Significant Outcomes
-
Agreeableness, a personality trait reflective of cooperation, compassion and empathy, was expressed to a different degree in similarly formed samples of two birth cohorts, being higher in the cohort exposed at an earlier age to a societal transition.
-
Whether Agreeableness scores were higher in the younger birth cohort was dependent on NPY genotype.
-
Further, this impact of NPY genotype was amplified by the serotonin transporter gene promoter polymorphism, suggesting that an interaction of the serotonergic and NPY-ergic neurons is involved in environmentally induced transformation of social behaviour.
Limitations
-
These findings have been obtained in a specific setting and that may need to be considered in the design of replication studies.
Introduction
Neuropeptide Y (NPY) is the most abundant and widely distributed neuropeptide in the mammalian brain, with particularly high expression in limbic areas, such as the hippocampus, amygdala and hypothalamus (Morris, Reference Morris1989; Fetissov et al., Reference Fetissov, Kopp and Hökfelt2004). It regulates essential biological functions such as blood pressure, food intake, neuroendocrine secretion, neuronal excitability and neuroplasticity (Stanley & Leibowitz, Reference Stanley and Leibowitz1984; Michalkiewicz et al., Reference Michalkiewicz, Michalkiewicz, Kreulen and McDougall2001; Magni, Reference Magni2003; Hökfelt et al., Reference Hökfelt, Stanic, Sanford, Gatlin, Nilsson, Paratcha, Ledda, Fetissov, Lindfors, Herzog, Johansen, Ubink and Pfenninger2008). NPY is a most potent orexigenic and has an essential role in regulating food intake, but centrally administered NPY also effectively reduces anxiety (Heilig, Reference Heilig2004), and endogenous NPY, as revealed by studies with NPY receptor antagonists, exerts anxiolytic effects through more than one receptor subtype and in several brain regions (Kask et al., Reference Kask, Harro, von Hörsten, Redrobe, Dumont and Quirion2002). Stress affects the expression level of NPY within the amygdala and cortex (Thorsell et al., Reference Thorsell, Svensson, Wiklund, Sommer, Ekman and Heilig1998; Thorsell et al., Reference Thorsell, Carlsson, Ekman and Heilig1999; Primeaux et al., Reference Primeaux, Wilson, Cusick, York and Wilson2005), suggesting that altered levels of NPY may influence the successful adaptation (Heilig & Thorsell, Reference Heilig and Thorsell2002; Sah & Geracioti, Reference Sah and Geracioti2013; Enman et al., Reference Enman, Sabban, McGonigle and Van Bockstaele2015). The mechanisms by which NPY promotes resilience are not established yet, but NPY is known to affect emotional learning and memory processing (Flood et al., Reference Flood, Hernandez and Morley1987) and to modify fear learning (Broqua et al., Reference Broqua, Wettstein, Rocher, Gauthier-Martin and Junien1995; Tasan et al., Reference Tasan, Verma, Wood, Lach, Hörmer, de Lima, Herzog and Sperk2016).
While the anxiety-reducing effect of NPY is observable in multiple models, a notable action of NPY is to reduce anxiety in tests based on social interaction (Kask et al., Reference Kask, Rägo and Harro1998; Kask et al., Reference Kask, Eller, Oreland and Harro2000). NPY also reduces the expression of fear in a mouse model of social fear conditioning after intracerebroventricular administration (Kornhuber & Zoicas, Reference Kornhuber and Zoicas2019) as well as if administered into the dorsolateral septum and central amygdala (Kornhuber & Zoicas, Reference Kornhuber and Zoicas2021). The role of NPY in social behaviour has been established even in Drosophila, as neuropeptide F (dNPF), the homologue of mammalian NPY, coordinates cooperation during development (Wu et al., Reference Wu, Wen, Lee, Park, Cai and Shen2003). Recently, Shiozaki et al. (Reference Shiozaki, Kawabe, Karasuyama, Kurachi, Hayashi, Ataka, Iwai, Takeno, Hayasaka, Kotani, Komatsu and Inui2020) demonstrated that NPY knockout zebrafish exhibit several anxiety-like behaviours, those including decreased social interaction.
