Significant outcomes
-
At admission schizophrenic patients had higher serum s100b protein concentrations than those of healthy subjects.
-
Schizophrenics’ s100b protein levels decreased between admission and discharge, but they still were higher than the s100b healthy subjects’ levels.
-
After 3 months of hospital discharge, the serum s100b protein levels normalised, reaching similar s100b serum level than those of the healthy subjects.
Limitations
-
The small number of patients precludes the generalisation of the results.
-
The s100b protein was measured in serum not in the cerebrospinal fluid.
-
The normalisation of s100b protein could have occurred before the 3 months after hospital discharge.
Introduction
Schizophrenia is a chronic psychiatric condition characterised by positive, negative and disorganisation symptoms as well as impaired cognition. Schizophrenia diagnosis relies on clinical interviews and is a multi-factorial disorder in its aetiopathogenesis and evolution. Several pathogenic mechanisms have been proposed (Marder and Cannon, Reference Marder and Cannon2019). Among them, neuroinflammation has been suggested as an important contributor in the schizophrenia pathogenesis (Buckley, Reference Buckley2019).
There is no single biological marker that helps clinicians with the diagnosis of schizophrenia, so, the search for peripheral markers that could help to reach a clinical diagnosis and management is welcomed.
Several molecules have been involved as biological markers in the clinical diagnosis of schizophrenia. The glial fibrillary acidic protein, the myelin basic protein, the neurone-specific enolase (Steiner et al., Reference Steiner, Bielau, Bernstein, Bogerts and Wunderlich2006), the total antioxidant capacity (Morera-Fumero et al., Reference Morera-Fumero, Díaz-Mesa, Abreu-Gonzalez, Fernandez-Lopez and Cejas-Mendez2017a), the complement (Ji et al., Reference Ji, Boerrigter, Cai, Lloyd, Bruggemann, O’Donnell, Galletly, Lloyd, Liu, Lenroot, Weickert and Shannon Weickert2021), the brain-derived neurotrophic factor (Xiu et al., Reference Xiu, Li, Chen, Chen, Curbo, Wu, Tong, Tan and Zhang2020) and the acute phase proteins (Morera et al., Reference Morera, Henry, García-Hernández and Fernandez-López2007) are mentioned just to name some of them.
In the last 20 years, the s100b protein has received attention as a peripheral biomarker of schizophrenia (Yelmo-Cruz et al., Reference Yelmo-Cruz, Morera-Fumero and Abreu-González2013). The s100b is a dimeric protein with a molecular weight of 21 KDa that has autocrine and paracrine actions on neurons and glia (Donato, Reference Donato2001). Lower concentrations of extracellular s100b act on glial and neuronal cells as a growth differentiating factor, while higher concentrations induce apoptosis (Fanò et al., Reference Fanò, Biocca, Fulle, Mariggiò, Belia and Calissano1995). The s100b protein may cross the blood-brain barrier (BBB) and its concentration in blood and CSF is considered a marker of brain injury (Gonçalves et al., Reference Gonçalves, Concli Leite and Nardin2008). It has been considered as the C-reactive protein of the brain (Sen and Belli, Reference Sen and Belli2007) and has been proposed as a biomarker directly related to the disease progression (Michetti et al., Reference Michetti, D’Ambrosi, Toesca, Puglisi, Serrano, Marchese, Corvino and Geloso2019)
In 1997, Green et al. measured the s100b concentration in the CSF of 154 neurology patients and 19 patients with no evidence of organic brain disease acted as control group. Among the neurology patients, a subgroup of 12 patients received the label of ‘patients with other neurology diseases’. In that group, there was one patient with schizophrenia that had higher values of s100b than the mean level of the control group (Green et al., Reference Green, Keir and Thompson1997). Two years later, the first specific s100b research on schizophrenic patients was published (Wiesmann et al., Reference Wiesmann, Wandinger, Missler, Eckhoff, Rothermundt, Arolt and Kirchner1999). It reported that patients had significantly higher plasma s100b concentrations than healthy blood donors. Since then several papers have been published on this topic (Chen et al., Reference Chen, Tian, Chen, Xiu, Wang, Yang, Wang, Yang and Tan2017; Deng et al., Reference Deng, Kahlon, Mohite, Amin, Zunta-Soares, Colpo, Stertz, Fries, Walss-Bass, Soares and Okusaga2018a; Milleit et al., Reference Milleit, Smesny, Rothermundt, Preul, Schroeter, von Eiff, Ponath, Milleit, Sauer and Gaser2016).
