Significant outcomes
-
Compared to control women, preeclamptic (PE) women exhibit significantly higher levels of depression, anxiety, and chronic fatigue syndrome (CFS), which are associated with immune-inflammatory response activation.
-
Soluble CTLA-4 (sCTLA-4), soluble CD80 (sCD80), and vitamin D are the three most significant biomarkers contributing to variations in depression, anxiety, and fatigue scores, with an inverse correlation between the sCTLA-4/sCD80 ratio and these neuropsychiatric symptoms.
-
Imbalances in soluble checkpoint molecules contribute to both hypertension and neuropsychiatric symptoms in PE, with sCTLA-4, membrane CTLA-4, sCD80, and membrane CD80 emerging as novel drug targets for treating PE-related conditions, including hypertension, depression, anxiety, and CFS.
Limitations
-
The study could have been strengthened by evaluating T effector and T regulatory cells through flow cytometry, as well as assessing membrane-bound CTLA-4, CD28, CD80, and CD86 expression on T cells.
-
Further analyses on oxidative and nitrosative stress markers would be beneficial to provide a more comprehensive understanding of their role in PE pathology.
-
Although the sample size may appear relatively small, it was determined through power analysis, achieving a power of 1.0 for primary outcome variables based on multiple regression analyses of biomarkers.
Highlights
-
Preeclamptic (PE) is accompanied by mood and chronic fatigue syndrome (CFS) symptoms
-
A lowered soluble CTLA-4 (sCTLA-4) / soluble CD80 (sCD80) ratio and metabolic disorders predict mood and CFS symptoms
-
Imbalances in soluble checkpoint molecules and metabolic pathways contribute to neuropsychiatric symptoms due to PE
Introduction
Preeclampsia (PE) is a common and potentially fatal condition that manifests during pregnancy. It is distinguished by the abrupt onset of hypertension, cephalalgia, and visual impairments (Brown et al., Reference Brown, Magee, Kenny, Karumanchi, McCarthy, Saito, Hall, Warren, Adoyi and Ishaku2018, Amon and Dickert, Reference Amon and Dickert2021, Narkhede and Karnad, Reference Narkhede and Karnad2021). Fifteen percent of annual maternal fatalities in developing countries are attributed to PE, according to estimates (Helmo et al., Reference Helmo, Lopes, Carneiro, Campos, Silva, dos Reis Monteiro, Rocha, dos Reis, Etchebehere, Machado and Corrêa2018). Reportedly, in addition to pain, hypertension, and oedema, women with PE experience chronic fatigue, depression, and anxiety (Hoedjes et al., Reference Hoedjes, Berks, Vogel, Franx, Bangma, Darlington, Visser, Duvekot, Habbema, Steegers and Raat2011b, Hu et al., Reference Hu, Li, Zhang, Yan and Coyne2015). Furthermore, several research studies have demonstrated a correlation between the severity of PE symptoms and an elevated prevalence of depression (Blom et al., Reference Blom, Jansen, Verhulst, Hofman, Raat, Jaddoe, Coolman, Steegers and Tiemeier2010, Hoedjes et al., Reference Hoedjes, Berks, Vogel, Franx, Bangma, Darlington, Visser, Duvekot, Habbema, Steegers and Raat2011a).
PE is characterised by endothelial dysfunctions, immune abnormalities, and syncytiotrophoblast stress (Jung et al., Reference Jung, Romero, Yeo, Gomez-Lopez, Chaemsaithong, Jaovisidha, Gotsch and Erez2022). PE is frequently associated with biomarkers of oxidative stress, inflammation, immune activation, and autoimmune responses (Grill et al., Reference Grill, Rusterholz, Zanetti-Dällenbach, Tercanli, Holzgreve, Hahn and Lapaire2009, Wang et al., Reference Wang, Li and Zhao2022). Placental apoptosis and necrosis may result from chronic hypoxia in the intervillous region, which may induce oxidative stress in the tissues (Soleymanlou et al., Reference Soleymanlou, Jurisica, Nevo, Ietta, Zhang, Zamudio, Post and Caniggia2005). Pro-inflammatory T helper (Th)1 and Th17 cytokines, along with suppressive Treg and Th2 cytokines (IL-10 and IL-4), have been found to be associated with PE at both the systemic and local levels (Toldi et al., Reference Toldi, Rigó, Stenczer, Vásárhelyi and Molvarec2011, Darmochwal-Kolarz et al., Reference Darmochwal-Kolarz, Kludka-Sternik, Tabarkiewicz, Kolarz, Rolinski, Leszczynska-Gorzelak and Oleszczuk2012, LaMarca et al., Reference Lamarca, Cornelius, Harmon, Amaral, Cunningham, Faulkner and Wallace2016). The transition to a Th1 response, characterised by increased IFN-γ secretion, is a critical element in PE (Laresgoiti-Servitje et al., Reference Laresgoiti-Servitje, GóMEZ-López and Olson2010).