In humans, cerebrospinal fluid NPY-like immunoreactivity has been shown to correlate with measures of impulsive aggression (Coccaro et al., Reference Coccaro, Lee, Liu and Mathé2012), and low CSF NPY-like immunoreactivity predicts future suicide attempts in patients with bipolar disorder (Sandberg et al., Reference Sandberg, Jakobsson, Pålsson, Landén and Mathé2014), suggesting that NPY signalling is altered in states of emotional dysregulation. As to whether NPY could be involved in persistent traits of personality and coping behaviour, very little information is available. No associations between NPY and personality traits have been reported. This should be considered surprising, given that several functional variants of the gene are known, that NPY plays a well-established role in the regulation of affect, and that personality measures from a large variety of samples are available to many investigators. What has come closest is the reporting (Melas et al., Reference Melas, Guban, Rahman, Lavebratt and Forsell2018) of slightly higher levels of conscientiousness, a trait referring to one’s inclination toward self-discipline, dutifulness, competence, thoughtfulness, and achievement-striving, and positively related to social support (Barańczuk, Reference Barańczuk2019), in NPY rs16147 T/T homozygotes, but even this association was not truly statistically significant. The NPY rs16147 T-allele promotes higher NPY expression levels both in lymphoblastoid cells and post-mortem brain (Zhou et al., Reference Zhou, Zhu, Hariri, Enoch, Scott, Sinha, Virkkunen, Mash, Lipsky, Hu, Hodgkinson, Xu, Buzas, Yuan, Shen, Ferrell, Manuck, Brown, Hauger, Stohler and Goldman2008). The NPY rs16147 T-allele has been considered a sign of autonomic flexibility to generate adequate responses to environmental stress by modifying heart rate, respiration and arousal (Chang et al., Reference Chang, Fang, Chang, Huang and Chang2016), thus increasing resilience and capability to cope with stress, and conferring resilience to intrusion symptoms of post-traumatic stress disorder (PTSD) in the context of cumulative traumatic stress (Watkins et al., Reference Watkins, Han, Krystal, Southwick, Gelernter and Pietrzak2017). Indeed, several studies have shown that depression, anxiety traits and anxiety disorders are associated with increased amygdala responsiveness to harmful stimuli (Etkin & Wager, Reference Etkin and Wager2007), that in turn is lower in NPY rs16147 T/T genotype (Domschke et al., Reference Domschke, Dannlowski, Hohoff, Ohrmann, Bauer, Kugel, Zwanzger, Heindel, Deckert, Arolt, Suslow and Baune2010). Furthermore, the rs16147 locus is in strong linkage disequilibrium with rs5574 (Yeung et al., Reference Yeung, Zhang, Chen, Bowers, Hu, Kang and Qi2011; Zain et al., Reference Zain, Mohamed, Jalaludin, Fauzi, Hamidi and Zaharan2015), and symptom scores in current PTSD patients were found associated with rs5574 (Ferić Bojić et al., Reference Ferić Bojić, Kučukalić, Džubur Kulenović, Avdibegović, Babić, Agani, Jakovljević, Kučukalić, Bravo Mehmedbašić, Šabić Džananović, Kravic, Babić, Pavlović, Aukst Margetic, Jaksic, Cima Franc, Rudan, Haxhibeqiri S.Goci Uka and Marjanović2019).
Risk-associated NPY rs16147 (NIH National Library of Medicine, 2022) and rs5574 (NIH National Library of Medicine, 2022) variants have a high prevalence. High prevalence of gene variants that are associated with psychiatric vulnerability suggests that gene–environment interactions shape the eventual significance of these variants, the “risk genes” thus rather acting as “plasticity genes” (Belsky et al., Reference Belsky, Jonassaint, Pluess, Stanton, Brummett and Williams2009). Almost by definition, if subjects are not stratified by environmental measures, then any association of variants of plasticity genes can be hidden. The hard problem here is, however, that there is no standard for selecting the critically important environmental variables to be included in analysis of gene–environment interaction. A large variety of environmental variables are potentially relevant. The contribution of a single environmental factor may, furthermore, be dependent on other environmental factors. One more global approach treats birth cohorts as proxy for total environment. Each birth cohort is, in global terms, exposed to a more similar environment than subjects of another birth cohort, owing to the dynamics of socio-economic background, societal values, and parental styles, and this may have impact on health, especially through changes in lifestyle (Holford, Reference Holford1991). Making a distinction between birth cohorts can be particularly relevant for social aspects of environment and health-related behaviour (Keyes et al., Reference Keyes, Li and Hasin2011; Phillips, Reference Phillips2014; Wedow et al., Reference Wedow, Zacher, Huibregtse, Mullan Harris, Domingue and Boardman2018; Virtanen et al., Reference Virtanen, Kaprio, Viken, Rose and Latvala2019). Birth cohort effects have long been noticed for, for example, the age of onset of major depression, alcohol use disorder and obsessive-compulsive disorder (but not several other psychiatric disorders) (Burke et al., Reference Burke, Burke, Rae and Regier1991). Importantly, social contacts were found to have