Significant outcomes
Major depressive disorder is the predominant cause of ‘Years of life lived with disability’ and ‘Years of life lost because of premature death’. Severity and prevalence of depression increase with age, but the molecular underpinnings of the disorder have only partially been elucidated. Since the NAD+-dependent histone deacetylases sirtuin-1 and sirtuin-2 affect the longevity in model organisms, we assessed Sirt1 and Sirt2 mRNA in the prefrontal cortex and hippocampus of young and old FSL rats, a well-characterised model of depression, and control FRL rats with the aim to explore the depressive-like ageing trajectory. Sirt1 levels were decreased in the young FSL rats compared to the aged FSL as well as to the age-matched young FRL rats (cf Table 1); the former finding is of interest for depression-like–ageing interaction and the latter for elucidating depression pathology. Concluding, this is the first demonstration that SIRT1 and SIRT2 are changed in brain of the FSL rats and that the changes are age-dependent. Thus, sirtuins are potential targets for treatment of age-related neurodegenerative diseases.
Limitations
(i) The small number of animals, (ii) due to lack of samples we could not determine the protein, and (iii) due to the loss of samples we could not compare hippocampal tissue from ‘young’ and ‘aged’ rats (cf Table 1).
Introduction
Major depressive disorder (MDD) is a devastating, life-threatening disorder with a major and increasing burden on the public health (Greenberg et al., Reference Greenberg, Fournier, Sisitsky, Pike and Kessler2015). The understanding of MDD aetiology and pathophysiology is limited (McEwen et al., Reference McEwen, Bowles, Gray, Hill, Hunter, Karatsoreos and Nasca2015) and optimal treatments are lacking. Using the currently available drugs, 30–40% of the patients fail to respond adequately (Tundo et al., Reference Tundo, de Filippis and Proietti2015). However, ketamine and esketamine (the latter approved by FDA and EMA in 2020 for treatment-resistant depression) have shown acute antidepressant effect (Henter et al., Reference Henter, Park and Zarate2021). Moreover, recent papers translationally based on rat studies (Cohen et al., Reference Cohen, Liu, Kozlovsky, Kaplan, Zohar and Mathé2012) have shown that insufflated NPY alleviated MDD symptoms (Mathé et al., Reference Mathé, Michaneck, Berg, Charney and Murrough2020) and PTSD symptoms (Sayed et al., Reference Sayed, Van Dam, Horn, Kautz, Parides, Costi, Collins, Iacoviello, Iosifescu, Mathé, Southwick, Feder, Charney and Murrough2018).
Individuals with depression have an enhanced risk of developing age-related diseases. Moreover, persons diagnosed with repeated episodes of MDD have decreased life expectancy, often due to cardiovascular disease and diabetes (Mezuk et al., Reference Mezuk, Eaton, Albrecht and Golden2008; Walker et al., Reference Walker, McGee and Druss2015). In a longitudinal study of ageing (ELSA), an association between the duration of depressive symptoms and mortality risk has been demonstrated (White et al., Reference White, Zaninotto, Walters, Kivimäki, Demakakos, Biddulph, Kumari, De Oliveira, Gallacher and Batty2016). However, the underlying biological mechanisms have only in part been elucidated. Of particular interest for our work that focuses on depression and ageing are sirtuins, a family of 7 NAD+-dependent histone deacetylases (HDACs) that alter the chromatin structure through deacetylation of histones resulting in altered gene expression (Sun et al., Reference Sun, Kennedy and Nestler2013; Lu et al., Reference Lu, Li, Zhang, Zhao, Yan and Yang2018). Sirtuins have several targets including the histone modifications H4K16ac and H3K18ac as well as H3K9m3 (Vaquero et al., Reference Vaquero, Scher, Lee, Erdjument-Bromage, Tempst and Reinberg2004, Reference Vaquero, Scher, Hwei-Ling, Alt, Serrano, Sterglanz and Reinberg2006; Vaquero et al., Reference Vaquero, Scher, Erdjument-Bromage, Tempst, Serrano and Reinberg2007; Eskandarian et al., Reference Eskandarian, Impens, Nahori, Soubigou, Coppée, Cossart and Hamon2013). Thus, as HDACs, sirtuins regulate several essential cellular processes, such as mitochondrial activity, synaptic plasticity, apoptosis, cellular stress