The cytotoxic T-lymphocyte antigen-4 (CTLA-4 or CD152) gene may serve as a risk factor for PE during pregnancy, according to the findings of prior research (Dehaghani et al., Reference Samsami Dehaghani, Doroudchi, Kalantari, Pezeshki and Ghaderi2005). CTLA-4 is a protein receptor that downregulates immunological responses and functions as an immune checkpoint (Syn et al., Reference Syn, Teng, Mok and Soo2017). An inhibitory signal is generated when CLTA-4 binds to cluster of differentiation 80 (CD80) (or CD86), which is expressed on antigen-presenting cells. This signal prevents the activation of CD28 (Qureshi et al., Reference Qureshi, Zheng, Nakamura, Attridge, Manzotti, Schmidt, Baker, Jeffery, Kaur and Briggs2011). CD80, classified as a B7, type I membrane protein within the immunoglobulin superfamily, functions as a costimulatory molecule for T-cells and is implicated in T-cell activation (Novelli et al., Reference Novelli, Benigni and Remuzzi2018, Garin et al., Reference Garin, Diaz, Mu, Wasserfall, Araya, Segal and Johnson2009). Interestingly, soluble CTLA-4 (sCTLA-4) and CD80 (sCD80) are measurable in serum (Magistrelli et al., Reference Magistrelli, Jeannin, Herbault, Benoit DE Coignac, Gauchat, Bonnefoy and Delneste1999, Hock et al., Reference Hock, O’Donnell, Taylor, Steinkasserer, McKenzie, Rothwell and Summers2006), and have roles in modulating the immune response and autoimmune responses (Simone et al., Reference Simone, Pesce, Antola, Rumbullaku, Bagnasco, Bizzaro and Saverino2014, Saverino et al., Reference Saverino, Simone, Bagnasco and Pesce2010). High concentrations of sCTLA-4 were observed in sera of patients with autoimmune thyroid diseases (Saverino et al., Reference Saverino, Brizzolara, Simone, Chiappori, Milintenda-Floriani, Pesce and Bagnasco2007), as well as in patients with type 1 diabetes, diffuse cutaneous systemic sclerosis (Sato et al., Reference Sato, Fujimoto, Hasegawa, Komura, Yanaba, Hayakawa, Matsushita and Takehara2004), systemic lupus erythematosus (Wong et al., Reference Wong, Lit, Tam, Li and Lam2005), and rheumatic arthritis (Cao et al., Reference Cao, Zou, luo, Chen and Zhang2012). An increase in sCD80 levels may lead to an increase in IFN-γ production by active T cells (Gu et al., Reference GU, AO, YANG, CHEN and XU2018). sCD80 levels increased significantly in patients with autoimmune disease including SLE (Pratama et al., Reference Pratama, Handono, Kalim and Susianti2023) and rheumatoid arthritis (Malkawi et al., Reference Malkawi, Nimer, Almogren, Masood, Alarfaj, Benabdelkamel, Abdel Rahman and Siaj2023) compared to the healthy population.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is an additional immune biomarker that should be considered in the context of PE. Endothelial cells, macrophages, mast cells, T cells, and fibroblasts are responsible for producing the latter glycoprotein (Cousins et al., Reference Cousins, Staynov and Lee1994, Nimer and Uchida, Reference Snimer and Uchida1995, Mukai et al., Reference Mukai, Tsai, Saito and Galli2018). The induction of differentiation and activation of macrophage and dendritic cells by GM-CSF suggests that it might play a crucial role in the pathogenesis of PE (Huang et al., Reference Huang, Zenclussen, Chen, Basar, Yang, Arcuri, Li, Kocamaz, Buchwalder, Rahman, Kayisli, Schatz, Toti and Lockwood2010). GM-CSF, lipopolysaccharide and/or IFN-γ polarise macrophages toward an M1 phenotype, which is characterised by the increased expression of proinflammatory cytokines and CD80 (Jaguin et al., Reference Jaguin, Houlbert, Fardel and Lecureur2013, Wisitpongpun et al., Reference Wisitpongpun, Potup and Usuwanthim2022). Prevalence estimates for vitamin D deficiency during pregnancy range from 8 to 70%, contingent upon factors such as UV exposure and skin pigmentation (Chacham et al., Reference Chacham, Rajput, Gurnurkar, Mirza, Saxena, Dakshinamurthy, Chaturvedi, Goyal and Chegondi2020, Judistiani et al., Reference Judistiani, Nirmala, Rahmawati, Ghrahani, Natalia, Sugianli, Indrati, Suwarsa and Setiabudiawan2019). Vitamin D deficiency is more prevalent among mothers with PE and their neonates; therefore, patients may be advised to take higher doses of vitamin D supplementation (Fogacci et al., Reference Fogacci, Fogacci, Banach, Michos, Hernandez, Lip, Blaha, Toth, Borghi and Cicero2020, Tammo and Yıldız, Reference Tammo and Yildiz2022).
An activated immune-inflammatory response (IRS), which includes Th1 and Th17 responses, has been found to be associated with affective disorders and chronic fatigue syndrome (CFS) (Maes, Reference Maes1993, Maes et al., Reference Maes, Almulla, Zhou, Algon and Sodsai2023b, Morris and Maes, Reference Morris and Maes2012). Reduced levels of albumin, zinc, calcium, and magnesium accompany this IRS response (Al-Dujaili et al., Reference Al-Dujaili, Al-Hakeim, Twayej and Maes2019, Al-Hakeim et al., Reference Al-Hakeim, Hadi, Jawad and Maes2022). Furthermore, it has been observed that prenatal chronic fatigue and perinatal depression are associated with decreased serum zinc and IRS activation (Roomruangwong et al., Reference Roomruangwong, Kanchanatawan, Sirivichayakul and Maes2017, Maes et al., Reference Maes, Abe, Sirichokchatchawan, Suwimonteerabutr, Sangkomkamhangd, Almulla and Satthapisit2023a). However, the correlations between affective symptoms and CFS due to PE and immune biomarkers, including sCTLA4, sCD80, vitamin D, zinc, copper, albumin, calcium, and magnesium, remain largely unknown.
Therefore, the current study aimed to examine the correlations between immune-related biomarkers (sCD80, sCTLA-4, GM-CSF, vitamin D, zinc, calcium, magnesium, copper) in women with PE versus controls and their associations with depression, anxiety, and CFS due to PE.
Material and methods
Subjects
From November 2022 to February 2023, the present study recruited sixty healthy expectant control women of comparable age and gestational age and ninety PE women with an average age of 32.67 ± 5.88 years. The participants were recruited from maternity teaching institutions and selected private clinics. The diagnosis of PE was made in accordance with the criteria established by the American College of Obstetricians and Gynecologists (Espinoza and Vidaeff, Reference ESPINOZA and VIDAEFF2019). After 20 weeks of pregnancy, PE was identified in women who exhibit proteinuria and have a systolic and diastolic blood pressure higher than 140 mmHg and 90 mmHg, respectively. Each patient in the investigation fulfilled the specified criteria, and proteinuria was detected in all cases using dipstick tests. In addition, the patients were administered methyldopa (Aldomet®) and were required to fast overnight. The patient’s gravidity was characterised as the cumulative count of pregnancies, encompassing abortions, ectopic pregnancies, and any other pregnancies recorded in the medical record. Parity denotes the count of births that transpire after the 28th week of gestation, encompassing stillbirths and intrauterine fetal fatalities (IUFD). Sixty women who were at least 20 weeks expectant and lacked any PE symptoms were chosen to comprise the control group. The controls were matched for gestational age to the PE patients. Their blood pressure was normal at<120/80 mmHg.