Increased s100b concentrations have been reported in schizophrenic patients compared to controls (Hong et al., Reference Hong, Zhao, Li, Peng, Wang, Li, Xiang, Su, Huang, Zhang, Zhao, Zhou, Mao, Lin, Fang, Zhang and Xie2016; Schmitt et al., Reference Schmitt, Bertsch, Henning, Tost, Klimke, Henn and Falkai2005; Steiner et al., Reference Steiner, Bielau, Bernstein, Bogerts and Wunderlich2006; Wiesmann et al., Reference Wiesmann, Wandinger, Missler, Eckhoff, Rothermundt, Arolt and Kirchner1999), but decreased (Gattaz et al., Reference Gattaz, Lara, Elkis, Portela, Gonçalves, Tort, Henna and Souza2000) and unchanged levels have also been reported (Hendouei et al., Reference Hendouei, Hosseini, Panahi, Khazaeipour, Barari, Sahebnasagh and Ala2016; Van Der Leeuw et al., Reference Van Der Leeuw, Marcelis, Peeters, Verbeek, Menheere, De Haan, Van Os and van Beveren2013; Uzbay et al., Reference Uzbay, Goktalay, Kayir, Eker, Sarandol, Oral, Buyukuysal, Ulusoy and Kirli2013). To add further confusion to these research area, several methodological approaches have been carried out. Transversal or cross-sectional designs, this is one point-time studies, the subjects are just studied once, these are the most common published ones(Kozłowska et al., Reference Kozłowska, Brzezińska-Błaszczyk, Agier, Wysokiński and Żelechowska2021; Milleit et al., Reference Milleit, Smesny, Rothermundt, Preul, Schroeter, von Eiff, Ponath, Milleit, Sauer and Gaser2016; Qi et al., Reference Qi, Xiu, Chen, Wang, Kosten, Kosten and Zhang2009; Schmitt et al., Reference Schmitt, Bertsch, Henning, Tost, Klimke, Henn and Falkai2005; Schroeter et al., Reference Schroeter, Abdul-Khaliq, Frühauf, Höhne, Schick, Diefenbacher and Blasig2003). Longitudinal designs are more demanding and time consuming; therefore, there are fewer of these kind of studies. Among the longitudinal designs, the most frequently published articles are those that study patients at two point-times, this is one basal measure and then one follow-up measure. The range between the basal measure and the follow-up measure varies between 6 weeks (Gerasimou et al., Reference Gerasimou, Tsoporis, Siafakas, Hatziagelaki, Kallergi, Chatziioannou, Parker, Parissis, Salpeas, Papageorgiou and Rizos2018; Morera-Fumero et al., Reference Morera-Fumero, Díaz-Mesa, Abreu-Gonzalez, Fernandez-Lopez and Cejas-Mendez2017b; Rothermundt et al., Reference Rothermundt, Missler, Arolt, Peters, Leadbeater, Wiesmann, Rudolf, Wandinger and Kirchner2001; Sarandol et al., Reference Sarandol, Kirli, Akkaya, Altin, Demirci and Sarandol2007; Steiner et al., Reference Steiner, Walter, Wunderlich, Bernstein, Panteli, Brauner, Jacobs, Gos, Rothermundt and Bogerts2009) and 12 weeks (Ling et al., Reference Ling, Tang, Jiang, Wiste, Guo, Weng and Yang2007). More than two point-time designs even fewer papers are available (Rothermundt et al., Reference Rothermundt, Ponath, Glaser, Hetzel and Arolt2004).
Apart from the time design of the studies (transversal vs longitudinal), it has been reported in healthy subjects that the serum concentration of s100b is significantly higher in summer than in winter with no differences in s100b concentrations between day and night in both seasons (Morera-Fumero et al., Reference Morera-Fumero, Abreu-Gonzalez, Henry-Benitez, Yelmo-Cruz and Diaz-Mesa2013). A day/night change in serum s100b concentrations of acutely relapsed paranoid inpatients has been reported when admitted to hospital. This day/night change was not present at discharge in spite that the serum s100b levels were still higher than the serum s100b level of the control group (Morera-Fumero et al., Reference Morera-Fumero, Díaz-Mesa, Abreu-Gonzalez, Fernandez-Lopez and Cejas-Mendez2017b).
The objective of the present research is to study the evolution of serum s100b levels in the same sample of schizophrenic patients at three different times, at hospital admission, discharge and 3 months after hospital discharge (3MAHD) both at midday (12:00) and midnight (24:00).
Methods
Subjects
Twenty-three paranoid schizophrenic inpatients meeting DSM-IV criteria participated in the study. Patients were recruited from the psychiatric ward of the Canary Islands University Hospital. They were hospitalised because of an acute psychotic relapse. Patients were diagnosed by two experienced clinical psychiatrists based on the Structured Clinical Interview for the DSM-IV.
A sample of 23 healthy controls, matched by age, gender and season of admission, without personal and family psychiatric history was recruited between the researchers’ acquaintances. Physical healthiness of the control group was evaluated by a short medical history and a general laboratory test. Mental healthiness was assessed informally by asking the subjects if they had received psychiatric treatment in the past or were receiving treatment at present or if any first-degree relative was in the past or at present receiving psychiatric treatment. Psychological treatment was considered as well as receiving psychiatric treatment.
