Psychostimulants are a broad class of drugs that reduce fatigue, promote alertness and wakefulness, and have possible mood-enhancing properties (Reference Orr and TaylorOrr 2007). Healthcare professionals have used amphetamine and methylphenidate since the early 1930s to treat people diagnosed with various conditions, including depression, fatigue, neurasthenia, weight loss and hypersomnolence. In the 1960s, multiple randomised controlled trials (RCTs) questioned the efficacy of psychostimulants in major depression against placebo, tricyclic antidepressants and monoamine oxidase inhibitors. Concerns were also raised about misuse and tolerance, leading to their decreasing use in general psychiatry (Reference Chiarello and ColeChiarello 1987).
In modern psychiatric practice, psychostimulants have a limited but important and well-identified role. The older agents (dexamphetamine, methylphenidate and pemoline) are used to treat attention-deficit hyperactivity disorder (ADHD) in both children and adults. A newer psychostimulant, modafinil, has US Food and Drug Administration and UK Medicines and Healthcare products Regulatory Agency (MHRA) approval for reducing sleepiness in obstructive sleep apnoea, narcolepsy and shift-work sleep disorder.
Research has continued into the use of psycho-stimulants to treat a range of affective, cognitive and somatic symptoms. This article will review the literature for psychostimulant use in adults for a variety of medical, neurological and psychiatric conditions that are relevant to modern psychiatric practice (see Box 1 for search method).
BOX 1 Search method for this review
We undertook a Medline search for articles published in English between January 1987 and October 2007 on psychostimulant use (key words: psychostimulants, dexamphetamine, amphetamine, methylphenidate, modafinil) in the treatment of symptoms and disorders of interest to psychiatrists (key words: depression, cognition, fatigue, apathy, schizophrenia, dementia).
We also searched the reference lists of all the articles found. Given the space constraints, this article focuses on topics that are most relevant to psychiatrists. The best evidence is presented, when available, including systematic reviews and randomised controlled trials. When such evidence was not available, open trials and case series have been referenced.
Pharmacology
Amphetamine
Amphetamine is an organic molecule that is an indirectly acting sympathomimetic, with its d-isomer, dexamphetamine, being three to four times more active than the l-isomer. Its mechanism of action is thought to be the increasing of noradrenaline and dopamine by blocking their reuptake and facilitating their release at central presynaptic neurons. It is also a weak monoamine oxidase inhibitor (Reference Chiarello and ColeChiarello 1987; Reference Masand and TesarMasand 1996). The stimulation of the dopaminergic mesocorticolimbic and nigrostriatal pathways may be responsible for the motor and behavioural effects (Reference Homsi, Walsh and NelsonHomsi 2000; Medsafe 2007).
Dexamphetamine
Dexamphetamine is rapidly absorbed and readily crosses the blood–brain barrier. Its central effects include anorexia, vasoconstriction and hyperthermia. Peripherally, it can elevate diastolic and systolic blood pressure and has a weak bronchodilatory action (Reference Homsi, Walsh and NelsonHomsi 2000). In healthy people, it increases wakefulness, alertness, motivation and initiative, and elevates mood (Reference Masand and TesarMasand 1996). Given its enhanced potency, the d-isomer is most commonly used in clinical practice and nearly all of the studies of psychostimulant use for depression have used dexamphetamine.
Methylphenidate
Methylphenidate is a piperidine derivative and its exact mechanism of action is unknown. It binds to dopamine transporters on the presynaptic membrane, particularly in the striatum, resulting in an increase in extrasynaptic dopamine levels. It may also regulate dopamine homeostasis at the nerve terminal and has some effect on noradrenaline reuptake and binds weakly to the serotonin transporter. Unlike dexamphetamine, it does not facilitate dopamine release at the nerve terminal (Reference Challman and LipskyChallman 2000). Clinically, methylphenidate is considered a milder psychostimulant than the amphetamine derivatives.