A comprehensive medical history evaluation was conducted on each participant to exclude any pre-existing systemic conditions that could potentially impact the results, including liver and renal disease, infection, and cardiovascular events. All female subjects who were taking immunosuppressants or had compromised immune systems were precluded from the study. There were no prenatal abnormalities observed in any of the participants. There were no reports of active ailments, including uterine contractions or membrane ruptures. Other exclusion criteria for patients and controls were autoimmune and immune disorders including diabetes mellitus type 1, psoriasis, CFS, lupus erythematosus, arthritis, and inflammatory bowel disease. All subjects with axis 1 neuropsychiatric disorders present before the pregnancy were excluded, such as major depression, bipolar disorder, autism, psycho-organic disorder, and substance use disorders. All subjects showed CRP levels below 6 mg/l (Al-Hakeim et al., Reference Al-Hakeim, Al-Rammahi and Al-Dujaili2015). Patients who ever had suffered from severe phase 2 (pneumonia) or phase 3 (admission into ICU) COVID-19 were excluded to participate. Women who had suffered from mild COVID-19 infection were allowed to participate if the symptoms had resided at least three months before inclusion in this study.
Before participating in the study, all control and patient participants, or their respective parents or legal guardians, provided written consent after receiving comprehensive information. Under Document No. 103/2022, the University of Hawler’s approval committee in Erbil, Iraq, granted ethical approval for the research endeavour. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Clinical assessments
The severity of CFS and fibromyalgia was assessed by a senior psychiatrist using the Fibro-Fatigue scale (Zachrisson et al., Reference Zachrisson, Regland, Jahreskog, Kron and Gottfries2002). The level of anxiety was evaluated using the Hamilton Anxiety Rating Scale (HAMA) (Hamilton, Reference Hamilton1959). Hamilton’s Depression Rating Scale (HAMD) (Hamilton, Reference Hamilton1960) was completed by every participant to measure severity of depression. The senior psychiatrist conducted semi-structured interviews to collect sociodemographic and clinical information. When diagnosing tobacco use disorder, DSM-IV-TR criteria were applied. By dividing weight in kilograms by length in metres, BMI was computed.
Measurements
Fasting venous blood samples were taken from the participants between 8.00 a.m. and 9.00 a.m. and collected into plain tubes. Samples were aliquoted and stored at -80 oC before assay. After separation, the sera were distributed into three Eppendorf® tubes. Serum albumin, calcium, magnesium, copper, and zinc were measured spectrophotometrically using kits supplied by Spectrum Diagnostics Co. (Cairo, Egypt). The CRP latex slide test (Spinreact®, Barcelona, Spain) was used for CRP assays in human serum. The test is based on the principle of latex agglutination. ELISA sandwich kits, supplied by Nanjing Pars Biochem Co., Ltd (Nanjing, China), were used to measure serum sCD80, GM-CSF, sTCLA-4, and vitamin D. The procedures were followed exactly without modifications according to the manufacturer’s instructions. The intra-assay coefficients of variation (precision within an assay) were<10.0%. We computed two indices: a) the ratio of sCTLA-4/sCD80; and b) a z-unit based composite as z sCTLA-4 + z sCD80.
Statistical analysis
The researchers utilised analysis of variance to examine the variations in scale variables between control and PE women, while analysis of contingency tables (χ2-test) was employed to determine the relationships between nominal variables. To ascertain the impact of diagnosis on the biomarkers, we utilised multivariate general linear model (GLM) analysis, which accounted for confounding variables such as age and BMI. As a result, we conducted between-subjects effect tests in order to examine the associations between the diagnosis and biomarkers. Estimated marginal mean (SE) values generated by the model using GLM analysis were calculated. By utilising manual and stepwise multiple regression analysis, the biomarkers that best predict the symptoms were identified. Collinearity was assessed in all regression analyses through the utilisation of tolerance and VIF values. We employed a manual method and an automatic stepwise approach that incorporated variables with a p-to-entry of 0.05 and a p-to-remove of 0.06. In cases where homoscedasticity was deemed invalid through comprehensive examination of plots comparing standardised residuals to standardised predicted values and the White and Breusch-Pagan test, we employed heteroscedasticity-consistent standard error (SE) or robust SE estimates (utilising the HC3 method). All analyses were checked using bootstrapped methods (n = 1000), and discrepancies between the bootstrapped and other approaches are reported if needed. For statistical significance, two-tailed tests were conducted using a p-value of 0.05. All statistical analyses were conducted utilising version 29 of IBM SPSS for Windows.
The estimated a priori sample size was calculated using G*Power 3.1.9.4 and applied to the primary analysis, which involved conducting a multiple regression analysis of the rating scale scores on the biomarkers. Based on an effect size of f = 0.11 (which accounts for approximately 10% of the variance), along with a maximum of 6 explanatory variables, an alpha value of 0.05, and a power of 0.8, it was determined that a minimum sample size of 130 was necessary. It should be added that the post-hoc estimated power for the same analysis was 1.0.
Results
Sociodemographic and clinical data
The results of demographic and clinical data of the healthy controls (HC) and PE patients are presented in Table 1. The duration of symptoms in the PE group is 8.0 ± 3.5 weeks and the age of onset at 29.3 ± 5.2 years. BMI, education level, residency, smoking status, number of pregnancies, and gestational age did not significantly differ between PE patients and the control group. PE patients show a significantly increase in systolic and diastolic blood pressure compared with the control group. PE patients have a significantly higher abortion rate, FF score, HAMA score, and HAMD score compared with the non-PE pregnant group. The PE group has a lower number of pregnancies and higher abortion rates than the control group.
Table 1. Sociodemographic and clinical parameters in preeclampsia (PE) women and healthy pregnant women groups

MWU: Mann-Whitney U test; BMI: body mass index, B.P: blood pressure, HAMA: Hamilton Anxiety Rating Scale, HAMD: Hamilton Depression Rating Scale, FF: fibro fatigue scale.
Biomarkers of PE
Table 2 shows the results of multivariate GLM analysis which examines the associations between the biomarkers (albumin, magnesium, calcium, Vitamin D, sCTLA-4, GM-CSF, sCD80, zinc, and copper) and the diagnosis (PE versus HC) while adjusting for age and BMI. Tests for between-subject effects showed that there were significant associations between diagnosis and (in descending order of importance) vitamin D, sCTLA-4, calcium, sCD80, copper, zinc, albumin, and magnesium.