Any subject with alcohol abuse, substance abuse, physical illness, pregnancy, severe trauma, head trauma, intake of anti-inflammatories or immunosuppressants, mental retardation and physical agitation was excluded in this study.
Psychopathological assessment
At admission, discharge and 3MAHD, psychopathology was measured with the Positive and Negative Syndrome Scale (PANSS) (Kay et al., Reference Kay, Fiszbein and Opler1987). Two clinical psychiatrists evaluated independently the psychopathology. They had attended to a training session in the use of the PANSS. After PANSS training, repeated assessments for the PANSS scores maintained an inter-rater correlation coefficient >0.8.
Study protocol
Blood was collected the day after admission, the day before discharge and in a 3MAHD appointment in the group of patients. The blood of the healthy controls was collected once in any day between the period of admission and discharge of the patients in order to match the patients for the s100b seasonal change (Morera-Fumero et al., Reference Morera-Fumero, Abreu-Gonzalez, Henry-Benitez, Yelmo-Cruz and Diaz-Mesa2013, Reference Morera-Fumero, Díaz-Mesa, Abreu-González and Fernandez-Lopez2021). Samples were collected at 12:00 and 24:00 h to minimise the interference with the hospitalisation routines and because midday and midnight represent two opposite times along the day. In order to reduce the physical and psychological stress induced by the blood extraction, subjects were relaxed in bed 1 h before the extraction (Gazzolo et al., Reference Gazzolo, Florio, Zullino, Giovannini, Scopesi, Bellini, Peri, Mezzano, Petraglia and Michetti2010). All subjects were Caucasian, so the racial bias was not present in our sample (Gannon et al., Reference Gannon, Kelly, Besch, Thakur, Khurana, Shurin, Shurin, Brar, Cihakova, Talor and Chengappa2020). After extraction, samples were placed in vacutainer tubes without anticoagulant and allowed to clot, then they were centrifuged at 3000 r.p.m. during 5 min. After that, serum was separated, aliquoted in Eppendorf tubes and stored frozen at −70° C until analysis.
In order to make all antipsychotic treatments comparable, each patient antipsychotic treatment was converted into chlorpromazine equivalent doses (CED) (Atkins et al., Reference Atkins, Burgess, Bottomley and Riccio1997; Woods, Reference Woods2003).
The study protocol was carried out following the Helsinki Declaration, and all subjects gave written informed consent before inclusion. The protocol was approved by the Ethics and Investigation Committee of the Canary Islands University Hospital.
Serum s100b measurements
Serum s100b levels were determined with an enzyme-linked immunoassay (ELISA) kit according to the manufacturer instructions (Bio Vendor, Candler, USA). The Bio Vendor Human s100b ELISA uses a polyclonal anti-cow s100b coated in microtitration wells. The absorbance of the resulting yellow colour product was measured spectrophotometrically at 450 nm in a microplate spectrophotometer reader (Benchmark Plus, Bio-Rad, Hercules, CA, USA). In this ELISA, the lowest detection limit was 10.8 pg/ml. Coefficients of variation was 3.92% and 5.03% for intra- and inter-assay variabilities, respectively. To minimise the assay variance, all serum samples were analysed the same day with the same laboratory batch and by the same analyst. The analyst was always blind with respect to the samples pertaining to day/night, admission/discharge/3MAHD and to patient/control groups.
Statistical analysis
Data were analysed using the 21st version of the Statistical Package for the Social Sciences (SPSS, Chicago, Illinois, USA). Patients and healthy subjects serum s100b differences were compared by means of a t-test for independent samples using the Bonferroni correction for multiple comparisons. Patients’ serum s100b differences and PANSS scores at admission, discharge and 3MAHD were analysed by an ANOVA for repeated measures. Bonferroni’s post hoc comparisons were carried out if the ANOVA results were statistically significant. The statistic chi-square was applied to study the association between qualitative variables. Because of the small sample size, the relationships between quantitative variables were analysed by means of the Spearman correlation coefficient. All statistical tests were two-tailed. Statistical significance level was set at 0.05. Quantitative data are presented as mean ± standard deviation (SD).
Results
Description of the samples’ characteristics
According to Table 1, patients and healthy controls were comparable according to age, body mass index and gender distribution.
Table 1. Samples’ characteristics. Results are presented as mean ± standard deviation and 95% confidence intervals

BMI: Body Mass Index; AIO: Age of Illness Onset in years; ID: Illness Duration in years; NPH: Number of Previous Hospitalisations; CED: Chlorpromazine Equivalent Dose in mg; LH: Length of Hospitalization in days; NA: Not Applicable.
* t-test for independent samples.
+ Chi-squared test.
