Summations
∙ Inflammatory bowel disease (IBD) patients are at an increased risk for developing an anxiety- or depression-related disorder.
∙ Animal models of IBD are a useful tool in understanding the physiological changes that occur in the brain in response to gastrointestinal (GI) inflammation.
∙ Due to the bidirectionality of the gut–brain axis stress, anxiety or depression may in turn exacerbate or trigger IBD. Treatment of psychological symptoms may improve health-related quality of life (HRQOL) and relapse rates for patients.
Considerations
∙ The increased risk of psychological symptoms during active IBD is generally agreed upon in the literature, however, further work is required during disease remission. It is possible that persistent psychological symptoms occur as a result of ongoing irritable bowel syndrome (IBS)-like symptoms.
∙ No one animal model or behavioural test can accurately represent the human condition in IBD or psychological illness. Translational tools such as magnetic resonance imaging (MRI) should be used for more direct comparisons between models and humans.
∙ The link between stress and IBD remains controversial and requires further study. The use of psychological treatments for IBD is unlikely to replace traditional anti-inflammatory treatments. Management of GI symptoms may also improve psychological outcomes.
Introduction
IBD is a chronic relapsing and remitting disorder of the GI tract. Crohn’s disease (CD) and ulcerative colitis (UC) are the two main subtypes of IBD. CD and UC have similar symptomatology, however, CD symptoms can depend on the site of inflammation. Symptoms common to both CD and UC include abdominal pain/cramping, loose stools, diarrhoea, bloody stools, rectal bleeding, fatigue and a loss of appetite or food avoidance. Extra-intestinal manifestations of IBD can affect joints, the skin and eyes (Reference Vavricka, Schoepfer, Scharl, Lakatos, Navarini and Rogler1). IBD patients are also at a higher risk of developing colorectal cancer (CRC) compared with the general population (Reference Kim and Chang2). CD and UC differ mainly in their histology and in terms of their location within the GI tract. CD affects all layers of the gut wall, whereas in UC inflammation is usually confined to the mucosa. The formation of intestinal granulomas and fistulae are hallmarks of CD. Inflammation in UC usually remains in the rectum and colon, whereas CD can affect any part of the GI tract, however, most commonly involves ileocaecal inflammation (Reference Lennard-Jones3,Reference Baumgart and Sandborn4).
The disease burden of IBD is challenging for patients and not only includes the physiological manifestations of the disease but also psychological and social burden. An IMPACT survey commissioned by the European Federation of Crohn’s and Ulcerative Colitis Associations in late 2010 assessed the impact that IBD can have on patients in terms of medical implications, emotional well-being, education and work, and overall quality of life. Almost half (48%) of European IBD patients surveyed indicated that even between flare-ups their lives are negatively affected by symptoms of IBD (Reference Lonnfors, Vermeire, Greco, Hommes, Bell and Avedano5). Although IBD can occur at any age, the disease has a peak in incidence rates in younger people between the ages of 15 and 30 years, meaning that the majority of patients are faced with this diagnosis during the most productive years of life. In CD, incidence declines sharply following this peak, however, in UC, peak incidence typically occurs 5 years later than in CD and plateaus, particularly in males, in whom incidence does not significantly decrease until the seventh decade of life (Reference Johnston and Logan6).
The aetiology of IBD remains unknown, however, the consensus is that immune dysfunction and inflammation occurs as a response to an environmental trigger in a genetically susceptible host. IBD is an incurable yet treatable disease typically managed with drugs (aminosalicylates, systemic corticosteroids and immunosuppressants) and surgery (such as bowel resection for CD or colectomy with ileostomy or ileo-anal pouch anastomosis) if necessary (Reference Carter, Lobo and Travis7,Reference Mowat, Cole and Windsor8). Surgery will generally be required in 70–80% of CD patients and up to 30% of UC patients (Reference Hwang and Varma9). Surgery may be curative for UC, whereas inflammation usually recurs following surgery in CD (Reference Sica and Biancone10). Mortality for IBD is slightly higher than the general population with a UK study of over 16 000 IBD patients with age- and sex-matched controls indicating 54% excess mortality associated with IBD diagnosis (Reference Card, Hubbard and Logan11).
Comorbid anxiety and depression in IBD
Historically there has been a long-standing interest in the comorbidity of psychological well-being, psychiatric illness and personality differences associated with IBD (Reference Straker12–Reference Murray15) in patients of all age groups. Many early studies in adults and children came to the conclusion that both UC and CD are related to higher incidence of psychological symptoms (Reference Farrokhyar, Marshall, Easterbrook and Irvine16–Reference Addolorato, Capristo, Stefanini and Gasbarrini18). Of particular interest is the study by Addolorato et al. (Reference Addolorato, Capristo, Stefanini and Gasbarrini18) as only patients naïve to steroid treatment and surgical intervention were included, and again IBD patients had significantly higher expression of depressive symptoms than controls (41.9% and 50% of CD and UC patients, respectively, show depressive symptoms compared with 11.1% of controls). They also report an association between mood and disease activation.