Modafinil
Modafinil is a mild psychostimulant derived from benzhydrylsulfinylacetamide and is structurally unrelated to methylphenidate and amphetamine. Its mechanism of action is unknown and evidence suggests that it is dissimilar to older psychostimulants. Animal studies indicate that modafinil's primary activity may be related to a decrease in gamma-aminobutyric acid-mediated neurotransmission. Other in vitro studies have demonstrated that modafinil also interacts with other neurotransmitter pathways such as glutamine in the hypothalamus, serotonin in the prefrontal cortex and hypothalamus, dopamine in the prefrontal cortex and noradrenaline in the ventrolateral preoptic nucleus. It also increases neuronal activity selectively in the hypothalamus, activating tuberomammillary nucleus neurons, which release histamine and neurons in the perifornical area, in turn releasing orexin/hypocretin. These cell groups are associated with the control of wakefulness (Reference Keating and RaffinKeating 2005). Modafinil is proposed to have less potential for misuse compared with methylphenidate (Reference JasinskiJasinski 2000).
Nearly all of the studies discussed below have used standard-release formulations of the psycho-stimulants. Table 1 shows the pharmacokinetics of dexamphetamine, methylphenidate and modafinil.
Major depression
Monotherapy
There have been several reviews of psychostimulants as monotherapy for primary unipolar depression (Reference Satel and NelsonSatel 1989; Reference Orr and TaylorOrr 2007). Most RCTs have compared the older psychostimulants with placebo or anti-depressants such as imipramine and phenelzine. There have been no RCTs with modafinil. Most of the trials are now more than 20 years old and have many methodological shortcomings, such as varying diagnostic criteria and the use of unvalidated rating scales or global impression alone (Reference Orr and TaylorOrr 2007). Most studies in non-elderly adults (under 65 years of age) were negative and, in the positive studies, stimulants were only modestly beneficial to certain subgroups compared with placebo.
In contrast to this there have been some early, but less than methodologically robust, positive trials of monotherapy psychostimulant use to treat depression in elderly people (Reference JacobsonJacobson 1958; Reference KaplitzKaplitz 1975). The most recent RCT (Reference Wallace, Kofoed and WestWallace 1995) was positive but involved only 16 medically ill patients with depression in a very short trial.
Overall, this treatment option has a poor evidence base. It may have some utility for elderly people or for treating depression in the medically unwell; this will be discussed below.
Adjunctive treatment
There are several positive open trials, audits and case reports regarding the use of psychostimulants to augment standard antidepressant treatment, including tricyclic antidepressants (Reference Gwirtsman, Szuba and TorenGwirtsman 1994), monoamine oxidase inhibitors (Reference FeinbergFeinberg 2004), selective serotonin reuptake inhibitors (Reference Ninan, Hassman and GlassNinan 2004), venlafaxine (Reference Masand, Anand and TanquaryMasand 1998) and mirtazapine (Reference Schillerstrom and SeamanSchillerstrom 2002).
The two negative RCTs using methylphenidate as an augmentation agent compared with placebo in adults with major depression showed no statistically significant improvement or accelerated response (Reference Postolache, Rosenthal and HellersteinPostolache 1999; Reference Patkar, Masand and PaePatkar 2006). Reference Patkar, Masand and PaePatkar and colleagues (2006) noted that in 60 out-patients with treatment-resistant depression, more participants responded to extended-release methylphenidate compared with placebo (40% v. 23.3%). However, this difference was not statistically significant. There were no differences in the rate of adverse events or the number of participants who dropped out of the trial.
Reference Lavretsky, Park and SiddarthLavretsky and colleagues (2006) conducted an RCT involving 16 elderly participants with major depression, comparing methylphenidate with placebo augmentation, commenced simultaneously with citalopram. There was an accelerated anti-depressant response by week 3 and a greater reduction in depression scores in the participants on the combination treatment. However, side-effects and intolerability caused a number of participants to drop out of the trial.