a role changing by birth cohort in the development of depression (Sjöberg et al., Reference Sjöberg, Östling, Falk, Sundh, Waern and Skoog2013). Nowadays, younger birth cohorts are increasingly more influenced by the rise of electronic media, the internet and social media, accompanied by a remarkable change in social communication. Importantly, birth cohort can moderate the relative contribution of genetic and environmental factors on behaviour. Thus, in a twin study, Virtanen et al. (Reference Virtanen, Kaprio, Viken, Rose and Latvala2019) have reported that heritability of alcohol consumption greatly varied in women by birth cohort (while it did not in men), and that alcohol abstinence was largely explained by shared environment in one birth cohort but by non-shared environment and additive genetic impact in another. Associations of specific common gene variants with behaviour have recently also been described as subject to birth cohort effects if the behaviour in question is socially motivated. For example, the serotonin transporter gene promoter genotype strongly interacted with gender and birth cohort in association with the debute of alcohol consumption so that the female 5-HTTLPR S/S homozygotes of the younger birth cohort of the Estonian Children Personality Behaviour and Health Study (ECPBHS) started alcohol use on average 3 years younger that their counterparts in the older birth cohort (Vaht et al., Reference Vaht, Merenäkk, Mäestu, Veidebaum and Harro2014). Genotype–birth cohort interactions for the debute of alcohol consumption or frequent alcohol use in early age were also found with other functional gene variants such as VMAT1 (rs1390938), NRG1 (rs6994992) and OXTR (rs53576) (see Harro & Vaht, Reference Harro, Vaht and Preedy2019 for review). Birth cohort can modify even the associations between genotype and somatic measures such as body mass index (Rosenquist et al., Reference Rosenquist, Lehrer, O'Malley, Zaslavsky, Smoller and Christakis2015). Given that NPY is related to anxiety regulation and social behaviour, we hypothesised that functional variants of NPY may interact with the birth cohort in shaping sociability-related traits.
We have previously found that the two variants of the NPY gene, rs16147 and rs5574, are associated with obesity, dietary intake, glucose, lipid metabolism and blood pressure from adolescence to young adulthood in the ECPBHS sample (Katus et al., Reference Katus, Villa, Ringmets, Veidebaum and Harro2021), and this suggestion of functionality of these genotypes making the sample appealing for further analysis of possible effects on anxiety-related measures and for possible birth cohort interaction. Thus, we examined the association of NPY rs16147 and rs5574 with personality traits in this representative sample of young adults 25 years of age separately in the two birth cohorts of ECPBHS. After the primary analyses revealed a genotype by birth cohort effect, we further considered a possible relationship with the serotonin transporter-linked polymorphic region (5-HTTLPR), given its role in the development of the 5-HT system, anxiety and putatively in neural plasticity involved in cohort effects (Lesch et al., Reference Lesch, Bengel, Heils, Sabol, Greenberg, Petri, Benjamin, Müller, Hamre and Murphy1996; Belsky et al., Reference Belsky, Jonassaint, Pluess, Stanton, Brummett and Williams2009; Vaht et al., Reference Vaht, Merenäkk, Mäestu, Veidebaum and Harro2014; Delli Colli et al., Reference Delli Colli, Borgi, Poggini, Chiarotti, Cirulli, Penninx, Benedetti, Vai and Branchi2022).
Materials and methods
Study sample
The study sample was formed for the European Youth Heart Study (EYHS; 1998/1999) and later incorporated into the ECPBHS. It is an ethnically homogeneous sample of Caucasian subjects, and the rationale and procedure of sample formation have previously been described (Harro et al., Reference Harro, Eensoo, Kiive, Merenäkk, Alep, Oreland and Harro2001). In brief, all schools of Tartu County, Estonia, that agreed to participate (54 of the total of 56) were included in the sampling using the probability proportional to the number of students of the respective age groups in the school and 25 schools were selected. In 1998/99, all children from grades 3 and 9 were invited to participate, and written informed consent was received from 79% of the requested subjects and their parents (original n = 1238). Before participation, written informed consent was obtained from the participants. The study was approved by the Ethics Review Committee on Human Research of the University of Tartu and conducted in accordance with the Declaration of Helsinki.
Personality measures
Personality traits of the five-factor model (Costa & McCrae, Reference Costa and McCrae1992) were measured by self-report at the age of 25 years (n = 856) with EE.PIP-NEO (Mõttus et al., Reference Mõttus, Pullmann and Allik2006), a semantically simplified 240-item version of the International Personality Item Pool (IPIP), which emulates the NEO-PI-R. In the five-factor model, traits are classified into five broad dimensions: Neuroticism, Extraversion, Openness, Agreeableness and Conscientiousness, each trait in the model comprising six facets (Costa & McCrae, Reference Costa and McCrae1992).