Comparison of s100b levels between patients and healthy controls
In order to control the effect of multiple comparisons between patients and healthy controls, the Bonferroni correction was used to correct the alpha error (Table 2). At admission and discharge patients presented significantly higher levels of serum s100b than healthy subjects at 12:00 h but not at 24:00 h (Table 2). At 3MAHD, the patients presented similar levels of serum s100b than the healthy subjects, both at 12:00 h and 24:00 h (Table 2), with no statistical difference between both groups.
Table 2. Comparison of serum s100b concentrations by time in patients and controls. Results are presented as mean ± standard deviation, 95% confidence of interval

3MAHD: Three Months After Hospital Discharge; PVABCMC: P-Value After Bonferroni’s Correction for Multiple Comparisons.
t-test for independent samples using the Bonferroni correction for multiple comparisons.
Comparison of serum s100b concentrations at admission, discharge and 3MAHD by time of the day
The comparison of serum s100b levels in patients at admission, discharge and 3MAHD (factor 1) by time of the day, 12:00 h and 24:00 h (factor 2) was carried out through an ANOVA for repeated measures in order to ascertain the effect of the main factors as well as their interaction.
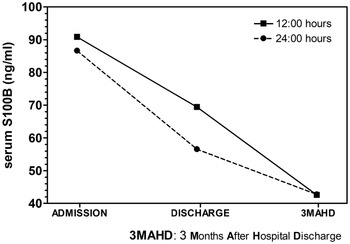
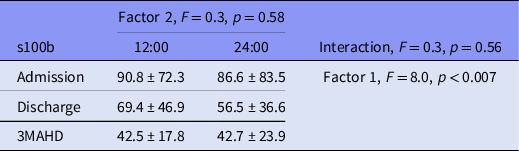
Factor 1 (s100b at admission, discharge and 3MAHD) elicited a significant result (Table 3). The post hoc Bonferroni’s comparisons are presented in Graph 1. Serum s100b levels at 12:00 and 24:00 h decreased significantly between admission and discharge as well as between discharge and 3MAHD.

Graph 1. Post-hoc Bonferroni’s comparisons of 12:00 h and 24:00 h serum s100b levels in patients at admission, discharge and 3 months after hospital discharge (3MAHD).
Table 3. ANOVA for s100b repeated measures by time of the day (factor 2) at admission, discharge and 3MAHD (factor 1). Results are presented as mean ± standard deviation
3MAHD: Three Months After Hospital Discharge.
Factor 2 (s100b at 12 h and 24:00 h) did not elicit a significant result (Table 3), and the interaction between factor 1 and 2 did not produce a significant result (Table 3).
Table 3 presents the results of the ANOVA for the s100b repeated measures in the patient group.
Comparison of serum s100b concentrations at 12:00 and 24:00 h in the control group
There were no differences between the serum s100b concentrations at 12:00 and 24:00 h in the control group (s100b 12:00: 42.3 ± 25.3, s100b 24:00: 45.4 ± 24.0, t: −1.423, p = 0.169).
Comparison of the PANSS scores at admission, discharge and 3MAHD
The comparison of the positive, negative, general and total scores at admission, discharge and 3MAHD through an ANOVA for repeated measures elicited significant results in the four scores (Table 4).
Table 4. Comparison of positive, negative, general and total PANSS scores at admission, discharge and 3 months after discharge (3MAHD)

ANOVA for repeated measures.
* Post hoc admission scores were significantly higher (p < 0.05) than scores at discharge and 3MAHD.
+ Post hoc scores at discharge and 3MAHD were not significantly different.
The post hoc Bonferroni’s comparisons elicited significant results in all measures (positive, negative, general and total scores). Patients had significantly higher scores at admission than at discharge and 3MAHD in all measures (Table 4). There were no significant differences between the scores at discharge and 3MAHD (Table 4).
Correlations between s100b concentrations and psychopathology
The correlations between serum s100b and PANSS are presented in Table 5. The 12:00 h serum s100b at admission correlated negatively with the total PANSS score at discharge and the negative and total PANSS scores at 3MAHD. Because the negative PANSS score contributes to the total PANSS score, we carried out a partial correlation between the s100b at 12:00 h and the total PANSS score at discharge and 3MAHD but controlling the effect of the negative score. The result shows that these correlations became not significant. The negative correlation between 12:00 h s100b levels at admission and the negative PANSS score at 3MAHD means that the higher the s100b concentration at admission the lower the negative PANSS score at 3MAHD was stated. The 24:00 h serum s100b at admission correlated negatively with the negative PANSS scores at admission and 3MAHD, in both cases, the higher the negative PANSS scores the lower the 24 h s100 levels at admission.
Table 5. Correlations between PANSS scores and serum s100b levels

PC: Partial Correlation; APC: After Partial Correlation.