resistance, inflammation and memory processes through epigenetic marking (Brunet et al., Reference Brunet, Sweeney, Sturgill, Chua, Greer, Lin, Tran, Ross, Mostoslavsky, Cohen, Hu, Cheng, Jedrychowski, Gygi, Sinclair, Alt and Greenberg2004; Gao et al., Reference Gao, Wang, Mao, Graff, Guan, Pan, Mak, Kim, Su and Tsai2010; Michan et al., Reference Michan, Li, Chou, Parrella, Ge, Long, Allard, Lewis, Miller, Xu, Mervis, Chen, Guerin, Smith, McBurney, Sinclair, Baudry, de Cabo and Longo2010; Yoshizaki et al., Reference Yoshizaki, Schenk, Imamura, Babendure, Sonoda, Bae, Oh, Lu, Milne, Westphal, Bandyopadhyay and Olefsky2010; Wątroba and Szukiewicz, Reference Wątroba and Szukiewicz2016). With regard to ageing, sirtuins also alter the longevity in model organisms, where an extra copy of Sirt2, an orthologue to the mammalian sirtuin genes, extended the replicative lifespan in yeast by 50%, while a deletion of the gene shortened the lifespan (Haigis and Guarente, Reference Haigis and Guarente2006). Thus, sirtuins may play an important role in ageing. Indeed, several studies suggest their role in the pathogenesis of many age-related diseases, including neurodegenerative diseases (reviewed in Wątroba and Szukiewicz, Reference Wątroba and Szukiewicz2016).
Furthermore, several studies have shown that alterations of sirtuin levels have been associated with depression-like behaviour in animal models and depression in humans. For example, a sparse whole genome sequencing of a large population of depressed patients identified alterations of two single nucleotide polymorphisms (SNPs) that contributed to risk of MDD, with one of them located near the SIRT1 gene (CONVERGE consortium, 2015). Moreover, a decrease in SIRT1 and SIRT2 expression in leukocytes was suggested to be a state marker in MDD and bipolar disorder patients (Abe et al., Reference Abe, Uchida, Otsuki, Hobara, Yamagata, Higuchi, Shibata and Watanabe2011). In accordance with this, another study reported decreased levels of SIRT1 in peripheral blood from MDD patients (McGrory et al., Reference McGrory, Ryan, Kolshus, Finnegan and McLoughlin2018). This was further supported by finding of reduced Sirt1 activity in the dentate gyrus in a mouse model of chronic stress. Results from rodent models regarding the role of Sirt1 in depression have been conflicting. A pharmacologic inhibition of hippocampal Sirt1 activity increased depression-like behaviour, while Sirt1 upregulation led to stress resilience, with corresponding effects on dendrite length and spine plasticity for dentate gyrus granule neurons (Abe-Higuchi et al., Reference Abe-Higuchi, Uchida, Yamagata, Higuchi, Hobara, Hara, Kobayashi and Watanabe2016). Ferland et al. (Reference Ferland, Hawley, Puckett, Wineberg, Lubin, Dohanich and Schrader2013), in contrast to Abe-Higuchi, demonstrated that mice subjected to chronic stress had increased Sirt1 activity in dentate gyrus. In addition, mice with a Sirt1 knockout in brain showed decreased anxiety and mice globally overexpressing Sirt1 showed increased anxiety (Libert et al., Reference Libert, Pointer, Bell, Das, Cohen, Asara, Bergmann, Preisig, Otowa, Kendler, Chen, Hettema, vandenOord, Rubio and Guarente2011), and the Sirt1 activator Resveratrol had an antidepressant-like effect in rat models of depression. However, resveratrol is hypothesised to also act through other pathways leading to antidepressant-like effect (Howitz et al., Reference Howitz, Bitterman, Cohen, Lamming, Lavu, Wood, Zipkin, Chung, Kisielewski, Zhang, Scherer and Sinclair2003; Hurley et al., Reference Hurley, Akinfiresoye, Kalejaiye and Tizabi2014; Liu et al., Reference Liu, Xie, Yang, Gu, Ge, Wang and Wang2014). With regard to Sirt2, mice exposed to chronic social defeat stress had increased Sirt2 mRNA and protein levels in the prefrontal cortex (PFC), whereas repeated injections of imipramine reduced the Sirt2 levels (Erburu et al., Reference Erburu, Muñoz-Cobo, Domínguez-Andrés, Beltran, Suzuki, Mai, Valente, Puerta and Tordera2015). Administration of a Sirt2 inhibitor into the mouse PFC reversed the chronic mild stress-induced elevated PFC Sirt2 levels and depression-like behaviour (Erburu et al., Reference Erburu, Muñoz-Cobo, Diaz-Perdigon, Mellini, Suzuki, Puerta and Tordera2017).