There have been two well-designed RCTs comparing modafinil with placebo as an augmentation agent to antidepressants in the treatment of non-elderly people with major depression. Participants in these trials had experienced a partial response to an antidepressant, usually a selective serotonin reuptake inhibitor, and had significant and persistent fatigue or sleepiness. At the end of both trials, there was no significant difference in depression scores between the two groups. A 6-week trial (Reference DeBattista, Doghramji and MenzaDeBattista 2003) involving 168 patients reported a significant improvement compared with placebo for sleepiness at week 1 and fatigue at week 2, but not at the end of the trial. An 8-week trial (Reference Fava, Thase and DeBattistaFava 2005) including 311 patients reported a non-significant trend towards greater mean reductions on depressive scores at the end of the trial for the modafinil group. Participants on modafinil experienced more adverse events, notably nausea and feeling jittery.
The evidence base for using psychostimulants as augmentation agents for major unipolar depression is limited (Reference Orr and TaylorOrr 2007). It remains to be seen whether psychostimulants may benefit a subgroup of people with depression who also have significant fatigue or sleep problems. A systematic review found inadequate evidence for modafinil as an augmentation agent in the treatment of fatigue in individuals with major depression who have had a partial response to antidepressants (Reference Lam, Freeman and CatesLam 2007).
Bipolar depression
Bipolar depression remains a major challenge in general adult psychiatry. The older psychostimulants have not been recommended owing to a lack of proven efficacy, mood-switching potential, tolerance and misuse. Modafinil has been used in open trials to treat bipolar depression, either as monotherapy or as an adjunct to standard antidepressant treatment (Reference Carlson, Merlock and SuppesCarlson 2004). Benefits over the older psychostimulants include less peripheral activation, decreased anxiogenic properties and lower misuse potential (Reference JasinskiJasinski 2000).
A subsequent RCT compared 6 weeks of adjunctive modafinil with placebo in 85 people with bipolar depression who were on a mood stabiliser, with or without concomitant antidepressant treatment (Reference Frye, Grunze and SuppesFrye 2007). The modafinil group had significantly higher remission (39% v. 18%) and response (44% v. 23%) rates, with significant improvements noted by week 2. There were no differences in treatment-emergent mania or hypomania during the study period between the two groups, and being on concomitant antidepressant treatment was not a factor in response or remission. Modafinil appeared to be well tolerated.
Although still preliminary and needing replication, this trial was well designed and provided evidence of a possible novel treatment option for bipolar depression. Modafinil's advantages included its relative safety, lack of mood-switching effect and relatively fast antidepressant response. Questions remain about the ideal dosage, any specific effects on energy and sleepiness, and the actual mechanism of action.
Depression in medical illness
An extensive literature exists regarding the use of dexamphetamine and methylphenidate as monotherapy for affective disorders in a wide variety of medically or surgically unwell patients. The case series and large retrospective audits summarised by Reference Masand and TesarMasand & Tesar (1996) reported clinical response rates between 48 and 70%. In individual patients, the results were sometimes dramatic, with an anti-depressant response within 2–3 days and with minimal side-effects. The recorded benefits include patients being discharged earlier than anticipated, engagement in rehabilitation, an absence of major side-effects and minimal drug interactions. The incidence of hypomania was rare (Reference Masand, Pickett and MurrayMasand 1995). Reported disadvantages include a lack of a solid evidence base, potential medication interactions and stigma surrounding psychostimulants (Reference Frierson, Wey and TablerFrierson 1991).
There have been few RCTs involving medically ill patients. Reference Landman, Peisig and PerlmanLandman and colleagues (1958) performed a crossover trial with 112 medically ill patients with mild depression, comparing methylphenidate with placebo. Methylphenidate was clearly superior and clearly differentiated from placebo. In another of these trials, Reference Wallace, Kofoed and WestWallace and colleagues (1995) conducted a small double-blind crossover trial in 16 medically ill elderly patients. Again, methylphenidate demonstrated a significant benefit over placebo. However, the trial's small numbers and high mortality rate make the results difficult to interpret. Modafinil has not undergone RCTs in medically ill people, although there have been positive case reports of its use (Reference Schwartz, Leso and BealeSchwartz 2002).