The Affective Neuroscience Personality Scale (ANPS)
Data on Affective Neuroscience Personality Scale (ANPS) (n = 925) were collected at the age of 25 or 33 years. We used the adaptation (Harro et al., Reference Harro, Laas, Eensoo, Kurrikoff, Sakala, Vaht, Parik, Mäestu and Veidebaum2019) of the short version of the ANPS (Davis et al., Reference Davis, Panksepp and Normansell2003), that is, a self-report instrument constructed bottom-up to correspond to the activity in neural circuits underlying basic emotive systems as defined in animal research (Panksepp, Reference Panksepp1998; Davis & Panksepp, Reference Davis and Panksepp2011). It comprises traits termed ANGER, FEAR, SADNESS, SEEKING, CARE and PLAY which correspond to six primary emotional systems that represent a tool for survival, endowing mammalian species with inherited behavioural programmes to react appropriately to complex environments (Panksepp, Reference Panksepp1998). ANPS has been translated to and validated in 10 different languages, and comparisons of the ANPS with five-factor personality assessments have uniformly shown close associations of these six emotions to the five-factor personality measures (for an overview, see Montag & Davis, Reference Montag and Davis2018). A meta-analytical analysis of 21 samples where both ANPS and five-factor measures have been administered provides evidence that high SEEKING relates to high Openness, high PLAY to high Extraversion, high CARE/low ANGER to high Agreeableness and high FEAR/SADNESS/ANGER to high Neuroticism (Marengo et al., Reference Marengo, Davis, Gradwohl and Montag2021).
Genotyping of NPY rs16147, NPY rs5574 and 5-HTTLPR
Genotyping of NPY rs16147 and rs5574 was performed as previously described (Katus et al., Reference Katus, Villa, Ringmets, Veidebaum and Harro2021). Genomic DNA was extracted from venous blood samples using Qiagen QIAamp® DNA Blood Midi Kit. The real-time polymerase chain reaction (RT-PCR) for genotyping the NPY rs5574 and rs16147 polymorphisms was performed using a TaqMan Pre- Designed SNP Genotyping Assay (Applied Biosystems; Foster City, CA, USA) containing primers and fluorescent probes. Context sequence [VIC/FAM] for rs5574 was as follows: TTTTTTCCAGATATGGAAAACGATC[C/T]AGCCCAGAGACACTGATTTCAGACC, and for rs16147: GCTTCCTACTCCGGCACCCAGTGGG[C/T]TGGTAGTCCTGTT GGCAGGAGACAA. Genotyping reactions were performed in a total volume of 10 μl with ∼25 ng of template DNA. RT-PCR reaction components and final concentrations were as follows: 1:5 5 × HOT FIREPol® Probe qPCR Mix Plus (ROX) (Solis BioDyne) and 1:20 80 × TaqMan Primers Probe. Reactions were performed on the Applied Biosystems ViiA™ 7 Real-Time PCR System. The amplification procedure consisted of an initial denaturation step at 95°C for 12 min and 40 cycles of 95°C for 15 s and 60°C for 1 min. Positive and negative controls were added to each reaction plate. No inconsistencies occurred. Genotyping was performed blind to all phenotypic data. Allele frequencies agreed with National Center for Biotechnology Information database and published reports.
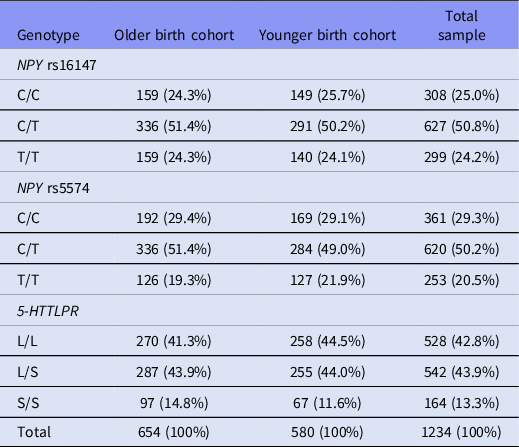
Genotyping for 5-HTTLPR biallelic classification was performed as described in Tomson et al., Reference Tomson, Merenäkk, Loit, Mäestu and Harro2011. The alleles at the 5-HTTLPR locus were amplified from genomic DNA using PCR as in previous studies (Anchordoquy et al., Reference Anchordoquy, McGeary, Liu, Krauter and Smolen2003). The polymorphic region was amplified using the primers 5-HTTLPR-F: 5′-6FAM-ATG CCA GCA CCT AAC CCC TAA TGT-3′ and 5-HTTLPR-R: 5′-GGA CCG CAA GGT GGG CGG GA-3′. PCR reaction components and final concentration were as follows: 1× of 5× HotFirepol BLEND with BSA 2.5 mM MgCl2 (Solis Biodyne); 5% of DMSO; 1 × of 10× Solution S (Solis Biodyne); 380 μM each of the forward and reverse primers; and 10–50 ng of template DNA. The amplification was conducted in a total volume of 20 μl. The touchdown PCR cycles were used as by Anchordoquy et al. (Reference Anchordoquy, McGeary, Liu, Krauter and Smolen2003). The electrophoresis was made on ABI PRISM 3130XL genetic analyser, and the components used were 1 μl PCR product, 10 μl Hi-Di formamide and 0.25 μl Liz 500 size standard. Genotypes were generated using ABI Gene-Mapper V 4.0 software. Genotype frequencies were in Hardy–Weinberg equilibrium and are shown in Table 1.