Neuropeptide Y (NPY) is the most abundant peptide in mammal brain and decreased NPY levels have been demonstrated in the cerebrospinal fluid of MDD and posttraumatic stress disorder (PTSD) patients as well as bipolar patients at risk for suicide. These findings have been replicated preclinically in models such as the Flinders Sensitive Line (FSL) an extensively characterised model of depression, displaying depressive-like behaviour, and Flinders Resistant Line (FRL) rats (Heilig et al., Reference Heilig, Zachrisson, Thorsell, Ehnvall, Mottagui-Tabar, Sjögren, Asberg, Ekman, Wahlestedt and Agren2004; Overstreet et al., Reference Overstreet, Friedman, Mathé and Yadid2005; Wu et al., Reference Wu, Feder, Wegener, Bailey, Saxena, Charney and Mathé2011; Cohen et al., Reference Cohen, Liu, Kozlovsky, Kaplan, Zohar and Mathé2012; Overstreet and Wegener, Reference Overstreet and Wegener2013; Sandberg et al., Reference Sandberg, Jakobsson, Pålsson, Landén and Mathé2014; Thorsell and Mathé, Reference Thorsell and Mathé2017). FSL rats display several features that resemble the human depression, including elevated REM sleep, psychomotor retardation and increased immobility in the forced swim test (Overstreet et al., Reference Overstreet, Friedman, Mathé and Yadid2005; Overstreet and Wegener, Reference Overstreet and Wegener2013). Additionally, several molecular features are shared with human depression, such as elevated proinflammatory cytokine IL-6, lower central expression of the glial-specific protein S100B and complement factor C3 in several brain regions; shortened telomere length, dysregulated NPY system as well as abnormalities in the glutamatergic system (Jimenez-Vasquez et al., Reference Jimenez-Vasquez, Overstreet and Mathé2000, Reference Jiménez-Vasquez, Diaz-Cabiale, Caberlotto, Bellido, Overstreet, Fuxe and Mathé2007; Hascup et al., Reference Hascup, Hascup, Stephens, Glaser, Yoshitake, Mathé, Gerhardt and Kehr2011; Melas et al., Reference Melas, Mannervik, Mathé and Lavebratt2012; Wei et al., Reference W, ei, Melas, Wegener, Mathé and Lavebratt2014, Reference Wei, Backlund, Wegener, Mathé and Lavebratt2015; Strenn et al., Reference Strenn, Suchankova, Nilsson, Fischer, Wegener, Mathé and Ekman2015; Du Jardin et al., Reference Du Jardin, Muller, Sanchez, Wegener and Elfving2017). Sirt2 has been shown to modulate the glutamate system (Erburu et al., Reference Erburu, Muñoz-Cobo, Diaz-Perdigon, Mellini, Suzuki, Puerta and Tordera2017). Thus, in the PFC of the FSL rat, no differences in glutamate dynamics were observed in brains of young (3–6 months old) FRL and FSL rats, but a significant increase in resting glutamate levels was found in the aged (12–15 months old) FSL compared with the young FSL and age-matched FRL rats. In FSL, percent change in glutamate release during stress was also higher in the aged rat compared to younger rat (Hascup et al., Reference Hascup, Hascup, Stephens, Glaser, Yoshitake, Mathé, Gerhardt and Kehr2011). These findings confirmed and extended interactions between depression and age in animal models. In view of these findings, we investigated the mRNA expression levels of Sirt1, Sirt2 as well as Npy in the FSL and the control FRL rat strains. The behavioural and associated brain differences between FSL and FRL, inter alia in NPY expression, are well documented and we measured NPY as a positive control. In addition, we have previously found NPY changes with age (Husum et al., Reference Husum, Aznar, Hoyer-Hansen, Larsen, Mikkelsen, Moller, Mathé and Wörtwein2006). The analyses were performed in the PFC and hippocampus, brain regions implicated in MDD and rodent depression models (Overstreet et al., Reference Overstreet, Friedman, Mathé and Yadid2005; Neumann et al., Reference Neumann, Wegener, Homberg, Cohen, Slattery, Zohar, Olivier and Mathé2011; Overstreet and Wegener, Reference Overstreet and Wegener2013).