Table 2. Results of multivariate general linear model analysis that examine the associations between the biomarkers and the diagnosis of preeclampsia
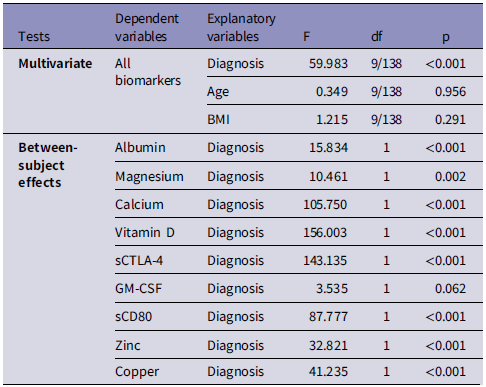
BMI: body mass index, sCTLA-4: soluble cytotoxic T lymphocyte-associated antigen-4, GM-CSF: Granulocyte-macrophage colony-stimulating factor.
Table 3 shows the model-generated estimated mean (± SE) biomarker values in the study groups. Serum levels of sCTLA-4, sCD80, copper, and z sCTLA-4 + z sCD80 (z score) show a significant increase in the PE group compared with the control group. There are significant decreases in serum levels of albumin, magnesium, calcium, Vitamin D, zinc, and sCTLA-4 / sCD80 ratio in PE women compared with the healthy pregnant women group.
Table 3. Model-derived estimated marginal means of the biomarkers in pre-eclampsia (PE) patients and control pregnant women

* Results of univariate GLM with age and body mass index as covariates; all other comparisons: results of tests of between-subjects effects after performing multivariate GLM (see Table 2). sCTLA-4: soluble cytotoxic T lymphocyte-associated antigen-4, GM-CSF: granulocyte-macrophage colony-stimulating factor.
Multiple regression analyses of clinical scores on biomarkers
Table 4 shows the results of different multiple regression analyses with the psychiatric rating scale scores as dependent variables and blood pressure and biomarkers as explanatory variables, while allowing for the effects of age, BMI, and education. Regression #1 shows that 68.2% of the variance in the total FF score was explained by the regression on systolic and diastolic blood pressure and sCD80 (all positively) and magnesium (inversely). In Regression #2, 62.4% of the variance in the HAMA score was explained by the regression on systolic and diastolic blood pressure, sCD80, and copper (all positively), and albumin (inversely). Regression #3 shows that 59.4% of the variance in the total HAMD score was explained by the regression on systolic and diastolic BP, sCD80, and sCTLA-4.
Table 4. Results of multiple regression analysis with neuropsychiatric rating scale scores as dependent variables and biomarkers and blood pressure (BP) data as explanatory variables

sCTLA-4: soluble cytotoxic T lymphocyte-associated antigen-4, HAMA: Hamilton Anxiety Rating Scale, HAMD: Hamilton Depression Rating Scale, FF: fibro fatigue scale.
In Table 5, we have recomputed these associations after deleting the blood pressure data. A significant part of the variance (58.0%) in the total HAMD score can be explained by the regression on sCTLA-4, sCD80, and BMI (all positively), vitamin D, calcium, and GM-CSF (negatively) (regression #1). Fig. 1 shows the partial regression plot of the HAMD total score on serum sCTLA-4. Regression #2 shows that 56.2% of the variance in the HAMA total score was explained by the regression on sCD80, sCTLA-4, copper (all positively), and albumin and vitamin D (both negatively). Fig. 2 shows the partial regression plot of the HAMA total score on serum sCD80. In Regression #3, 55.8 % of the variance in the FF score could be explained by the regression on sCTLA-4, copper, sCD80 (all positively), vitamin D, calcium, and magnesium (inversely associated).

Figure 1. Partial regression plot of the hamilton depression rating scale (HAMD) score on serum soluble cytotoxic T-lymphocyte antigen-4 (CTLA-4) (after adjusting for age, body mass index, education) p < 0.001.
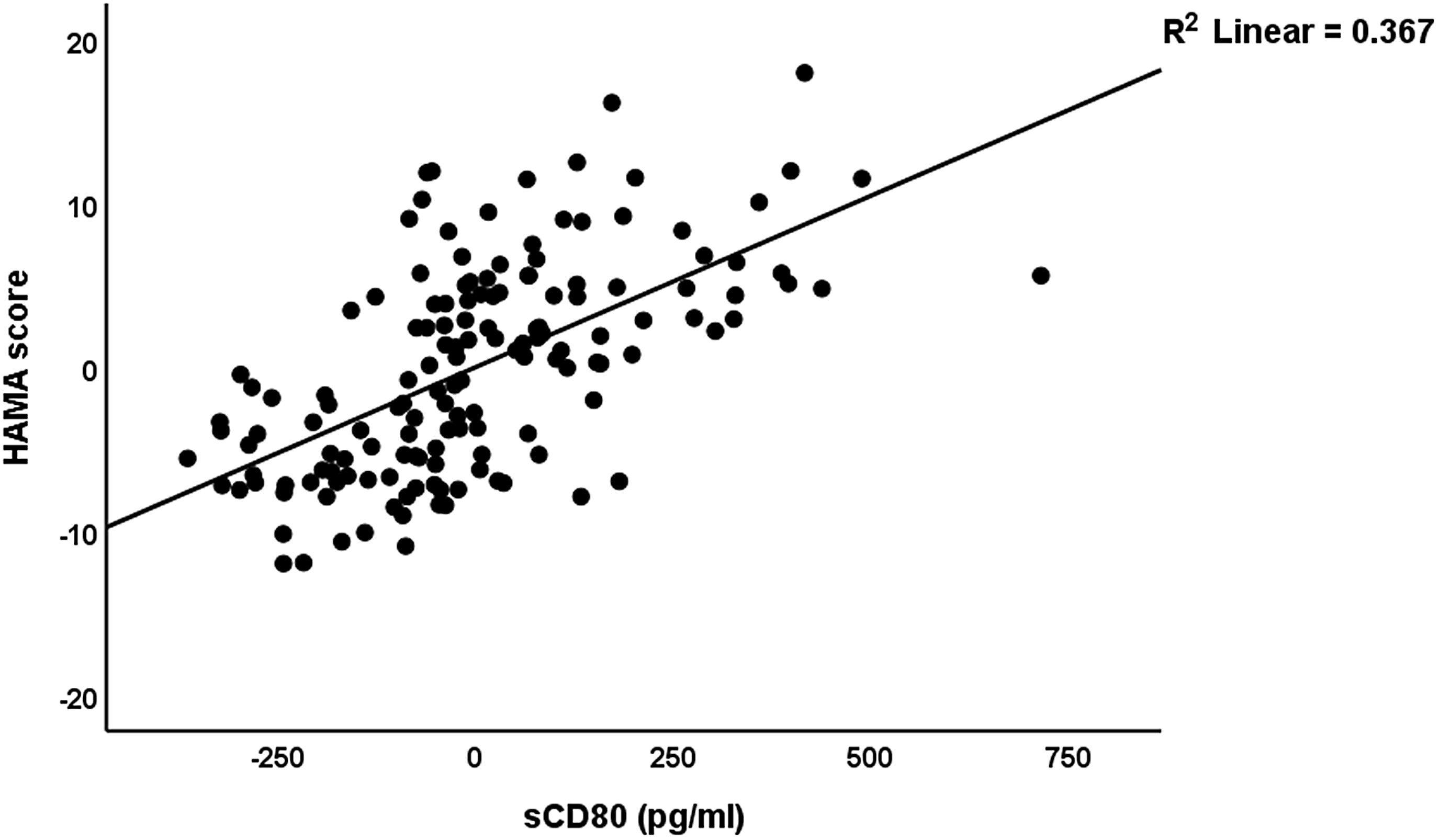
Figure 2. Partial regression plot of the Hamilton Anxiety Rating Scale score on soluble CD80 (sCD80) (after adjusting for age, body mass index, education) p < 0.001.