Both the two trials and the retrospective audits above demonstrate a significant short-term anti-depressant effect with psychostimulants in medically ill people, but the long-term benefits and risks associated with extended treatment are still unknown. Although depressive symptoms have been the main indication for psychostimulant prescription in these cases, the diagnosis has not necessarily always been major depression or its equivalent.
Cancer and palliative care
Psychostimulants, particularly methylphenidate, have been studied for treating depression associated with cancer in a wide variety of populations, from ambulatory to palliative care patients. Retrospective audits and open studies report that between 27 and 78% of patients experience some improvement in depressive symptoms on a psychostimulant (Reference Rozans, Dreisbach and LertoraRozans 2002). None of the studies in depression has been an RCT, which may be difficult to undertake in this population. Furthermore, there may have been a non-specific euphoriant effect. Despite the lack of robust evidence, many authorities have recommended the use of psychostimulants, especially in depression in palliative care, when a fast response is required in a limited amount of time (Reference BlockBlock 2000).
Fatigue is also a significant symptom in this patient population and may be due to the cancer itself or the related treatments. Open studies were initially encouraging but an RCT of 31 patients comparing methylphenidate with placebo found no significant difference between the two groups at 1 and 4 weeks of open-label extension (Reference Bruera, Valero and DriverBruera 2006).
One open study and one RCT found significant benefits in cognition for people with cancer treated with psychostimulants (Reference Rozans, Dreisbach and LertoraRozans 2002). Cognitive impairment in cancer may be due to the process of the disease, metabolic abnormalities, opiate use or the side-effects of radiation or chemotherapy. Finally, methylphenidate has been of benefit for hypoactive delirium in palliative care settings (Reference Keen and BrownKeen 2004).
Stroke
Post-stroke depression
There have been positive retrospective audits and open trials of psychostimulants, predominantly methylphenidate, in post-stroke depression (Reference KrausKraus 1995). Partial or full remission was reported in 40 to 80% of patients, with doses ranging from 5 to 40 mg daily, over 2–4 weeks. Stimulants were well tolerated and treatment response usually occurred within 1–3 days.
Reference Lazarus, Moberg and LangsleyLazarus and colleagues (1994) noted no difference in the degree of response to methylphenidate compared with nortriptyline. However, the average speed of response was significantly different (2.4 days for methylphenidate compared with 27 days for nortriptyline). Reference Grade, Redford and ChrostowskiGrade and colleagues (1998) noted a significant improvement with 3 weeks of methylphenidate in Hamilton Rating Scale for Depression scores. In contrast, a Cochrane review (Reference Martinsson, Hårdemark and EksborgMartinsson 2007) found no benefit for dexamphetamine for depression in the context of post-stroke rehabilitation.
An important consideration in using stimulants in the post-stroke period is the potential for an elevation in blood pressure. In the absence of robust post-stroke data, it is worth considering a brain-injury study (Reference Alban, Hopson and LyAlban 2004) that reported minimal adverse effects of methylphenidate on pulse rate and blood pressure (which increased by 7 bpm and 2.5 mmHg respectively).
Stroke rehabilitation
Reference Martinsson, Hårdemark and EksborgMartinsson and colleagues' (2007) Cochrane review on the use of dexamphetamine given over 1–5 weeks in the post-stroke period, usually in combination with physical or cognitive training, showed benefits of treatment on motor and language functioning, but not on neurological functioning, performance of activities of daily living or depression. A non-significant increase in mortality was observed with dexamphetamine. The authors concluded that dexamphetamine could not be recommended routinely in stroke rehabilitation.