The 12:00 h serum s100b at discharge correlated negatively with the negative PANSS scores at admission and 3MAHD as well as with the total PANSS score at discharge and 3MAHD. The partial correlation between the s100b at 12:00 h and total PANSS score at discharge and 3MAHD controlling the effect of the negative score elicited that both correlations became not significant (Table 5). Again, the higher the negative PANSS scores, the lower the s100b level at 12.00 h. The 24:00 h serum s100b at discharge correlated positively with the positive and general PANSS scores at admission. Patients with higher positive and general PANSS scores tended to have higher serum s100b levels at 24:00 h. There was a negative correlation between the 24:00 h serum s100b at discharge and the negative PANSS scores at admission.
There were no correlations between the 12:00 and 24:00 h serum s100b levels and any PANSS scores.
Correlations between s100b levels and clinical variables
The correlations between serum s100b levels and the clinical variables are presented in Table 6. Only one correlation was statistically significant. There was a negative correlation between the 24:00 h s100b concentration at discharge and the CED, that is, patients treated with higher CED tended to present lower 24:00 h serum s100b levels at discharge.
Table 6. Correlations between serum S100B levels and clinical variables

AIO: Age of Illness Onset in years; ID: Illness Duration in years; NPH: Number of Previous Hospitalisations; CED: Chlorpromazine Equivalent Dose in mg; LH: Length of Hospitalisation in days; 3MAHD: 3 Months After Discharge.
Discussion
Three time points s100b measurements
To our knowledge, this is the first time that it is reported that serum s100b levels at 12:00 and 24:00 h in acutely relapsed paranoid schizophrenia patients show a normalisation of s100b levels after 3 months of hospital discharge (3MAHD). Regarding the s100b serum concentrations, at admission, s100b levels were significantly higher at 12:00 h in patients than those observed in healthy controls. At discharge, 12:00 h s100b levels in patients had decreased with respect to admission levels, but they were still higher at 12:00 h than the s100b levels of the healthy subjects. Serum s100b levels at admission and discharge were higher at 24:00 h in patients than the 24:00 h serum s100b levels of the healthy group, but the difference did not show a statistical significance. Serum s100b levels at 24:00 h also decreased between admission and discharge. Three months after hospital discharge, s100b levels in patients had decreased with respect to the discharge time point, but the patients’ 3MAHD s100b levels were similar to the s100b levels of the healthy subjects at 12:00 and 24:00 h.
As far as we know, there is only one paper with a similar design to our research that studied patients at three time points (Rothermundt et al., Reference Rothermundt, Ponath, Glaser, Hetzel and Arolt2004). Patients were studied at intake (T0), 12 (T12) and 24 (T24) weeks after intake. The authors reported higher levels of serum s100b in patients than in healthy controls at T0, T12 and T24, but there were no differences in s100b levels in patients at T0, T12 and T24. Methodological differences could explain the opposite results.
First, the paper of Rothermundt et al. (Reference Rothermundt, Ponath, Glaser, Hetzel and Arolt2004) was a ‘multicentre study’ (31 psychiatric centres throughout Germany and Austria), while our study is a monocentre study. Second, our sample was comprised at T0 (admission) and T1 (discharge) by inpatients with an acute clinical relapse, while at T3 (3MAHD), the same patients were living in the community as outpatients. In the study of Rothermundt et al. (Reference Rothermundt, Ponath, Glaser, Hetzel and Arolt2004), the whole sample was comprised by outpatients, so patients could have milder psychopathology. Third, the patients’ sample of Rothermundt et al. research (Reference Rothermundt, Ponath, Glaser, Hetzel and Arolt2004) comprises of paranoid, undifferentiated, residual and unspecified schizophrenia patients, while our patients’ sample was only composed of paranoid schizophrenia patients. Fourth, in our study, the antipsychotic treatment was transformed into CED since the beginning of the research, while in the study of Rothermundt et al. (Reference Rothermundt, Ponath, Glaser, Hetzel and Arolt2004), former medication was discontinued 1 week prior to the initial investigation time point (T0) and then patients were assigned to treatment with risperidone or flupenthixol. And fifth, in the research of Rothermundt et al. (Reference Rothermundt, Ponath, Glaser, Hetzel and Arolt2004), patients were assigned to 2 to 6 mg of risperidone or to 4 to 12 mg of flupentixol. Considering that all patients were assigned to the maximum dose of risperidone (6 mg) or flupenthixol (12 mg), the CED would be 300 mg of chlorpromazine for the risperidone group and 600 mg of chlorpromazine for the flupenthixol group. In our research, the mean CED was 811.6 mg, so a higher dose of antipsychotics was administered.