Table 5. Results of multiple regression analysis with neuropsychiatric rating scale scores as dependent variables and biomarkers (without blood pressure data) as explanatory variables

BMI: body mass index, sCTLA-4: soluble cytotoxic T lymphocyte-associated antigen-4, GM-CSF: Granulocyte-macrophage colony-stimulating factor, HAMA: Hamilton Anxiety Rating Scale, HAMD: Hamilton Depression Rating Scale, FF: fibro fatigue scale.
The z unit based composite z sCTLA4 + z CD80 was significantly correlated with the FF (r = 0.645, p < 0.001), HAMA (r = 0.684, p < 0.001), and HAMD (r = 0.702, p < 0.001) scores. The sCTLA4 / sCD80 ratio was inversely and significantly correlated with the FF (r = −0.229, p = 0.005), HAMA (r = −0.222, p = 0.006), and HAMD (r = −0.210, p = 0.010) score.
Multiple regression analyses of BP data on biomarkers
Table 6 shows the results of the multiple regression analyses with blood pressure as dependent variable and biomarkers and clinical data as explanatory variables. Regression #1 shows that 71.6% of the variance in the systolic blood pressure can be explained by the regression on sCTLA-4, copper, and having a child (positively) and calcium and vitamin D (inversely). Fig. 3 shows the partial regression plot of the systolic blood pressure on the serum sCTLA-4. Regression #2 shows that 48.0% of the variance in the diastolic blood pressure was explained by sCTLA-4 and serum copper (both positively), and vitamin D, calcium, and GM-CSF (all negatively).
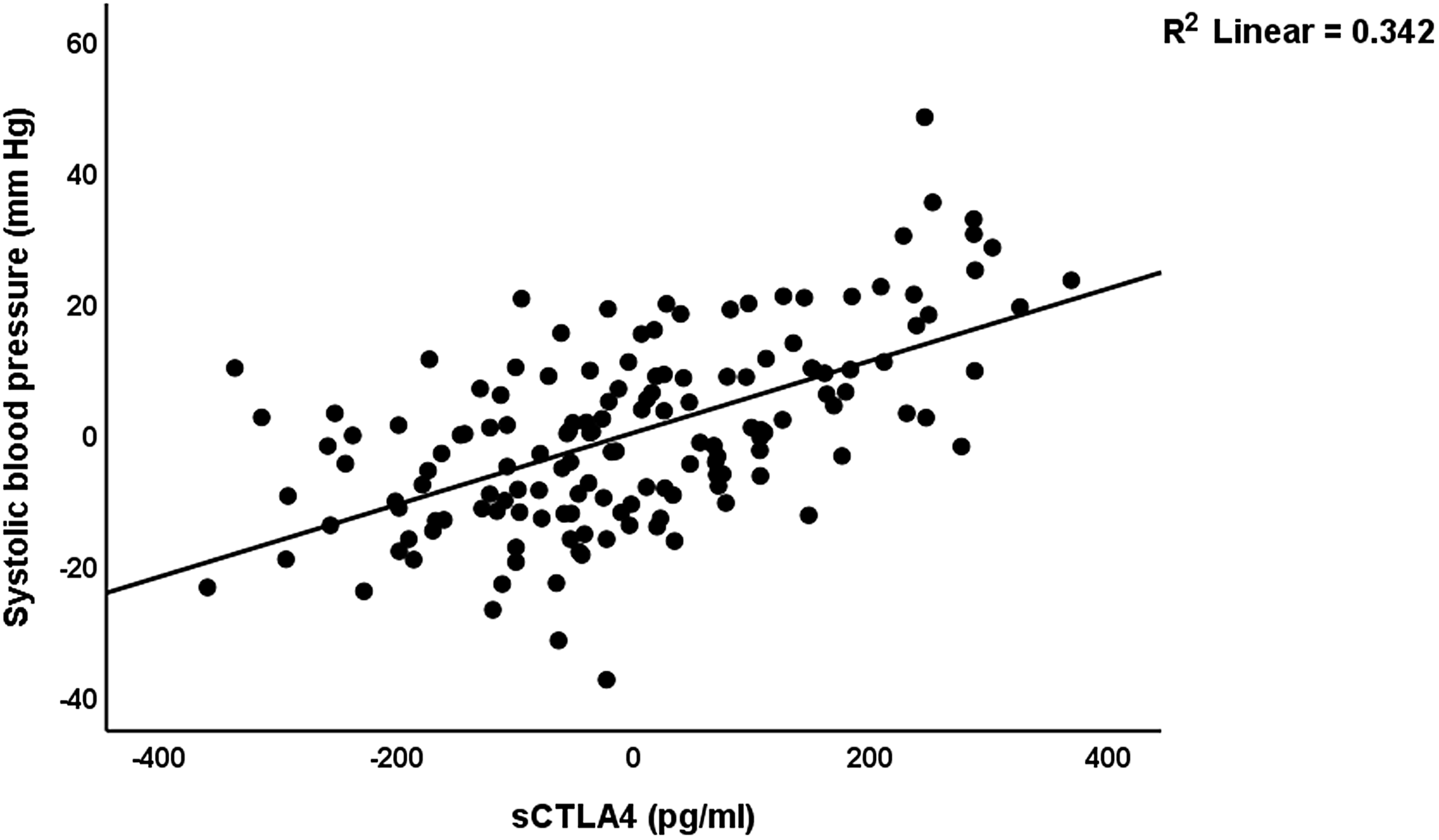
Figure 3. Partial regression plot of the systolic blood pressure on the serum soluble cytotoxic T-lymphocyte antigen-4 (sCTLA-4) (after adjusting for age, body mass index, calcium, vitamin D, zinc) p < 0.001.