In a recent systematic review of the comorbidity of psychological disorders with IBD, Mikocka-Walus et al. (Reference Mikocka-Walus, Knowles, Keefer and Graff19) evaluated 66 articles published between 2005 and 2014. Rates of anxiety and depression were estimated to be greater in IBD patients compared with healthy individuals, with rates of both being higher during the active IBD phase compared with remission. Mean rates of anxiety and depression were significantly higher in CD compared with UC but only modestly so.
Validated questionnaires including the Hospital Anxiety and Depression Scales (HADS), Beck Depression Inventory (BDI), State-Trait Anxiety Inventory (STAI) and Hamilton anxiety and depression scales have been used to confirm an increased risk of psychological disturbances in IBD patients (Reference Gandhi, Jedel, Hood, Mutlu, Swanson and Keshavarzian20–Reference Calvet, Gallardo and Coronas23). Differences between studies suggest that factors such as the level of disease activity may influence anxiety and depression scores. Depression and/or anxiety are consistently reported to be increased in the active phase of IBD (Reference Gandhi, Jedel, Hood, Mutlu, Swanson and Keshavarzian20,Reference Clark, Srinath and Youk24–Reference Ben Thabet, Charfi and Mnif26).
A detailed summary of reports documenting anxiety and depression symptoms in IBD patients is summarised in Table 1.
Table 1 Reports of anxiety- and depression-related symptoms in inflammatory bowel disease (IBD) patients
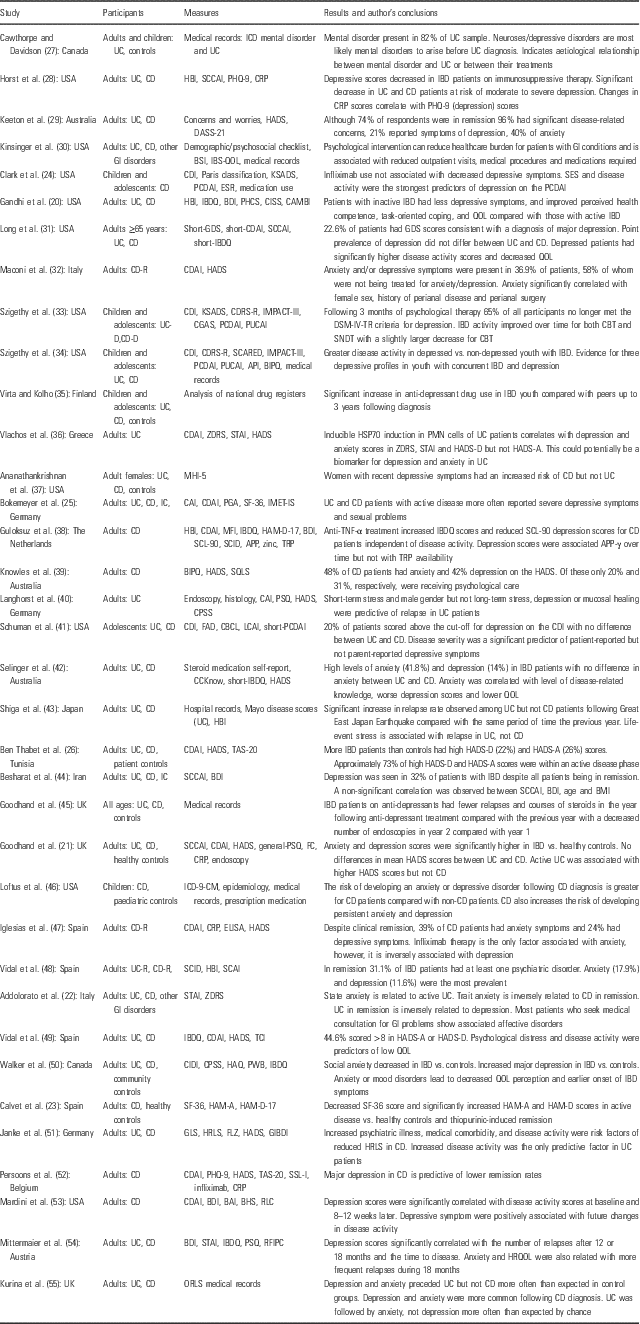
API, Abdominal Pain Index; APPγ, acute phase protein – gamma; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; BHS, Beck Hopelessness Scale, BIPQ, Brief Illness Perception Questionnaire; BSI, Brief Symptom Inventory; CRP, C-reactive protein; CES-D, Center for Epidemiologic Studies-Depression Scale; CBCL, Child Behaviour Checklist; CDI, Children’s Depression Inventory; CDRS-R, Children’s Depression Rating Scale – Revised; CGAS, Children’s Global Assessment Scale; CAI, Clinical Activity Index; CBT, Cognitive Behavioural Therapy; CPSS, Cohen Perceived Stress Scale; CAMBI, Complementary and Alternative Medicine Beliefs Inventory; CIDI, Composite International Diagnostic Interview; CISS, Coping Inventory for Stressful Situations; CCKnow, Crohn’s and Colitis Knowledge score; CD/CD-R/-D, Crohn’s disease/in remission/with depression; CDAI, Crohn’s Disease Activity Index; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision; DASS, Depression Anxiety and Stress Scale; ELISA, enzyme-linked immunosorbent assay; ESR, erythrocyte sedimentation rate; FC, faecal calprotectin; FAD, Family Assessment Device; FLZ, Fragen zur Lebenszufriedenheit – German questionnaire on life satisfaction; GI, gastrointestinal; GLS, General Life Satisfaction; GP, general population; GDS, Geriatric Depression Scale; GIBDI, German Inflammatory Bowel Disease activity Index; HAM-A/-D, Hamilton rating scale for Anxiety/Depression; HBI, Harvey Bradshaw Index; HAQ, Health Anxiety Questionnaire; HRLS, Health-Related Life Satisfaction; HRQOL, Health-Related Quality of Life; HSP70, heat shock protein 