Two time points s100b measurements
Eight papers were carried out with a design that included two time point measurements and a control group (Gerasimou et al., Reference Gerasimou, Tsoporis, Siafakas, Hatziagelaki, Kallergi, Chatziioannou, Parker, Parissis, Salpeas, Papageorgiou and Rizos2018; Hendouei et al., Reference Hendouei, Hosseini, Panahi, Khazaeipour, Barari, Sahebnasagh and Ala2016; Ling et al., Reference Ling, Tang, Jiang, Wiste, Guo, Weng and Yang2007; Morera-Fumero et al., Reference Morera-Fumero, Díaz-Mesa, Abreu-Gonzalez, Fernandez-Lopez and Cejas-Mendez2017b; Rothermundt et al., Reference Rothermundt, Missler, Arolt, Peters, Leadbeater, Wiesmann, Rudolf, Wandinger and Kirchner2001; Sarandol et al., Reference Sarandol, Kirli, Akkaya, Altin, Demirci and Sarandol2007; Schroeter et al., Reference Schroeter, Abdul-Khaliq, Krebs, Diefenbacher and Blasig2009; Steiner et al., Reference Steiner, Walter, Wunderlich, Bernstein, Panteli, Brauner, Jacobs, Gos, Rothermundt and Bogerts2009) almost all studies lasting 6 weeks except one study that lasted 12 weeks (Ling et al., Reference Ling, Tang, Jiang, Wiste, Guo, Weng and Yang2007). The results are controversial with five possible evolutions.
First, a decrease of s100b concentrations was observed between T0 and T1 was observed, although at T1 s100b concentrations remained higher than the s100b concentrations in the control group (Gerasimou et al., Reference Gerasimou, Tsoporis, Siafakas, Hatziagelaki, Kallergi, Chatziioannou, Parker, Parissis, Salpeas, Papageorgiou and Rizos2018; Ling et al., Reference Ling, Tang, Jiang, Wiste, Guo, Weng and Yang2007; Morera-Fumero et al., Reference Morera-Fumero, Díaz-Mesa, Abreu-Gonzalez, Fernandez-Lopez and Cejas-Mendez2017b; Sarandol et al., Reference Sarandol, Kirli, Akkaya, Altin, Demirci and Sarandol2007).
Second, no change was observed between T0 and T1 with both measures, but they were higher than those detected in the control group (Ling et al., Reference Ling, Tang, Jiang, Wiste, Guo, Weng and Yang2007).
Third, no change was found between T0 and T1, both measures were similar to those of the control group (Ling et al., Reference Ling, Tang, Jiang, Wiste, Guo, Weng and Yang2007; Sarandol et al., Reference Sarandol, Kirli, Akkaya, Altin, Demirci and Sarandol2007; Hendouei et al., Reference Hendouei, Hosseini, Panahi, Khazaeipour, Barari, Sahebnasagh and Ala2016).
Fourth, an increase of s100b between T0 and T1 was observed with both measures, showing higher values than those of the control group (Schroeter et al., Reference Schroeter, Abdul-Khaliq, Krebs, Diefenbacher and Blasig2009).
Fifth, a decrease of s100b between T0 and T1 was recorded but at T1 patients s100b concentrations were similar to the s100b concentrations of the control group (Rothermundt et al., Reference Rothermundt, Missler, Arolt, Peters, Leadbeater, Wiesmann, Rudolf, Wandinger and Kirchner2001; Steiner et al., Reference Steiner, Walter, Wunderlich, Bernstein, Panteli, Brauner, Jacobs, Gos, Rothermundt and Bogerts2009).
In our opinion, the differences in the results may be explained by several factors.
First, some patients did a treatment washout before entering the study (Ling et al., Reference Ling, Tang, Jiang, Wiste, Guo, Weng and Yang2007; Sarandol et al., Reference Sarandol, Kirli, Akkaya, Altin, Demirci and Sarandol2007), while in other studies patients were drug-naïve (Gerasimou et al., Reference Gerasimou, Tsoporis, Siafakas, Hatziagelaki, Kallergi, Chatziioannou, Parker, Parissis, Salpeas, Papageorgiou and Rizos2018; Sarandol et al., Reference Sarandol, Kirli, Akkaya, Altin, Demirci and Sarandol2007) or acutely relapsed inpatients (Schroeter et al., Reference Schroeter, Abdul-Khaliq, Krebs, Diefenbacher and Blasig2009; Hendouei et al., Reference Hendouei, Hosseini, Panahi, Khazaeipour, Barari, Sahebnasagh and Ala2016; Morera-Fumero et al., Reference Morera-Fumero, Díaz-Mesa, Abreu-Gonzalez, Fernandez-Lopez and Cejas-Mendez2017a).