Table 6. Results of multiple regression analyses with blood pressure (BP) data as dependent variables and biomarkers and clinical data as explanatory variables

sCTLA-4: soluble cytotoxic T lymphocyte-associated antigen-4, GM-CSF: Granulocyte-macrophage colony-stimulating factor.
Discussion
Increased neuropsychiatric symptoms in PE
One significant discovery from this study is that PE women exhibit higher scores in all three neuropsychiatric areas (depression, anxiety, and CFS) compared to healthy pregnant women. In this study, women with pre-existing depression, anxiety, and chronic fatigue were not included. This suggests that the findings of this research demonstrate a link between PE and the development of new neuropsychiatric symptoms.
Previous research has found that there is a significant connection between PE and the development of depression, as well as an increase in the severity of depressive symptoms (Caropreso et al., Reference Caropreso, de Azevedo Cardoso, Eltayebani and Frey2020, Hu et al., Reference Hu, Li, Zhang, Yan and Coyne2015). A significant proportion of women with PE experience perinatal or postpartum depression (Mbarak et al., Reference Mbarak, Kilewo, Kuganda and Sunguya2019). Furthermore, it has been established that PE itself is a contributing factor to the development of post-partum depression, as highlighted by the study conducted by Ye et al. (Reference Ye, Chen, Xu, Dai, Luo, Shan and Qi2021).
Research has indicated a significant increase in anxiety levels among women who have been diagnosed with PE (Abedian et al., Reference Abedian, Soltani, Mokhber and Esmaily2015). However, a comprehensive review indicated that there seemed to be a connection between PE and depression, while no link was found with anxiety (Delahaije et al., Reference Delahaije, Dirksen, Peeters and Smits2013). Women who have experienced PE tend to report higher levels of depression and fatigue compared to those who have not had PE (Mommersteeg et al., Reference Mommersteeg, Drost, Ottervanger and Maas2016, Agrawal and Yamamoto, Reference Agrawal and Yamamoto2015).
One could make the case that the concern over potential fetal loss and other future repercussions would lead to increased levels of depression, anxiety, and fatigue in patients with PE. In addition, unexpected medical procedures and the possibility of mortality can cause feelings of depression and anxiety in pregnant women (Szita et al., Reference Szita, Baji and Rigó2015). However, it is worth noting that a significant portion of the variation in the severity of these neuropsychiatric symptoms can be attributed to immune-inflammatory biomarkers. This suggests that these biological factors may hold more significance than psychological factors, as will be explored in the following section.
Biomarkers of PE
In the present study, it was observed that the PE group had higher levels of serum sCTLA-4, sCD80, and copper, while magnesium, calcium, zinc, and albumin were found to be significantly lower in PE. These findings suggest a correlation between PE and activation of immune-inflammatory responses system. PE is commonly recognised as an immune-inflammatory disorder (Redman et al., Reference Redman, Sacks and Sargent1999, Harmon et al., Reference Harmon, Cornelius, Amaral, Faulkner, Cunningham, Wallace and LaMarca2016). Administering anti-inflammatory compounds could potentially provide benefits for women experiencing PE, as it may help address issues related to maternal immune system failure and excessive inflammation (Chatterjee et al., Reference Chatterjee, Chiasson, Seerangan, Tobin, Kopriva, Newell-Rogers and Mitchell2015).
In previous studies, researchers explored the potential link between gene polymorphisms in CTLA-4 and the risk of PE. However, a meta-analysis did not yield any significant association between the two (Liu et al., Reference Liu, Song, Zhao and Meng2022). In a separate study, RT-PCR revealed a decrease in the expression of checkpoint inhibitory markers, such as CTLA-4, in the decidual tissue of women with PE compared to the control group (Madadi et al., Reference Madadi, Mohammadinejad, Alizadegan, Hojjat-Farsangi, Dolati, Samadi Kafil, Jadidi-Niaragh, Soltani-Zangbar, Motavalli, Etemadi, Eghbal-Fard, Aghebati-Maleki, Danaii, Taghavi and Yousefi2022). Lower levels of CTLA-4 expression were observed in women with miscarriages, specifically on peripheral lymphocytes, T regulatory cells, and decidual lymphocytes. Additionally, the ratio of CTLA-4+/CD28+ in Treg cells was found to be decreased (Jin et al., Reference Jin, Chen, Zhang, Guo and Li2009, Jin et al., Reference Jin, Fan, Zhang, Guo and Li2011). In patients with preeclampsia, dendritic cells exhibit elevated expression of CD80 (and CD86), which is linked to enhanced differentiation of Th1 and Th17 cells (Wang et al., Reference Wang, Tao, Cheng, Zhu, Chen, Yao and Su2014). Women with recurrent spontaneous abortion also show elevated levels of sCD80 (Zych et al., Reference Zych, Roszczyk, Dąbrowski, Kniotek and Zagożdżon2023). All in all, in the pathophysiology of PE, there is an imbalance among immune checkpoint molecules (such as CTLA-4) and stimulatory signals (Wang et al., Reference Wang, Tao, Cheng, Zhu, Chen, Yao and Su2014, Collier et al., Reference Collier, Modest, Aguayo, Bondzie, Patel, Hacker and Barouch2023, Boulanger et al., Reference Boulanger, Bounan, Mahdhi, Drouin, Ahriz-saksi, Guimiot and Rouas-freis2024).