Table 1. NPY rs16147, NPY rs5574 and 5-HTTLPR genotype frequencies n (% within cohort) in the ECPBHS sample
Statistical methods
Statistical analysis was conducted using the SPSS software v27 (IBM Corp, Armonk, NY). General linear model univariate analysis of variance was used when analysing the effect of NPY rs16147, NPY rs5574, 5-HTTLPR and birth cohort on personality measures. The interaction effect of NPY genotype and birth cohort on Agreeableness was examined by using a univariate full factorial interaction model with birth cohort and NPY genotype as factors. The models were performed separately for 5-HTTLPR S/S genotype and L-allele carriers. ANGER and CARE were included in the models as covariates to measure their possible confounding effect. Pearson’s correlation was used to measure the strength of associations between ANGER, CARE, Agreeableness, and its facets. All analyses were performed in the whole sample with males and females combined. In the statistical analysis, the conventional 5% level was used to assess the significance. No specific correction for multiple testing was used as many measures are inter-correlated, and the research was meant to be exploratory.
Results
We found no significant independent effect of the NPY rs16147 and rs5574 genotypes on the five-factor personality domains (Neuroticism, Extraversion, Openness, Agreeableness and Conscientiousness) or dimensions measured by the Affective Neuroscience Personality Scale (ANGER, FEAR, SADNESS, SEEKING, CARE and PLAY) in the total sample of ECPBHS (Supplementary Table 1). Similarly, no effect of 5-HTTLPR on five-factor personality domains or ANPS dimensions were found (Supplementary Table 2).
Given the focus on social anxiety in this study, data on Agreeableness were analysed in more detail. Females had higher Agreeableness scores than males in both younger and older birth cohorts: F (1,361) = 33.13 and F (1,466) = 45.43, respectively, p = 0.001. The score of Agreeableness was higher [F(1,828) = 14.05, p < 0.001] in the younger birth cohort compared to the older birth cohort (Table 1). The interaction analysis of the birth cohort and the NPY rs16147 or rs5574 genotype revealed significant interaction effects on Agreeableness: F (2, 823) = 5.27 and (F(2, 823) = 4.45, respectively, p = 0.005. Participants with C/T and T/T genotypes of NPY rs16147 belonging to the younger birth cohort scored significantly higher in Agreeableness (Fig. 1A) compared to those with the same genotype in the older birth cohort. In the case of NPY rs5574, participants with C/C genotype belonging to the younger birth cohort scored significantly higher in Agreeableness than those of the older cohort with the same genotype (Fig. 1B). Contrasting 298 individuals with double polymorphism of NPY rs16147 T/T and rs5574 C/C to the rest of the participants did not reveal a significant difference in Agreeableness in the total sample. However, as expected, a significant genotype × birth cohort interaction effect [F(1,829) = 9.07, p = 0.003] on Agreebleness was found, indicating that double polymorphism of rs16147 T/T and rs5574 C/C contributes to similar large difference in Agreeableness between the birth cohorts.