Materials and methods
Animals and tissue samples
FSL and FRL rats were maintained at the Animal Facility, Karolinska Institutet, Huddinge, under controlled conditions of temperature (22 ± 1°C), relative humidity (45–55%) and daylight cycle (12:12 h, lights on at 6:00 am). Standard rat chow and tap water were available ad libitum. The PFC and hippocampi were dissected according to Glowinski and Iversen (Reference Glowinski and Iversen1966) and stored at −80°C until subsequent analyses. Expression levels of Sirt1, Sirt2 and Npy were determined in the PFC of male (’young’ = 3-month-old and ‘aged’ = 14–15 months old) FSL young n = 4, FSL aged n = 7, FRL young n = 5 and FRL aged n = 9. Since hippocampal tissue from the aged rats was lost, we could only analyse tissue from the 3-month-old rats (FSL n = 8; FRL n = 7). All experimental work was approved by the Ethical Committee for protection of animals at the Karolinska Institutet.
RNA extraction and reverse transcription
Tissue-Tearor (Biospec Products Inc., Bartlesville, OK, USA) was used to homogenise the brain samples. Total RNA was extracted using AllPrep DNA/RNA mini kit according to the manufactures protocol (Qiagen, Hilden, Germany) and was treated with DNase I to eliminate the contamination of DNA (Qiagen). The total RNA concentrations were measured spectrophotometrically using NanoDrop 2000 (Thermo Fisher Scientific, Rockford, IL, USA). The RNA quality of 10 samples was examined by Agilent 2100 BioAnalyzer (Agilent Technologies, Germany). Complementary DNA (cDNA) was synthesised by reverse transcription of total RNA using SuperScript III First-Strand Synthesis System for qPCR with random hexamers according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). cDNA was stored at −20°C and RNA at −80°C.
Gene expression by quantitative RT-PCR
Quantative RT-PCR was applied to measure mRNA levels of the genes Sirt1, Sirt2 and Npy in PFC and hippocampal tissue. Amplifications of these genes and the housekeeping gene Actin were performed in triplicates of the cDNA samples using Power-SYBR Green (Applied Biosystems Inc., Foster City, CA, USA) and the QuantStudioTM flex 6/7 System (Thermo Fisher Scientific). Sample to sample variation in Sirt1, Sirt2 and Npy expression levels were corrected for by normalising to that of Actin, and interplate variation was corrected for by including a positive control/calibrator sample in each plate. The relative gene expression, fold change, was calculated according to the comparative Ct method using the formula: 2−ΔΔCt. The primers sequences, restricting the amplification to mRNA, were (written 5′ to 3′) Sirt1 Fw:TACCCCATGAAGTGCCTCAA, Sirt1 Rv:AAGTTTGGCATACTCGCCAC, Sirt2 Fw: GCAGGAATCCCTGACTTCCG, Sirt2 Rv: TGCCCAGGATAGAGCTCCTTA, Npy Fw:CGCTCTGCGACACTACATCA, Npy Rv:TGGGGGCATTTTCTGTGCTT, beta-actin Fw: TAAGGCCAACCGTGAAAAGAT and beta-actin Rv: GTGGTACGACCAGAGGCATAC.
Statistical analysis
The Levene’s test and Shapiro−Wilk test were used for assessing the homogeneity of variance and the normality of the data, respectively. The statistical significance of differences in PFC gene expression between the four groups (young and aged FRL and FSL rats) were tested applying two way-ANOVA followed by a post hoc analysis with a Bonferroni-corrected independent sample t-test. As the beforehand mentioned assumptions were met for hippocampus, a two-tailed independent test was used. Statistical significance was assigned for p < 0.05. Outliers marked in the figure legends were identified based on the values below Q1-1.5*IQR or above Q3 + 1.5*IQR. The analyses were made in R (Rstudio version 1.2.1335).
