may, in fact, be examining psychopathy and not antisocial personality disorder alone.
Borderline personality disorder
Neuropsychological assessments of individuals with borderline personality disorder have reported deficits in those tasks reliant on frontal and temporal brain areas (Reference Swirsky-Sacchetti, Gorton and SamuelSwirsky-Sacchetti 1993), such as executive function and memory tasks. Emotional processing deficits are also seen in borderline personality disorder, with impaired recognition of emotional faces (Reference Levine, Marziali and HoodLevine 1997) and impairments of emotion recognition when integrating facial expressions and prosody (Reference Minzenberg, Poole and VinogradovMinzenberg 2006). The increased startle response to aversive stimuli in borderline personality disorder (Reference Hazlett, Speiser and GoodmanHazlett 2007) contrasts with the pattern found in psychopathy (Reference Patrick, Bradley and LangPatrick 1993).
Structural abnormalities
Structural anomalies found in borderline personality disorder predominantly involve frontal and limbic regions. Significant frontal volume reductions have been found in whole frontal lobe (Reference Lyoo, Han and ChoLyoo 1998), cingulate cortex (Reference Hazlett, New and NewmarkHazlett 2005) and orbitofrontal cortex (Reference Chanen, Velakoulis and CarisonChanen 2008). Significant reductions in anterior cingulate cortex (Reference Minzenberg, Fan and NewMinzenberg 2008) and cingulate gyrus (Reference Soloff, Nutche and GoradiaSoloff 2008) grey matter concentration have also been reported.
A number of MRI studies report structural differences of the amygdala in people with borderline personality disorder compared with healthy controls. However, the direction of these differences varies between studies, with reduced volume (Reference Weniger, Lange and SachsseWeniger 2009), increased (Reference Minzenberg, Fan and NewMinzenberg 2008) and decreased (Reference Soloff, Nutche and GoradiaSoloff 2008) grey matter concentration, and no volumetric difference (Reference New, Hazlett and BuchsbaumNew 2007) being variously reported. Lack of consistency among these findings may arise from methodological differences between studies, including use of mixed- v. single-gender cohorts (for discussion of further limitations see Reference Minzenberg, Fan and NewMinzenberg 2008).
Structural abnormalities of the hippocampus are also seen in borderline personality disorder, with individuals showing reduced hippocampal volume (Reference Zetzsche, Preuss and FrodlZetzsche 2007; Reference Weniger, Lange and SachsseWeniger 2009) and reduced grey matter concentration (Reference Soloff, Nutche and GoradiaSoloff 2008) compared with healthy controls. Additional medial temporal and limbic structural findings include reduced grey matter concentration of parahippocampal gyrus and uncus (Reference Soloff, Nutche and GoradiaSoloff 2008). These findings suggest that common traits of borderline personality disorder and psychopathy (e.g. impulsivity, poor behavioural controls) (Box 1) may be due to similar patterns of frontal and limbic abnormality (Reference Blair, Mitchell and MitchellBlair 2005).
Differences between activity in frontal and limbic areas
Functional studies also find activity in frontal and limbic areas to differ in borderline personality disorder compared with healthy controls. For example, anterior cingulate cortex hypoactivation is seen during emotional processing (Reference Wingenfeld, Rullkoetter and MensebachWingenfeld 2009). Coupled with findings of increased amygdala blood-oxygen level dependency activation during emotional processing (Reference Herpertz, Dietrich and WenningHerpertz 2001; Reference Donegan, Sanislow and BlumbergDonegan 2003; Reference Koenigsberg, Siever and LeeKoenigsberg 2009), these results support frontolimbic deficit theories of borderline personality disorder. These propose that amygdala hyperactivation and anterior cingulate cortex hypoactivation to emotional stimuli produce heightened emotional arousal in combination with a reduced ability to inhibit and regulate emotional expression (Reference Wingenfeld, Rullkoetter and MensebachWingenfeld 2009). These linked processes could account for the increased intensity of affective experience shown by individuals with borderline personality disorder.
Clinical implications
From this discussion it can be seen that not only are there common traits between psychopathy and each of the Cluster B personality disorders, but that there is also some overlap in brain anomalies found in the three conditions for which neuroimaging data exist. Shared features of borderline personality disorder and psychopathy may reflect similar cognitive and neural mechanisms. For example, ‘emotion’ executive dysfunction associated with prefrontal structural and functional abnormalities may contribute to impulsivity, disinhibition, and increased risk of reactive aggression (although other factors may influence whether aggression is directed to self or others). Conversely, the increased affective intensity of borderline personality disorder in contrast to the reduced affective experience of individuals with psychopathy may reflect respective differences in amygdala reactivity. However, it should be noted that clinical features such as ‘reactive aggression’, ‘disinhibition’ and ‘affective intensity’ are not caused by single brain structures acting in isolation. This raises the possibility that the relative contribution of a given structure (e.g. orbitofrontal cortex, amygdala) to a clinical feature (e.g. reactive aggression) may vary depending on the characteristics of other components of relevant brain systems (e.g. anterior cingulate cortex).
Future research
In general, psychiatric research is increasingly focused on understanding reciprocal influences within and between levels at which people are constituted (e.g. genes, cells, brain systems, social environment), and may ultimately reveal a range of mechanisms by which clinical features such as reactive aggression or impulsivity are produced.
Despite the volume of imaging data related to these conditions, there still remains a great deal to be learnt about the neurobiology of these complex disorders. Identification of neural mechanisms underlying clinical features of psychopathy and other personality disorders may enable improved understanding of diagnosis, aetiology, treatment and prognosis. Future research would benefit from the establishment of common technical parameters, study populations and clinical definitions to facilitate comparisons between studies. The distinct, yet overlapping, nature of these diagnoses from a neurobiological as well as a clinical perspective further supports the view that personality disorders may be better classified with dimensional rather than categorical methods.
MCQs
Select the single best option for each question stem
-
1 According to DSM-IV-TR, psychopathy is:
-
a an associated feature of antisocial personality disorder
-
b a distinct personality disorder
-
c a subcategory of borderline personality disorder
-
d the same as antisocial personality disorder
-
e a and c.
-
-
2 Borderline personality disorder is associated with:
-
a greater male to female incidence
-
b high risk of self-harm and suicide
-
c amygdala dysfunction
-
d emotional processing deficits
-
e b–d.
-
-
3 Neuroimaging research findings can:
-
a measure differences in brain function between groups
-
b be compared directly with all other neuroimaging studies
-
c clearly distinguish psychopathy from antisocial personality disorder
-
d be used to diagnose borderline personality disorder
-
e b and c.
-
-
4 Antisocial personality disorder:
-
a is more prevalent than psychopathy
-
b is associated with greater and more severe criminality than psychopathy
-
c can be diagnosed in children
-
d can be diagnosed using the PCL-R
-
e cannot co-occur with psychopathy.
-
-
5 Adult psychopathy is associated with:
-
a reduced prefrontal grey matter
-
b altered visual cortical responses to fearful expressions
-
c reduced amygdala volume
-
d reduced prefrontal grey matter and amygdala volume
-
e b–d.
-
MCQ answers
| 1 | d | 2 | e | 3 | a | 4 | a | 5 | e |



eLetters
No eLetters have been published for this article.