However, in this study, we focused on measuring the soluble forms of CTLA-4 and CD80 molecules, specifically sCTLA-4 and sCD80. It is important to note that these soluble forms do not per se possess the same functions as the cell-bound molecules. sCTLA-4 can suppress immune responses both in laboratory settings, animal studies, and individuals diagnosed with rheumatoid arthritis (Kremer et al., Reference Kremer, Westhovens, Leon, DI Giorgio, Alten, Steinfeld, Russell, Dougados, Emery and Nuamah2003, Linsley et al., Reference Linsley, Brady, Urnes, Grosmaire, Damle and Ledbetter1991, Oaks et al., Reference Oaks, Hallett, Penwell, Stauber, Warren and Tector2000, Linsley, Reference Linsley1995). Increased sCD80 may restore CD4+ and CD8+ T cell activation (Haile et al., Reference Haile, Dalal, Clements, Tamada and OSTRAND-ROSENBERG2013). Based on our findings, it seems that the decreased sCTLA-4 / sCD80 ratio in PE could suggest a shift towards heightened immune activation, possibly due to a decrease in immunosuppressive signals and a relative increase in immune-stimulatory signals. These findings align with the inflammatory biomarkers (such as decreased albumin, zinc, and magnesium) identified in our study and support the immune-inflammatory theory of PE (Toldi et al., Reference Toldi, Rigó, Stenczer, Vásárhelyi and Molvarec2011, Darmochwal-Kolarz et al., Reference Darmochwal-Kolarz, Kludka-Sternik, Tabarkiewicz, Kolarz, Rolinski, Leszczynska-Gorzelak and Oleszczuk2012, LaMarca et al., Reference Lamarca, Cornelius, Harmon, Amaral, Cunningham, Faulkner and Wallace2016).
Our study found no notable variation in GM-CSF levels between women with PE and those in the control group. Prior research has indicated a notable rise in serum and placental GM-CSF levels in women with preeclampsia when compared to the control group (Hayashi et al., Reference Hayashi, Hamada and Ohkura2004). In a study conducted by Gratacós et al., a similar lack of significant difference in GM-CSF was observed during the second trimester, which aligns with the findings of our own study (Gratacós et al., Reference Gratacós, Filella, Palacio, Cararach, Alonso and Fortuny1998). Nevertheless, at other gestational ages, there may be a significant increase in GM-CSF compared with controls (Gratacós et al., Reference Gratacós, Filella, Palacio, Cararach, Alonso and Fortuny1998).
It has been suggested that the decrease in calcium, magnesium, and zinc levels in the blood during pregnancy could potentially play a role in the development of PE. Therefore, adding these elements to the diet through supplementation may be beneficial in preventing PE (Jain et al., Reference Jain, Sharma, Kulshreshtha, Mohan and Singh2010). Possible reasons for the decline in these elements could be heightened inflammatory reactions and the demands of the developing fetus (Kumru et al., Reference Kumru, Aydin, Simsek, Sahin, Yaman and Ay2003, Sukonpan and Phupong, Reference Sukonpan and Phupong2005, Ma et al., Reference Ma, Shen and Zhang2015). One likely reason for the decrease in vitamin D levels in women who are pregnant may be the increased need for calcium metabolism to support the growth of the fetus. Several studies have consistently shown a strong link between vitamin D deficiency and a higher risk of PE (Serrano et al., Reference Serrano, Guío, Quintero-Lesmes, Becerra-Bayona, Luna-Gonzalez, Herrera and Prada2018, Achkar et al., Reference Achkar, Dodds, Giguère, Forest, Armson, Woolcott, Agellon, Spencer and Weiler2015, Bodnar et al., Reference Bodnar, Catov, Simhan, Holick, Powers and Roberts2007), although there are some authors who do not agree with this finding (Mirzakhani et al., Reference Mirzakhani, Litonjua, Mcelrath, O’connor, Lee-Parritz, Iverson, Macones, Strunk, Bacharier, Zeiger, Hollis, Handy, Sharma, Laranjo, Carey, Qiu, Santolini, Liu, Chhabra, Enquobahrie, Williams, Loscalzo and Weiss2016).
Associations between biomarkers and severity of neuropsychiatric symptoms
An essential discovery in this study is the connection observed between the neuropsychiatric rating scales and the serum biomarker levels. Therefore, a significant portion of the variation in the clinical scores (ranging from 55.8% to 58.0%) could be attributed to the presence of a combination of up to six distinct biomarkers. When it comes to predicting depression, anxiety, and chronic fatigue caused by PE, sCD80, sCTLA-4, and vitamin D are the top-3 most important predictors.
As previously mentioned, affective disorders such as major depression and associated generalised anxiety disorder, as well as CFS, are classified as neuro-immune disorders (Maes et al., Reference Maes, Bosmans, Suy, Vandervorst, DE Jonckheere and Raus1990, Maes et al., Reference Maes, Vandoolaeghe, Ranjan, Bosmans, Bergmans and Desnyder1995, Maes and Carvalho, Reference Maes and Carvalho2018, Twisk and Maes, Reference Twisk and Maes2009, Maes, Reference Maes2011). Thus, the inverse associations between the sCTLA4 / sCD80 ratio and the FF, HAMA, and HAMD scores may play a role in the immune pathophysiology of depression, anxiety, and CFS associated with PE. It is important to emphasise that a higher T effector /T regulatory ratio plays a significant role in major depression (Maes et al., Reference Maes, Zhou, Jirakran, Vasupanrajit, Boonchaya-anant, Tunvirachaisakul, Tang, Li and Almulla2024). CTLA-4 is found on the surface of both T regulatory and conventional T cells, playing a crucial role in regulating the activation of T effector cells by providing negative feedback (Gardner et al., Reference Gardner, Jeffery and Sansom2014). In addition, CTLA-4 has the ability to compete with CD28 for ligand binding, effectively acting as a counteractive force against CD28-mediated co-stimulation (Walker and Sansom, Reference Walker and Sansom2011). Interestingly, some, but not all studies reported a significant association between CTLA-4 gene polymorphisms and major depression (Liu et al., Reference Liu, Li, Li, Wang, Li, Zeng, Li, Chen, Hu, Zheng, Lin, Feng and Shi2011, Jun et al., Reference Jun, Pae, Chae, Bahk and Kim2001). Decreased levels of zinc, magnesium, and vitamin D, along with elevated copper, are significant indicators of conditions such as depression, perinatal depression, anxiety, CFS, and perinatal fatigue (Maes et al., Reference Maes, D’haese, Scharpé, D’hondt, Cosyns and DE Broe1994, Maes et al., Reference Maes, Mihaylova and DE Ruyter2006, Roomruangwong et al., Reference Roomruangwong, Anderson, Berk, Stoyanov, Carvalho and Maes2018, Jung et al., Reference Jung, Spira, Steinhagen-thiessen, Demuth and Norman2017, Wang et al., Reference Wang, Um, Dickerman and Liu2018b, Wessels et al., Reference Wessels, Maywald and Rink2017, Kanwar and Sharma, Reference Kanwar and sharma2022, McCarty, Reference Mccarty2010, Lee et al., Reference Lee, Tajar, Pye, Boonen, Vanderschueren, Bouillon, O’neill, Bartfai, Casanueva, Finn, Forti, Giwercman, Han, Huhtaniemi, Kula, Leanmr, Pendleton, Punab, Wu and Group2012, Aghajafari et al., Reference Aghajafari, Letourneau, Mahinpey, Cosic and Giesbrecht2018, Wang et al., Reference Wang, Liu, Sun, Chen, Zhao and Zhang2018a).