This review and an RCT (Reference Sonde and LökkSonde 2007) suggest that psychostimulants should not be used routinely in post-stroke rehabilitation. However, they might be tried in individual patients to target symptoms such as depression, fatigue and sleepiness, if other treatments have been ineffectual or are contra-indicated.
Traumatic brain injury
Psychostimulants have a potential therapeutic role in ameliorating the neuropsychiatric sequelae of traumatic brain injury. Most studies have used methylphenidate at doses of 0.3 mg/kg given twice daily to patients with moderate to severe traumatic brain injury (Reference Whyte, Vaccaro and Grieb-NeffWhyte 2002).
Although there is a dearth of RCTs with sufficient power to draw any definitive conclusions or to allow psychostimulants to be recommended as routine therapy, this group of agents may be worth considering for impairing symptoms that have failed to respond to conventional treatment.
Acute and subacute recovery
A meta-analysis of controlled single-subject trials involving individuals included at a mean of 7.5 days after traumatic brain injury showed no effect of methylphenidate on command-following or yes/no communication abilities (Reference Martin and WhyteMartin 2007). Reference Kaelin, Cifu and MatthiesKaelin and colleagues (1996) reported an improvement in a number of attentional domains in a prospective study of 11 adults who received 10 days of methylphenidate, at a mean duration of 20 days after traumatic brain injury.
In an RCT of methylphenidate over 30 days in the subacute phase of traumatic brain injury, significant differences were seen on measures of attention, motor performance and disability after treatment, but this was not the case at 90 days (Reference Plenger, Dixon and CastilloPlenger 1996). Reference Lee, Kim and KimLee and colleagues (2005) described a 4-week RCT involving 30 patients who had experienced a major depressive episode, comparing methylphenidate with sertraline or placebo in treatment that began a mean of 35 days after traumatic brain injury. The study showed significant improvements in post-concussional symptoms, daytime wakefulness and reaction time with methylphenidate compared with placebo. Sertraline led to impaired performance in these domains.
Cognition
The rationale for the use of psychostimulants in cognitive rehabilitation after traumatic brain injury is derived from their use in ADHD, where methylphenidate has been shown to reduce hyperactivity, increase on-task behaviour and enhance working memory (Reference Whyte, Hart and SchusterWhyte 1997).
A number of small crossover RCTs of methylphenidate in adults with traumatic brain injury have been reported. Treatment duration varied between 10 days and 6 weeks, with a mean time since injury of 1.4–3.9 years. Two of the studies were negative (Reference Gualtieri and EvansGualtieri 1988; Reference Speech, Rao and OsmonSpeech 1993). However, Reference Whyte, Hart and SchusterWhyte and colleagues (1997, Reference Whyte, Hart and Vaccaro2004) reported significant improvements in domains related to cognitive speed and response rate, but not in attentional domains or motor speed. They concluded that methylphenidate improved ‘initial performance’, but may not enhance patients' ability to maintain performance over time.
One parallel-group RCT has been conducted, with cognitive functioning as a secondary outcome measure. Reference Mooney and HaasMooney & Haas (1993) studied the effects of methylphenidate over 6 weeks at a mean of 2 years after traumatic brain injury. No benefits were noted regarding attention or memory, but participants with higher pre-treatment anger scores did show a significant improvement on a verbal learning task.
Depression
Reference Lee, Kim and KimLee and colleagues (2005) noted significant improvements in mood with methylphenidate over 4 weeks; sertraline had a similar efficacy but a higher total number of adverse events. Thus, methylphenidate may be both beneficial and tolerable in the early stages of treatment for a depressive episode after traumatic brain injury, but it is unclear whether these results would be sustained over a longer period of time.
Behaviour
Reference Mooney and HaasMooney & Haas (1993) reported significant improvements in anger control and psychopathology with 6 weeks of methylphenidate treatment. Patients with higher pre-treatment anger scores showed the greatest benefit. Reference Speech, Rao and OsmonSpeech and colleagues (1993) found no effects of methylphenidate for differences in ‘social personality’ functioning on the Katz Adjustment Scale, despite the majority of patients and observers reporting improvements during the drug condition.