Results
Prefrontal cortex
Expression levels of Sirt1, Sirt2 and Npy in PFC of young and old FSL and FRL rats are depicted in Fig. 1. Sirt1 levels were significantly decreased in the FSL compared to the FRL rats (F[1,21]= 13.8; p = 0.001, two-way ANOVA). Sirt1 levels were also significantly different between the age groups (F[1,21]= 6.22; p = 0.022), and a significant interaction between age and genotype was found (F[1,21]= 5.26; p = 0.032); the young FSL rats had a significantly lower Sirt1 expression with respect to both age (p = 0.026, post hoc test) and genotype (p = 0.001, post hoc test). Sirt2 levels were significantly lower in the FSL (F[1,21]= 14,1; p = 0.001) but did not differ between the age groups. With regard to Npy , in similarity to Sirt2, Npy mRNA levels in PFC were downregulated in the FSL compared to the FRL rats (F[1,21]= 31.3; p < 0.001) but did not differ between the age groups.
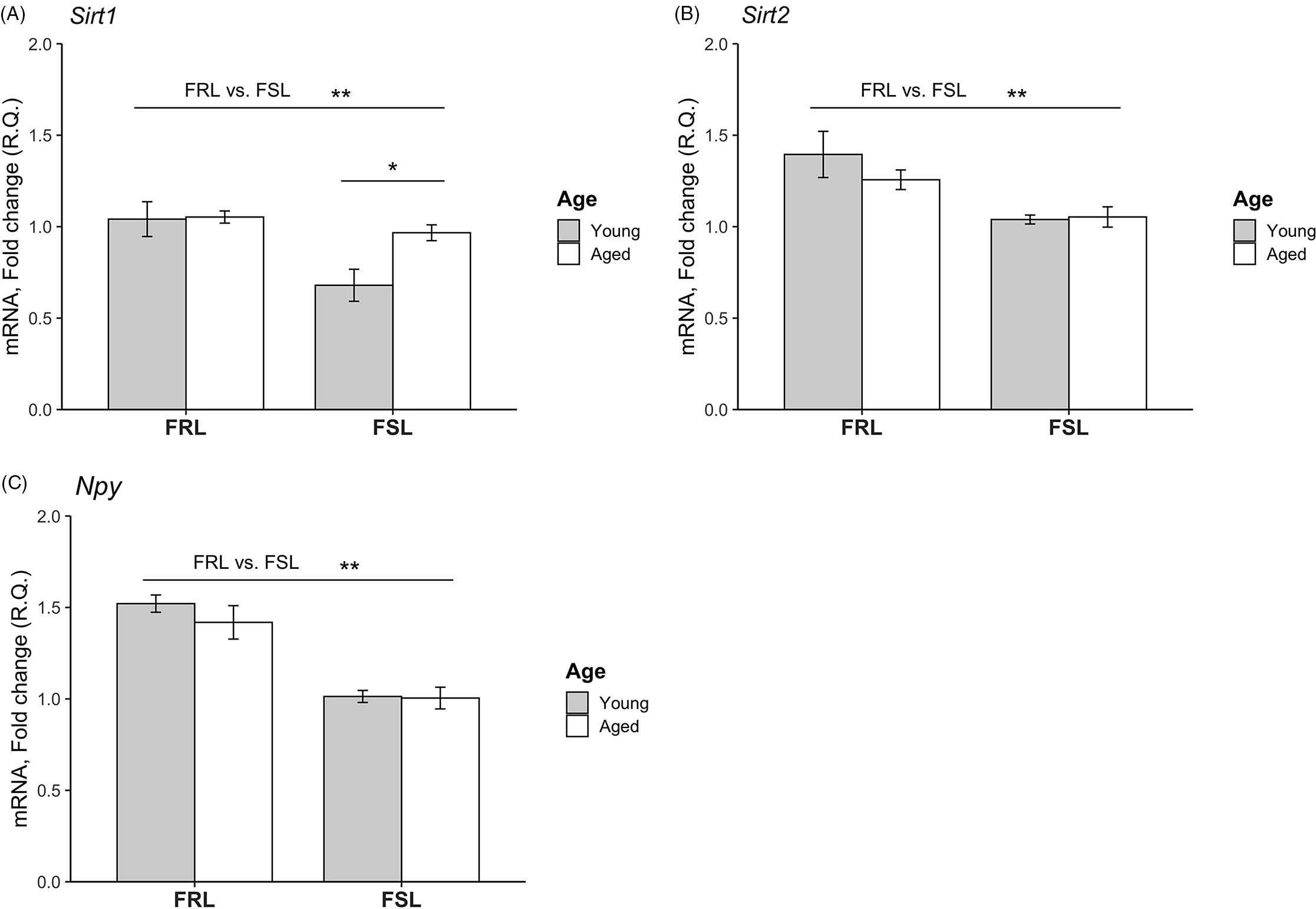
Fig. 1. Relative mRNA levels of the Sirt1, Sirt2 and Npy genes in the prefrontal cortex (PFC) of young and aged FSL and FRL rats. (A) Sirt1 levels were significantly decreased in FSL compared to FRL. Moreover, the levels were lower in the young FSL rats compared to the aged FSL. (B) Sirt2 levels were lower in FSL compared to FRL. No difference with regard to age was found. (C) Npy levels were reduced in FSL compared to FRL. No difference with regard to age was found. The relative quantification (R.Q) bars represent mean values and the error bars represent the standard error of the mean (SEM). n = 4 young FSL; n = 7 old FSL, n = 5 young FRL; n = 9 old FRL; * p < 0.05, ** p < 0.01.
Hippocampus
Expression levels of Sirt1, Sirt2 and Npy in hippocampus of young FSL and FRL rats are depicted in Fig. 2. Since samples from the old FSL and FRL rats were lost in processing, effects of ageing could not be investigated. In similarity to findings in the PFC, hippocampal Sirt1 levels were reduced in young FSL compared to young FRL rats (p = 0.005). There was no detectable difference in Sirt2 levels between the FSL and FRL. Lastly, Npy levels were decreased in the hippocampus of the young FSL compared to young FRL rats (p = 0.003).
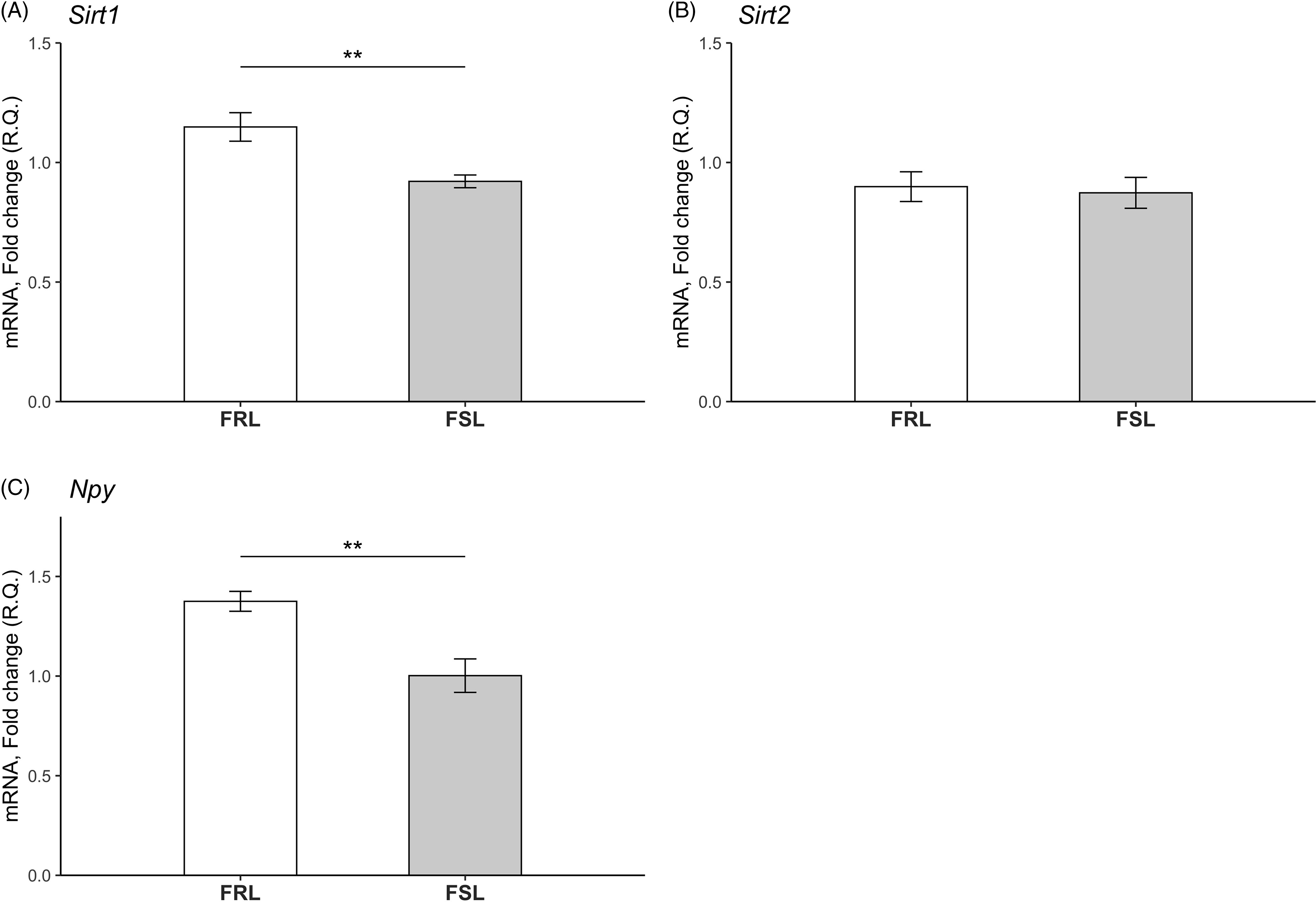