One noteworthy discovery from the present study is the strong predictive power of sCTLA-4, copper, calcium, and vitamin D in relation to systolic and diastolic hypertension. Previously, it was shown that there is a connection between depressive symptoms in early pregnancy and the mother’s blood pressure during the first trimester (Bilbul et al., Reference Bilbul, Caccese, Horsley, Gauvreau, Gavanski, Montreuil, Konci, Lai, Da Costa, Zelkowitz, Shen, Gryte, Larosa, Brown, Suarthana and Nguyen2022). Other researchers have found that women with higher blood pressure in the third trimester tend to experience increased depression (Hoedjes et al., Reference Hoedjes, Berks, Vogel, Franx, Bangma, Darlington, Visser, Duvekot, Habbema, Steegers and Raat2011a, Ye et al., Reference Ye, Chen, Xu, Dai, Luo, Shan and Qi2021). There is a correlation between immune activation and oxidative and nitrosative stress, which has been linked to hypertension in individuals with depression (Bonifácio et al., Reference Bonifácio, Barbosa, Moreira, Coneglian, Vargas, Nunes, Moraes and Maes2021). Inflammation is linked to hypertension through the activation of pathways related to oxidative stress, immune activation caused by sodium, and the inflammasome (Patrick et al., Reference Patrick, Van Beusecum and Kirabo2021). Vitamin D plays a crucial role in promoting angiogenesis and reducing blood pressure by affecting the renin-angiotensin system (Bodnar et al., Reference Bodnar, Catov, Simhan, Holick, Powers and Roberts2007, Evans et al., Reference Evans, Bulmer, Kilby and Hewison2004, Fischer et al., Reference Fischer, Schroer, Ludders, Cordes, Bucker, Reichrath and Friedrich2007, Halhali et al., Reference Halhali, Tovar, Torres, Bourges, Garabedian and Larrea2000, Tarcin et al., Reference Tarcin, Yavuz, Ozben, Telli, Ogunc, Yuksel, Toprak, Yazici, Sancak and Deyneli2009, Robinson et al., Reference Robinson, Alanis, Wagner, Hollis and Johnson2010). Therefore, vitamin D is expected to play a role in repairing the endothelium and promoting angiogenesis, while also regulating blood pressure (Behjat Sasan et al., Reference BEHJAT SASAN, ZANDVAKILI, SOUFIZADEH and Baybordi2017).
Limitations of the study
It would have been intriguing to evaluate T effector and T regulatory cells through flow cytometry, as well as assess membrane-bound CTLA-4, CD28, CD80, and CD86 on T cells. It would be beneficial to conduct further analyses on oxidative and nitrosative stress. One could make the case that the sample size is relatively small. Nevertheless, the sample size was determined through power analysis, and the subsequent power achieved in the primary outcome variables (as analysed through multiple regression on the biomarkers, as shown in Table 5) was 1.0.
Conclusions
Compared to control women, PE women exhibit higher depression, anxiety, and CFS scores. Approximately 55.8%–58.0% of the variance in the HAMD, HAMA, and FF scores was accounted for by the regression on biomarkers, and sCTLA-4, sCD80, and vitamin D were the three most significant biomarkers. The HAMD/HAMA/FF scores exhibited a significant and inverse correlation with the sCTLA-4/sCD80 ratio. Approximately 70% of the variance in systolic blood pressure was predicted by copper, sCTLA-4, vitamin D, and calcium. The results emphasise that symptoms of depression, anxiety, and chronic fatigue associated with PE are accompanied by immune-inflammatory response activation. Imbalances among soluble checkpoint molecules contribute to the pathogenesis of both hypertension and neuropsychiatric symptoms associated with PE. sCTLA-4 and membrane CTLA-4 as well as sCD80 and membrane CD80 are new drug targets to treat PE and depression, anxiety, and CFS due to PE. Moreover, sCTLA-4 and copper, and lowered calcium and vitamin D are new drug targets to treat hypertension in PE women.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgements
We acknowledge the assistance of the workers at the Middle Euphrates Center for Neurological Sciences, Najaf City, Iraq, in sample collection and lab measurements. No other persons or third-party services were involved in the research or manuscript preparation.
Author’s contributions
Jangir Sami Omar: the acquisition, analysis, have drafted the work. Niaz Albarzinji: the acquisition, analysis, substantively revised it. Mengqi Niu: interpretation of data, substantively revised it. Naz Hawree Taher: the acquisition, analysis, substantively revised it. Bayar Aram: the acquisition, analysis, substantively revised it. Mohammed Salam Sulaiman: the acquisition, analysis, substantively revised it. Shatha Rouf Moustafa: the acquisition, analysis, substantively revised it. Hussein Kadhem Al-Hakeim: conception, substantively revised it. Michael Maes: conception, substantively revised it. All the contributing authors have participated in the preparation of the manuscript, and have approved the submitted version. All the contributing authors have agreed both to be personally accountable for the author’s own contributions.
Funding statement
No funding was received for this research.
Competing interests
The authors declare no conflicts of interest with any industrial or other organisation regarding the submitted paper.
Ethics approval and consent to participate
Before participating in the study, all control and patient participants, or their respective parents or legal guardians, provided written consent after receiving comprehensive information. Under Document No. 103/2022, the University of Hawler’s approval committee in Erbil, Iraq, granted ethical approval for the research endeavour. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.