Psychiatric symptoms in other neurological disorders
Depression
Psychostimulants have been used to treat depression in people with neurological disorders, including dementia, Parkinson's disease, epilepsy, neuropathy, normal pressure hydrocephalus and multiple sclerosis. However, data have been restricted to case series or large retrospective audits involving medically ill patients.
Apathy
Apathy is a common and disabling symptom seen in a range of neuropsychiatric and mental disorders. A number of case reports have noted the successful use of methylphenidate, dexamphetamine and modafinil for treating apathy in a range of neurological conditions (Reference Padala, Burke and BhatiaPadala 2007).
Early studies suggested that psychostimulants may be useful for apathy in people with varying degrees of cognitive impairment or dementia, with or without depression, but it was unclear whether the improvements were due to changes in affect, motivation or cognition, or a combination of the three (Reference MooreMoore 1981). Reference Galynker, Ieronimo and MinerGalynker and colleagues (1997) prospectively involved 17 individuals with dementia and found a significant improvement in negative symptoms with methylphenidate treatment.
Reference KaplitzKaplitz (1975) remains the only RCT of psychostimulants for apathy. It reported a positive effect of methylphenidate v. placebo in ‘withdrawn, apathetic geriatric patients’.
In light of the above evidence, it might be worth considering prescribing a psychostimulant for treatment-resistant apathy, regardless of aetiology, particularly in older adults.
Chronic fatigue syndrome
Psychostimulants have been trialled in people with chronic fatigue syndrome. Reference Olson, Ambrogetti and SutherlandOlson and colleagues (2003) conducted a 6-week RCT in 20 patients, comparing dexamphetamine (up to 15 mg twice a day) with placebo. The medication group showed a statistically significant reduction on the Fatigue Severity Scale. Other outcome measures, including quality-of-life patient-determined outcomes, were negative. The authors concluded that dexamphetamine may be useful in managing chronic fatigue syndrome.
There have been two crossover trials since: one with modafinil, which was negative (Reference Randall, Cafferty and ShneersonRandall 2005), and one with methylphenidate, which was modestly positive (Reference Blockmans, Persoons and van HoudenhoveBlockmans 2006). These studies suggest that psychostimulants do not have a routine role in chronic fatigue syndrome.
HIV/AIDS
Evidence from RCTs provides support for the efficacy of psychostimulants in the treatment of HIV/AIDS-associated depression. Significant improvements in mood, energy levels and quality of life were seen in patients with depressive-spectrum disorders and fatigue treated with 2 weeks of dexamphetamine (Reference Wagner and RabkinWagner 2000). Reference Fernandez, Levy and SamleyFernandez and colleagues (1995) found no difference over 6 weeks between methylphenidate and desipramine in HIV-positive individuals with a major depressive episode. Both groups showed significant improvements in mood, although methylphenidate did not have a more rapid onset of efficacy than desipramine.
Subjective fatigue occurs in up to 50% of patients during the course of HIV/AIDS (Reference Breitbart, Rosenfeld and KaimBreitbart 2001). There is reasonable RCT evidence to support the use of methylphenidate, pemoline and modafinil for HIV-related fatigue, with associated improvements seen in mood, distress levels and quality of life (Reference Breitbart, Rosenfeld and KaimBreitbart 2001; Reference Rabkin, McElhiney and RabkinRabkin 2004).
Small prospective and crossover studies have reported positive effects of methylphenidate, dexamphetamine and modafinil on measures of cognition, including memory, processing speed and executive functioning in HIV/AIDS (Reference Rabkin, McElhiney and RabkinRabkin 2004). However, negative trials also exist (Reference Hinkin, Castellon and HardyHinkin 2001) and improvement has been observed in placebo groups, suggesting the possibility of carry-over and/or practice effects.