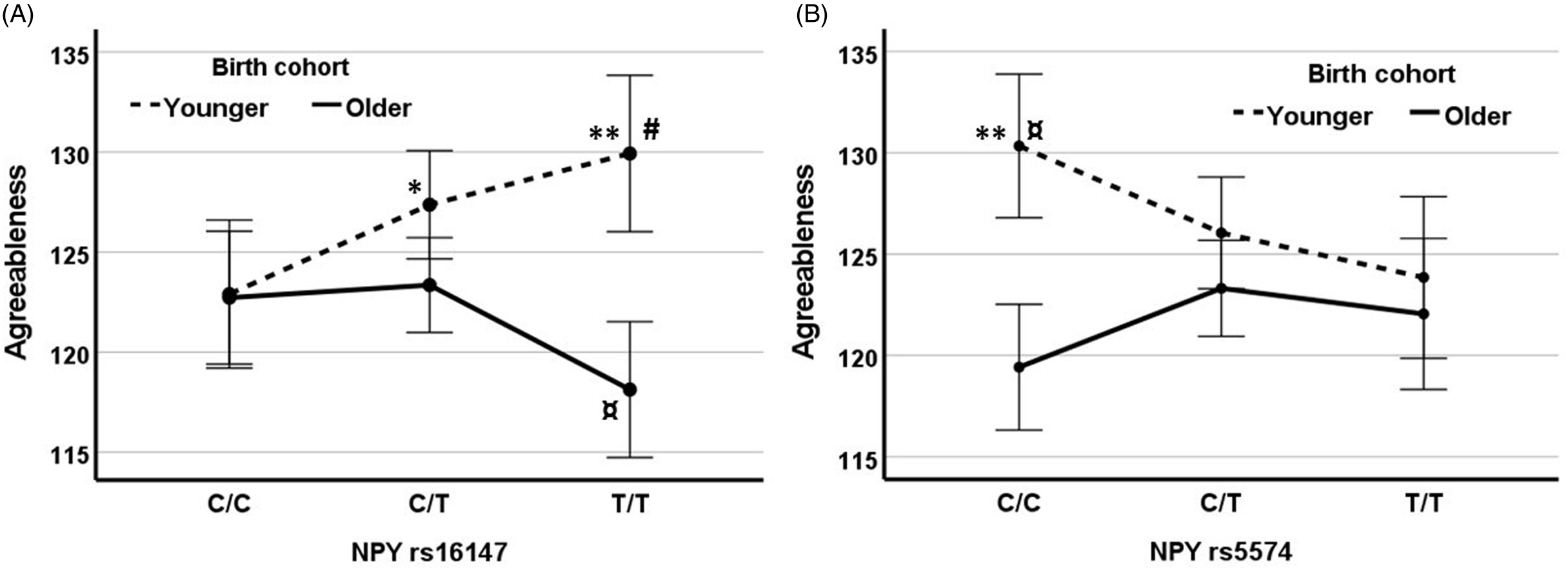
Fig. 1. The NEO-PI Agreeableness scores in participants of the younger and older ECPBHS birth cohorts by NPY rs16147 (A) and NPY rs5574 (B) genotypes (mean±SE). *p < 0.05 and **p < 0.001 different from the participants of the older cohort with the same genotype; #p < 0.01 different from the participants from the older cohort with the C/C genotype; ¤p < 0.01 different from the participants with other genotype groups of the same birth cohort.
Agreeableness comprises six facets: Trust, Straightforwardness, Altruism, Compliance, Modesty and Tender-Mindedness (Costa and McCrae, Reference Costa and McCrae1992). Having found this genotype × birth cohort interaction effect on Agreeableness, we looked further into the facets of Agreeableness and observed that the interaction effects of NPY rs16147 and rs5574 genotypes and birth cohort were present for Straightforwardness: F (2, 848) = 3.52, p = 0.03 and F (2, 848) = 4.07, p = 0.02, Altruism: F (2, 846) = 5.03, p = 0.007; F (2, 846) = 3.79, p = 0.02 and Tender-Mindedness: F (1, 842) =3.92, p = 0.02; F (1, 842) = 4.11, p = 0.02, respectively. In contrast, no statistically significant interaction effect of NPY genotypes and birth cohort were found on Trust, Compliance or Modesty.
The Five-Factor Model Agreeableness domain is most closely related to the ANPS CARE system, which is associated with high levels of Agreeableness, and on the other hand with the ANGER system, related to low Agreeableness levels. Because the genotype × birth cohort interaction was resting on distinct subset of facets of Agreeableness, we examined the correlation of each facet with these two personality domains of ANPS, ANGER and CARE. ANGER and CARE were not significantly associated with each other (r = −0.01; p = 0.70). Inter-correlations between Agreeableness, its subscores, ANGER and CARE are presented in Table 2. In brief, positive correlations between Agreeableness facets and CARE were by far the strongest with those that had the interaction effect between the genotype and birth cohort, that is, Straightforwardness, Altruism and Tender-Mindedness.
Table 2. Pearson’s correlations between Agreeableness, its subscales (EE.PIP-NEO), ANGER and CARE (ANPS)
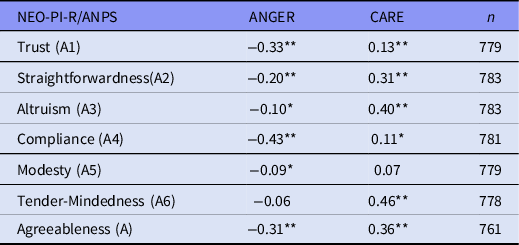
*p < 0.01; **p < 0.001.
NPY rs16147 and NPY rs5574 genotypes had no independent effect on ANGER or CARE in the total sample of ECPBHS. However, when cohorts were studied separately, it was found that in the older birth cohort, NPY rs16147 C/C homozygotes scored higher on ANGER than NPY rs16147 T-allele carriers (F(1,502) = 4.02; p < 0.05). When ANPS ANGER and CARE were added to the models as covariates, the interaction effect of the cohort and NPY rs16147 was still significant on Agreeableness F (7,760) = 4.62; p = 0.01 as well as the facets Altruism F (7,780) = 5.22; p = 0.006 and Tender-Mindedness F (7,777) = 3.07; p = 0.047, after controlling for the covariates. In the case of NPY rs5574 and birth cohorts, the interactions were turned insignificant, except for Altruism: F (7,780) = 3.25; p = 0.036 with ANGER and CARE.