Second, the clinical diagnosis sometimes included different types of schizophrenia or no clinical type was mentioned. A combination of undifferentiated, paranoid and residual schizophrenia (Hendouei et al., Reference Hendouei, Hosseini, Panahi, Khazaeipour, Barari, Sahebnasagh and Ala2016), first-episode psychosis (Gerasimou et al., Reference Gerasimou, Tsoporis, Siafakas, Hatziagelaki, Kallergi, Chatziioannou, Parker, Parissis, Salpeas, Papageorgiou and Rizos2018), paranoid, hebephrenic, catatonic and undifferentiated schizophrenia (Schroeter et al., Reference Schroeter, Abdul-Khaliq, Krebs, Diefenbacher and Blasig2009), schizophrenics (Ling et al., Reference Ling, Tang, Jiang, Wiste, Guo, Weng and Yang2007; Sarandol et al., Reference Sarandol, Kirli, Akkaya, Altin, Demirci and Sarandol2007) or paranoid schizophrenia (Rothermundt et al., Reference Rothermundt, Missler, Arolt, Peters, Leadbeater, Wiesmann, Rudolf, Wandinger and Kirchner2001; Steiner et al., Reference Steiner, Walter, Wunderlich, Bernstein, Panteli, Brauner, Jacobs, Gos, Rothermundt and Bogerts2009; Morera-Fumero et al., Reference Morera-Fumero, Díaz-Mesa, Abreu-Gonzalez, Fernandez-Lopez and Cejas-Mendez2017b).
Third, in the clinical type of the sample, most studies were carried out with acutely admitted inpatients (Gerasimou et al., Reference Gerasimou, Tsoporis, Siafakas, Hatziagelaki, Kallergi, Chatziioannou, Parker, Parissis, Salpeas, Papageorgiou and Rizos2018; Hendouei et al., Reference Hendouei, Hosseini, Panahi, Khazaeipour, Barari, Sahebnasagh and Ala2016; Morera-Fumero et al., Reference Morera-Fumero, Díaz-Mesa, Abreu-Gonzalez, Fernandez-Lopez and Cejas-Mendez2017b; Rothermundt et al., Reference Rothermundt, Missler, Arolt, Peters, Leadbeater, Wiesmann, Rudolf, Wandinger and Kirchner2001; Schroeter et al., Reference Schroeter, Abdul-Khaliq, Krebs, Diefenbacher and Blasig2009), but one study (Ling et al., Reference Ling, Tang, Jiang, Wiste, Guo, Weng and Yang2007) was accomplished with chronic inpatients, while in another study (Sarandol et al., Reference Sarandol, Kirli, Akkaya, Altin, Demirci and Sarandol2007) the sample comprises of a combination of outpatients and inpatients.
We do not exclude other sources that may explain the variety of the results, such as treatment duration, type and dose of antipsychotics or seasonality of serum s100b concentrations.
PANNS scores and s100b concentrations
In general, in our study, the significant correlations occurred between the negative psychopathology and the s100b concentrations, with a negative sign. The only positive correlations were observed between the 24:00 h level of s100b at discharge and the positive and general PANSS scores. By using the PANSS, some studies reported an absence of any correlation between s100b and total, general, positive and negative scores (Deng et al., Reference Deng, Kahlon, Mohite, Amin, Zunta-Soares, Colpo, Stertz, Fries, Walss-Bass, Soares and Okusaga2018; Hendouei et al., Reference Hendouei, Hosseini, Panahi, Khazaeipour, Barari, Sahebnasagh and Ala2016; Kozłowska et al., Reference Kozłowska, Brzezińska-Błaszczyk, Agier, Wysokiński and Żelechowska2021; Qi et al., Reference Qi, Xiu, Chen, Wang, Kosten, Kosten and Zhang2009; Steiner et al., Reference Steiner, Walter, Wunderlich, Bernstein, Panteli, Brauner, Jacobs, Gos, Rothermundt and Bogerts2009), while an absence of correlation between the PANSS total, positive and negative scores and s100b levels has been reported without mentioning the general subscale score (Lara et al., Reference Lara, Gama, Belmonte-de-Abreu, Portela, Gonçalves, Fonseca, Hauck and Souza2001).
The measurement of clinical symptoms with different clinical scales has also produced confusing results. By using the BPRS, an absence of correlation between the sum and the five BPRS subscores and s100b levels was reported (Schroeter et al., Reference Schroeter, Abdul-Khaliq, Krebs, Diefenbacher and Blasig2009), and a positive correlation between the total BPRS and the third BPRS item score (though disturbance) and serum concentrations of s100b (Schroeter et al., Reference Schroeter, Abdul-Khaliq, Frühauf, Höhne, Schick, Diefenbacher and Blasig2003) has been reported as well. A negative correlation between serum s100b and the scale for assessment of negative symptoms (SANS) has been reported too (Lara et al., Reference Lara, Gama, Belmonte-de-Abreu, Portela, Gonçalves, Fonseca, Hauck and Souza2001; Schmitt et al., Reference Schmitt, Bertsch, Henning, Tost, Klimke, Henn and Falkai2005). No correlation between s100b and the BPRS and the Negative Symptoms Rating Scale (NSRS) has also been reported (Gattaz et al., Reference Gattaz, Lara, Elkis, Portela, Gonçalves, Tort, Henna and Souza2000).