Fig. 2. Relative mRNA levels of the Sirt1, Sirt2 and Npy genes in the hippocampi of young FSL and FRL rats. (A) Sirt1 levels were significantly decreased in young FSL compared to young FRL. (B) No differences in Sirt2 levels between young FRL and young FSL were detected. n = 8 FSL; n = 7 FRL (one outlier in the FSL group in the Sirt1 gene has been excluded) *p < 0.05, **p < 0.01. (C) Npy levels were significantly decreased in young FSL compared to young FRL. The relative quantification (R.Q) bars represent mean values and the error bars represent the standard error of the mean (SEM) n = 8 FSL; n = 7 FRL (one outlier in the FSL group in the Sirt1 gene has been excluded) *p < 0.05, ** p < 0.01.
Discussion
Our main findings are lower expressions of Sirt1 and Sirt2 in the PFC of the FSL compared to the FRL strain. Moreover, Sirt1 was also decreased in young FSL rats. Relationships of these changes to pathology and the disease trajectory with age are not clear but point to new targets to elucidate depression pathophysiology and develop novel treatments. Vicissitudes of Sirt1 and Sirt2 expression have been reviewed in the Introduction section. Differences in species used (mouse and rat) as well as a variety of experimental procedures have led to divergent, sometimes conflicting results. Nevertheless, a tentative conclusion is that reduced Sirt1 and Sirt2 expressions are associated with depression and anxiety-like behaviours. The downregulated Sirt1 levels in PFC and hippocampus of young FSL rats were partly in agreement with reports showing a reduction of Sirt1 mRNA levels in hippocampus but not in medial PFC using stress-induced behavioural models of depression in 2-month-old mice (Abe-Higuchi et al., Reference Abe-Higuchi, Uchida, Yamagata, Higuchi, Hobara, Hara, Kobayashi and Watanabe2016). In line with this, 2-month-old mice susceptible to chronic social defeat stress had reduced Sirt1 mRNA levels in hippocampus compared to the controls and stress-resilient mice (Kim et al., Reference Kim, Hesterman, Call, Magazu, Keeley, Armenta, Kronman, Neve, Nestler and Ferguson2016). With regard to the downregulated Sirt2 levels, we found in the PFC of the FSL rats, studies in mice focusing on chronic stress and the disrupted vesicular glutamate transporter 1 (VGLUT1+/−) models reported a link between reduction in Sirt2 mRNA and Sirt2 activity in PFC (Erburu et al., Reference