In an attempt to elucidate the mechanisms behind these birth cohort effects, we performed an interaction analysis of NPY rs16147 genotype and 5-HTTLPR genotype on Agreeableness. No 5-HTTLPR main effect on Agreeableness was found in either younger or older cohort: F (2,361) = 0.49 and F (2,466) = 0.55), p = 0.61 and 0.57, respectively. In the total sample, a significant interaction effect of NPY rs16147 and 5-HTTLPR genotype on Agreeableness was found, F (3,828) = 5.11, p = 0.02, indicating that participants with NPY rs16147 T/T and 5-HTTLPR S/S genotype scored highest on Agreeableness (Table 3). More specifically, in the younger birth cohort, the NPY rs16147 T/T plus 5-HTTLPR S/S group had by far the highest agreeableness and the NPY rs16147 T/T plus 5-HTTLPR L-allele carrier group still had somewhat higher average Agreeableness than the NPY rs16147 C-allele carriers. In contrast, in the older birth cohort, the main contrast with other groups was with the NPY rs16147 T/T plus 5-HTTLPR L-allele carriers that had lower Agreeableness than others. Taken together, the 5-HTTLPR S/S genotype amplified the NPY rs16147 genotype effect on Agreeableness, but this varied between the cohorts in terms of which 5-HTTLPR genotype made the difference.
Table 3. Agreeableness (Mean± SD) scores in the participants of the older and the younger birth cohort and the total sample of ECPBHS with different combinations of NPY rs16147 and 5-HTTLPR genotype
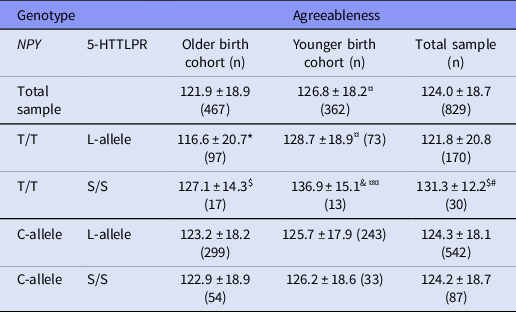
*p < 0.005 different from the participants with NPY C-allele/5-HTTLPR L-allele, same birth cohort; $ p < 0.05 different from the participants with NPY T/T genotype/5-HTTLPR L-allele, same birth cohort; #p < 0.05 different from the participants with NPY C-allele/5-HTTLPR S/S genotype, total sample; & p = 0.07 different from NPY T/T genotype/5-HTTLPR L allele, same birth cohort; ¤p < 0.001 difference between the birth cohorts; ¤¤p = 0.08 difference between the birth cohorts.
Discussion
The present study tested whether two functional variations in the NPY gene (rs16147 and rs5574) impact human personality. No simple association of the NPY genotypes with traits such as Neuroticism or FEAR was found, which is not surprising given the absence of any relevant hits in genome-wide association studies. Previously, the NPY rs16147 C-allele and rs5574 T-allele were found significantly and a similar manner associated with body composition and blood pressure in the ECPBHS sample (Katus et al., Reference Katus, Villa, Ringmets, Veidebaum and Harro2021). Given the consistent findings in animal models demonstrating the role of NPY in emotion and social anxiety, we examined the possibility that these gene variants have dissimilar by birth cohort association in traits that reflect social sensitivity.
Indeed it was found that association between the NPY gene variants and the personality domain Agreeableness is strongly influenced by the birth cohort: NPY rs16147 T-allele homozygotes and rs5574 C-allele homozygotes were very different in the older (born in 1983) and the younger (born in 1989) birth cohort. It may seem a brief period between the cohorts, but it coincides with major societal changes that have led to large behavioural differences, for example, in alcohol use, that were sensitive to functional gene variants (Vaht et al., Reference Vaht, Merenäkk, Mäestu, Veidebaum and Harro2014; Harro & Vaht, Reference Harro, Vaht and Preedy2019). In the interval of these birth cohorts, the study area was a theatre for the fastest transition amongst the Countries of Central and Eastern Europe from central planning to free market economy (Allaste & Bennett, Reference Allaste, Bennett and Allaste2013). Birth cohort effects occur, for example, in conditions of major economic shifts (Sutin et al., Reference Sutin, Terracciano, Milaneschi, An, Ferrucci and Zonderman2013). A recent study demonstrated that amongst the Big Five personality traits, only Agreeableness was a subject of cohort effects (Brandt et al., Reference Brandt, Drewelies, Willis, Schaie, Ram, Gerstorf and Wagner2022). Agreeableness is one of the most salient and influential personality constructs that emphasises cooperation, compassion and empathy (John & Srivastava, Reference John, Srivastava, Pervin and John1999). Agreeable people tend to be thoughtful, sympathetic, cooperative and often see other people this way. They are likely to avoid interpersonal conflict, experience less social stress (Asendorpf & Wilpers, Reference Asendorpf and Wilpers1998; Yu et al., Reference Yu, Zhao, Li, Zhang and Li2021) and are more prone to prosocial behaviour (Habashi et al., Reference Habashi, Graziano and Hoover2016). In general, people who score highly for Agreeableness are more likely to cope by seeking social support (O'Brien & DeLongis, Reference O'Brien and DeLongis1996; Penley & Tomaka, Reference Penley and Tomaka2002).