Clinical variables and s100b concentrations
There were no significant correlations between illness duration (ID), the number of previous hospitalisations (NPH), the length of hospitalisation (LH) and the age of illness onset (AIO) and serum s100b at admission, discharge and 3 months after hospital discharge (3MAHD) at 12:00 and 24:00 h.
Several studies have reported the absence of relationship between ID and s100b levels (Rothermundt et al., Reference Rothermundt, Missler, Arolt, Peters, Leadbeater, Wiesmann, Rudolf, Wandinger and Kirchner2001; Schroeter et al., Reference Schroeter, Abdul-Khaliq, Frühauf, Höhne, Schick, Diefenbacher and Blasig2003, Reference Schroeter, Abdul-Khaliq, Krebs, Diefenbacher and Blasig2009) as we have also found in this study. However, a recent meta-regression analysis (Schümberg et al., Reference Schümberg, Polyakova, Steiner and Schroeter2016) has reported a positive correlation between ID and s100b.
Regarding the NPH, the absence of correlation has been reported (Schroeter et al., Reference Schroeter, Abdul-Khaliq, Frühauf, Höhne, Schick, Diefenbacher and Blasig2003, Reference Schroeter, Abdul-Khaliq, Krebs, Diefenbacher and Blasig2009). Several authors have reported no significant correlation between s100b and AIO (Rothermundt et al., Reference Rothermundt, Missler, Arolt, Peters, Leadbeater, Wiesmann, Rudolf, Wandinger and Kirchner2001; Schroeter et al., Reference Schroeter, Abdul-Khaliq, Frühauf, Höhne, Schick, Diefenbacher and Blasig2003).
The chlorpromazine equivalent dose (CED) had a negative and significant correlation with the s100b concentrations at 24:00 h at discharge. Patients that were treated with higher doses of CED had lower serum s100b at discharge at 24:00 h. However, some studies have reported no significant correlations between s100b and the antipsychotic medication (Rothermundt et al., Reference Rothermundt, Missler, Arolt, Peters, Leadbeater, Wiesmann, Rudolf, Wandinger and Kirchner2001; Schroeter et al., Reference Schroeter, Abdul-Khaliq, Krebs, Diefenbacher and Blasig2009) or CED after 6 weeks of treatment (Schroeter et al., Reference Schroeter, Abdul-Khaliq, Krebs, Diefenbacher and Blasig2009). Another study reported increased serum s100b concentrations in treated schizophrenic patients compared to untreated patients, and the s100b was similar in the control group and the unmedicated patients (Schroeter et al., Reference Schroeter, Abdul-Khaliq, Frühauf, Höhne, Schick, Diefenbacher and Blasig2003). No correlation between serum s100b and cumulative drug dosage has also been reported (Steiner et al., Reference Steiner, Walter, Wunderlich, Bernstein, Panteli, Brauner, Jacobs, Gos, Rothermundt and Bogerts2009).
Our study has several limitations. The first limitation has to do with the small number of patients. Second, some intermediate measures of s100b between discharge and 3MAHD may shed some light about the evolution of the s100b levels in this time period.
It is worth mentioning that our study has some strengths. The fact that the research was carried out in one centre, that the patients were the same subjects at the three time points and that the biological samples were analysed at the same time give consistency to our results. Second, all the s100b analyses were carried out by the same technician, who was blind with respect to the group samples (patients vs controls) as well as to the time of the day (12:00 and 24:00 h) and to the time point (intake, discharge and 3MAHD) of the blood extractions.
In conclusion, considering the limitations of our research, significant alterations of the s100b protein serum levels in patients with paranoid schizophrenia at hospital admission compared to the s100b serum levels of the healthy control group were found. When patients were discharged, approximately 3 weeks later, serum s100b levels had decreased and at 3 months after hospital discharge, the s100b levels were similar to those of the healthy control subjects, thus reaching a normalisation of the serum s100b levels. Our results point out to the fact that the acute inflammatory response produced in patients acutely relapsed is reversed after 3 months of hospital discharge. Therefore, the variations of serum s100b concentrations when the patients suffer from an acute relapse episode may be a useful predictor of disease evolution. However, our study needs to be replicated with bigger samples, and future studies should consider longer follow-up periods in order to establish the serum s100b levels as a variable with prognostic value.
Acknowledgements
We would like to thank Dr. Aram Morera-Mesa (freelance translator) for his assistance in the translation of this article.
Author contributions
ALMF and LFL designed the study. EDM and MSHB collected the data. PAG carried out the biochemical analyses. ALMF y LFL wrote the first draft. All authors have participated in the analysis and interpretation of data and revised the manuscript. All authors have approved the final version of the manuscript.
Financial support
This work was partly supported by Fundación Canaria de Investigación y Salud (FUNCIS) of the Government of the Canary Islands under a Grant (PI: 08/115).
Conflict of interest
The authors report no conflict of interest.