Erburu, Muñoz-Cobo, Domínguez-Andrés, Beltran, Suzuki, Mai, Valente, Puerta and Tordera2015, Reference Erburu, Muñoz-Cobo, Diaz-Perdigon, Mellini, Suzuki, Puerta and Tordera2017; Munoz-Cobo et al., Reference Munoz-Cobo, Belloch, Diaz-Perdigon, Puerta and Tordera2017). In those experiments, both antidepressants and a selective Sirt2 inhibitor reduced Sirt2 levels in PFC as well as depression-like behaviour. Probable explanations are that the discrepancies are due to species differences and that FSL rats represent a genetic model of depression clearly different from the stress models and the model of disrupted VGLUT transporter. Furthermore, the differences between our Sirt2 findings and the aforementioned studies in PFC could reflect the heterogeneity in underlying molecular pathways of depression-like behaviour. Another variable that has to be considered in interpreting the results is that of circadian rhythm. For instance, SIRT1 expression also shows circadian rhythm (Chang and Guarente, Reference Chang and Guarente2013) and, in a different context, we have shown that circadian rhythm fluctuations in NPYergic system and HPA) axis underlie differences in vulnerability to stress responses (Cohen et al., Reference Cohen, Vainer, Matar, Kozlovsky, Kaplan, Zohar, Mathé and Cohen2015). However, since we controlled for the time of sacrifice, this factor can be ruled out with regard to results within our study but could have a bearing on comparison with other studies. The Sirt1 mRNA expression in the PFC region was decreased in young FSL rats only, while the levels in the older group did not significantly differ between the FSL and FRL. These findings suggest that during the ageing trajectory, there are compensatory mechanisms that counteract the reduction of Sirt1 levels found in young depressed FSL rats; elucidation of such mechanisms should contribute to understanding the depression–ageing interaction. Finally, our findings are consistent with the reports of reduced SIRT1 and SIRT2 levels in leukocytes from patients with MDD compared to healthy controls (Abe et al., Reference Abe, Uchida, Otsuki, Hobara, Yamagata, Higuchi, Shibata and Watanabe2011; McGrory et al., Reference McGrory, Ryan, Kolshus, Finnegan and McLoughlin2018).