Regarding the facets of Agreeableness, the NPY gene variant and birth cohort interaction effects were present for Straightforwardness, Altruism and Tender-mindedness, but not for Trust, Compliance or Modesty. We found that Straightforwardness, Altruism and Tender-Mindedness are significantly correlated with CARE. According to Costa and McCrae (Reference Costa and McCrae1992), people scoring high on Straightforwardness tend to interact with others directly and honestly, and those scoring low are less direct, tend to be high in self-monitoring and are generally deceitful or manipulative. Individuals who score high on Altruism tend to be helpful, considerate and intrinsically motivated, while low scorers tend to be uninvolved and more self-interested. Tender-Mindedness is the extent to which an individual’s judgments and attitudes are determined by emotion and primarily characterised by sympathy. Individuals scoring high for Straightforwardness, Altruism and Tender-Mindedness experience a great deal of empathy and tend to get pleasure out of taking care of others, and they are considering and helpful and in active interaction with other people. Low scorers, on the other hand, are less likely to be sympathetic to the needs of people around them and tend to be less moved by emotions of others. Unlike the facets mentioned above, Trust, Compliance and Modesty are rather passive traits, less dependent on environmental changes.
The fact that NPY genotypes are not directly associated with personality traits but interact with birth cohort to predict Agreeableness and its facets suggests that these genetic associations relate to the variation in environmental contexts. It is likely that the impact of environmental effects is modulated by genetic pathways, causing some individuals or population groups to be differentially affected by composite changes in the environment leading to birth cohort effects (Rosenquist et al., Reference Rosenquist, Lehrer, O'Malley, Zaslavsky, Smoller and Christakis2015). Possibly, the NPY gene variants might have an effect either on coping styles with stress through personality-dependent choices or through modifying the interpretation of stressful events.
The domain of Agreeableness is socially oriented and relates to the way people interact with others. Because of its role in interpersonal relations, the influence of Agreeableness is essential in social adjustment. Social behaviour, in general, is defined as all behaviour that influences, or is influenced by, other members of the same species. According to Grant (Reference Grant1963), social behaviour covers reproductive activities and all behaviour that brings individuals together and all forms of aggressiveness. Therefore, social behaviour is critical to successfully interacting with other members of the species, obtaining food and mates, and avoiding predation. Human social behaviour is more complex but no less essential for health and survival and is determined by the person’s individual characteristics and environmental factors. It is generally recognised that social behaviour is not a unitary behaviour with a unitary neurological basis, but different aspects of social behaviour have different neural and endocrine underpinnings (Moyer, Reference Moyer1968).
Furthermore, in search of putative mechanisms involved in this interaction effect of NPY genotype and birth cohort on Agreeableness, it was found dependent on 5-HTTLPR variants. In this sample, we had previously revealed a qualitatively different effect of the 5-HTTLPR S/S genotype on initiation of drinking alcohol in the two birth cohorts (Vaht et al., Reference Vaht, Merenäkk, Mäestu, Veidebaum and Harro2014). Serotonin has been associated with a wide range of aspects of social behaviour regulation, and NPY plays a role in the modulation of serotonergic pathways in the central nervous system (Gehlert et al., Reference Gehlert, Thompson, Hemrick-Luecke and Shaw2008; Rezitis et al., Reference Rezitis, Herzog and Ip2022). This refers to the possibility that the different contribution of NPY genotype on Agreeableness in the two birth cohorts is modulated by central serotonergic activity.
In conclusion, the association between the NPY gene variants and a personality domain reflecting social desirability depends in times of rapid societal changes on the birth cohort, serving as an example of the relationship between the plasticity genes (Belsky et al., Reference Belsky, Jonassaint, Pluess, Stanton, Brummett and Williams2009) and environment.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/neu.2023.23.
Acknowledgements
We are grateful to all ECPBHS participants and the whole ECPBHS team.
Author contributions
Evelyn Kiive – data analysis, visualisation and writing original draft; Margus Kanarik – review and editing; Toomas Veidebaum – funding, data curation, review and editing; Jaanus Harro – funding, data curation, supervision, review and editing.
Financial support
This study was supported by the Estonian Research Council (PRG1213, IUT 42-2) and the European Commission Horizon 2020 Program Project Eat2beNICE (no 728018).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.