Positive effects have not yet been found in RCTs for methylphenidate (Reference Fernandez, Levy and SamleyFernandez 1995) or dexamphetamine (Reference Wagner and RabkinWagner 2000) for cognitive functioning in HIV. Therefore, psychostimulants cannot currently be recommended as a treatment for this symptom cluster.
Other areas of psychiatry
The successful use of modafinil and methylphenidate to treat sedation caused by psychotropic drugs has been noted in case reports and uncontrolled studies in relation to sodium valproate (Reference BeriganBerigan 2004), antipsychotics, including clozapine (Reference MillerMiller 1996), and selective serotonin reuptake inhibitors (Reference Schwartz, Azhar and ColeSchwartz 2004). The possibility of adverse effects must be considered whenever using psychostimulants in people with psychotic disorders, with case reports noting the exacerbation of psychotic symptoms with modafinil (Reference Narendran, Young and ValentiNarendran 2002) and movement disorder symptoms with methylphenidate (Reference MillerMiller 1996).
Reference Rosenthal and BryantRosenthal & Bryant (2004) described benefits of modafinil on clinical presentation, global functioning and fatigue in a prospective study of individuals with schizophrenia and schizoaffective disorder. However, an RCT of modafinil for the alleviation of fatigue and cognitive deficits in a similar population found no benefit of modafinil over placebo (Reference Sevy, Rosenthal and AlvirSevy 2005). One participant withdrew from each of these studies because of worsening psychosis.
One RCT reported benefits of modafinil on abstinence rates in cocaine dependence (Reference Dackis, Kampman and LynchDackis 2005), whereas another (Reference Grabowski, Roache and SchmitzGrabowski 1997) found no benefits with methylphenidate.
Adverse effects
Common side-effects of older psychostimulants include insomnia, agitation, anxiety and confusion (Reference Orr and TaylorOrr 2007). Although such side-effects are generally considered uncommon and short-lived when treatment is continued, one must be cautious when interpreting these results and drawing conclusions from the limited evidence available, which is usually related to children and adolescents treated for ADHD. The potential for misuse must always be considered, but many authors who have prescribed psychostimulants regularly for medical purposes have not noted misuse to be a significant problem (Reference Masand and TesarMasand 1996; Reference Olson, Ambrogetti and SutherlandOlson 2003).
The cardiovascular changes associated with older psychostimulants have been highlighted and concerns about sudden death led the US Food and Drug Administration to recommend that psychostimulant drugs used to treat ADHD include a black-box warning (Reference NissenNissen 2006). It was speculated that older psychostimulants could increase heart rate and blood pressure, leading to chronic heart failure and arrhythmias with long-term use. The warning is primarily directed at the use of psychostimulants to treat children and adolescents with ADHD. It is not clear how psychostimulant use would affect adults being treated for conditions other than ADHD. Heart rate and blood pressure changes associated with psychostimulant use are modest but measurable (Reference Alban, Hopson and LyAlban 2004). Furthermore, psychostimulant use in adults may be only short term and at different doses than for ADHD treatment. The UK's MHRA has recently issued drug safety advice regarding methylphenidate and potential cardiovascular and cerebrovascular risks (Medicines and Healthcare products Regulatory Agency 2009).
Side-effects of modafinil include headache, nausea and nervousness, with only the first symptom reaching significantly greater frequency than placebo in clinical trials. Most adverse effects are mild to moderate and large overdoses of modafinil alone have had no fatal outcomes (Reference Keating and RaffinKeating 2005). Despite a generally benign safety profile, the MHRA has recently issued concerns about modafinil and the risk of serious skin reactions and possible exacerbation of psychiatric symptoms (Medicines and Healthcare products Regulatory Agency 2008).
There has always been a theoretical risk of inducing mania or hypomania when using psychostimulants and case reports have been published, but these are considered rare in the adult population (Reference Masand, Pickett and MurrayMasand 1995; Reference Orr and TaylorOrr 2007). In a trial for bipolar depression, modafinil did not have higher rates of mania or hypomania compared with placebo (Reference Frye, Grunze and SuppesFrye 2007).