Findings of reduced Npy levels in both PFC and hippocampus of the FSL rats are in agreement with previous results (Jiménez-Vasquez et al., Reference Jimenez-Vasquez, Overstreet and Mathé2000 and Reference Jiménez-Vasquez, Diaz-Cabiale, Caberlotto, Bellido, Overstreet, Fuxe and Mathé2007; Wu et al., Reference Wu, Feder, Wegener, Bailey, Saxena, Charney and Mathé2011; Thorsell and Mathé, Reference Thorsell and Mathé2017) and are thus a quality indicator of procedures used in this study. With regard to the effect of age, in one study (Husum et al., Reference Husum, Aznar, Hoyer-Hansen, Larsen, Mikkelsen, Moller, Mathé and Wörtwein2006), we found that ageing caused an exacerbated loss of NPY immunoreactive cells in the dentate gyrus in the FSL strain compared with FRL. The aged FSL rats also had shortened 5-HT-IR fibres in the dorsal hippocampus, indicative of an impaired 5-HT innervation of this area, compared with FRL. These results are not contradictory due to the basic difference in methodology and call for in depth study of ageing effect on the NPYergic system.
The strengths of the study– the first of its kind – are the findings of significant decrease in Sirt1 and Sirt2 expression in the FSL compared to the FRL strain as well as an increase in Sirt1 with ageing in brain regions of relevance for depression. In addition, we confirmed the decreased Npy expression in FSL versus FRL strains. These findings contribute to mapping the molecular underpinnings of depression and the ageing–depression temporal trajectory and have a potential to contribute to development of novel therapeutic targets.
Table 1. Significant outcomes and limitations

Authors contributions
AAM: concept and initiative, breeding of animals, preparation of brains, and tissue sample contribution; MS and PE: experimental work: all authors: data analysis and interpretation; MS and AAM: draughting the manuscript. MS, PE, CL, AL and AAM: final manuscript and approval.
Financial support
Supported by the Swedish Medical Research Council grant 10414 (AAM, 2016-02955) and the Centre for Psychiatry Research, SLL-KI (AAM).
Conflict of interest
None.
Statement of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Animal welfare ethical statement
All experimental work was approved by the Ethical Committee for protection of animals at the Karolinska Institutet.