Conclusions
Currently, routine use of psychostimulants cannot be recommended for any of the conditions described above. Most trials have been very short and maintenance and follow-up data are non-existent. The evidence gathered to date would suggest, however, that these agents should be considered for specific symptoms such as depression, apathy, fatigue and sleepiness in a range of disorders. In clinical practice, the use of psychostimulants would be most appropriate where traditional agents have been ineffectual or contraindicated, a rapid response may be life-saving or lead to a greatly improved outcome or quality of life, and adverse events can be carefully monitored and managed.
MCQs
-
1 The primary mechanism of action of modafinil:
-
a releases dopamine at the nerve terminal
-
b may be through gamma-aminobutyric acid-mediated neurotransmission
-
c targets the dopamine transporter
-
d releases serotonin at the nerve terminal
-
e releases noradrenaline at the nerve terminal.
-
-
2 Methylphenidate's half-life in adults is:
-
a 1–2 hours
-
b 2–3 hours
-
c 2–7 hours
-
d 7–10 hours
-
e 11–14 hours.
-
-
3 In major depression, psychostimulants:
-
a are effective as a monotherapy antidepressant
-
b always precipitate mania or hypomania
-
c have good evidence as an augmentation agent to standard antidepressants
-
d have good evidence for treating fatigue and sleepiness in depression
-
e may accelerate an antidepressant response in elderly patients.
-
-
4 There is RCT evidence to support the use of psychostimulants in treating:
-
a apathy in dementia
-
b fatigue in HIV/AIDS
-
c cognitive deficits in HIV/AIDS
-
d cognitive deficits in schizophrenia
-
e clozapine-related sedation.
-
-
5 The most commonly recommended dose of methylphenidate in traumatic brain injuries is:
-
a 0.15 mg/kg/day
-
b 0.15 mg/kg twice a day
-
c 0.3 mg/kg/day
-
d 0.3 mg/kg twice a day
-
e 0.5 mg/kg/day.
-
MCQ answers
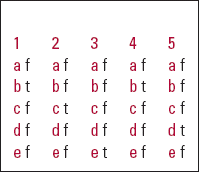
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | f | a | f | a | f | a | f | a | f |
| b | t | b | f | b | f | b | t | b | f |
| c | f | c | t | c | f | c | f | c | f |
| d | f | d | f | d | f | d | f | d | t |
| e | f | e | f | e | t | e | f | e | f |
TABLE 1 Pharmacokinetics of the major psychostimulants
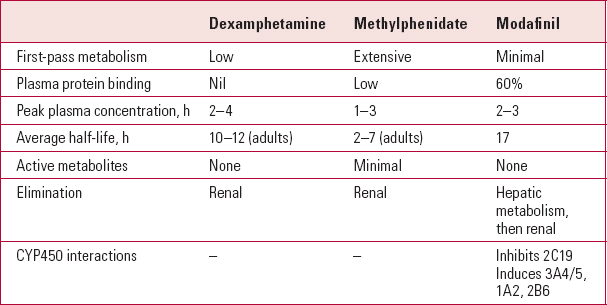
| Dexamphetamine | Methylphenidate | Modafinil | |
|---|---|---|---|
| First-pass metabolism | Low | Extensive | Minimal |
| Plasma protein binding | Nil | Low | 60% |
| Peak plasma concentration, h | 2–4 | 1–3 | 2–3 |
| Average half-life, h | 10–12 (adults) | 2–7 (adults) | 17 |
| Active metabolites | None | Minimal | None |
| Elimination | Renal | Renal | Hepatic metabolism, then renal |
| CYP450 interactions | – | – | Inhibits 2C19 Induces 3A4/5, 1A2, 2B6 |




eLetters
No eLetters have been published for this article.