70; HADS/HADS-A/HADS-D, Hospital Anxiety and Depression Scale/-Anxiety subscale/-Depression subscale; IBQ, Illness Behaviour Questionnaire; IF, immunofluorescent; IMET-IS, IMET – Impairments in Sexuality; IC, indeterminate colitis; ICD/ICD-CM, International Classification of Diseases/-Clinical Modification; IBD, inflammatory bowel disease; IBDQ, Inflammatory Bowel Disease Questionnaire; IBS, irritable bowel syndrome; LCAI, Lichtiger Colitis Activity Index; MHI-5, Mental Health Index-5; MFI, Multidimensional Fatigue Inventory; ORLS, Oxford Record Linkage Study; PHQ, Patient Health Questionnaire; PCDAI, Paediatric Crohn’s Disease Activity Index; PUCAI, Paediatric Ulcerative Colitis Activity Index; PHCS, Perceived Health Competence Scale; PSQ, Perceived Stress Questionnaire; PGA, Physician Global Assessment; PMN, polymorphonuclear cells; PWB, Psychological Well-Being Manifestations Scale; QOL, Quality of Life; RFIPC, Rating Form of IBD Patient Concerns; RLC, Holmes Recent Life Changes; SCARED, Screen for Child Anxiety-Related Disorders; SF, Short-Form Health Survey; SCCAI, Simple Clinical Colitis Activity Index ; SSL-I, Social Support List – Interactions; STAI, State-Trait Anxiety Inventory; SQLS, Stoma Quality of Life Scale; SCID, Structured Clinical Interview for Axis-I DSM-IV Disorders; SNDT, Supportive Non-Direct Therapy; SCL, Symptom Distress Checklist; TAS, Toronto Alexithymia Scale; TRP, tryptophan; UC/UC-R/-D, ulcerative colitis/in remission/with depression; ZDRS, Zung Depression Rating Scale.
Appending number on abbreviations in table, if present, indicates number of items on test
Anxiety/depression in paediatric IBD
Increased risk for psychiatric disorders is not unique to adults with IBD; adolescents and children with IBD are also reported to have increased risk of anxiety and depression. In a large paediatric patient study; Loftus et al. (Reference Loftus, Guerin and Yu46) compared 2144 paediatric patients with CD to 10 720 age- and sex-matched controls. As with the adult studies, young patients with CD were found to be at greater risk of developing persistent anxiety disorders and depression. Interestingly, they found steroids to be a risk factor for developing anxiety disorders, however, even after controlling for this, CD itself was found to be a risk factor for mood disorders. Their analysis of prescription drug types suggest that the psychiatric conditions observed in the CD patients are being managed to some degree, as the usage of anti-depressants, anti-psychotics, anxiolytics, mood stabilisers and benzodiazepines was higher in CD patients compared with controls (Reference Virta and Kolho35). Szigethy et al. (Reference Szigethy, Youk and Benhayon34) have reported that three distinct profiles of depression exist in youths with IBD (n=217): mild depression (in 75% of participants) encompassing diverse, low-grade depressive symptoms and possessing the highest quality of life; somatic depression (in 19%) displaying severe fatigue, appetite change, anhedonia, decreased motor activity and depressed mood with concurrent high-dose steroid therapy and the highest IBD activity; and cognitive despair (in 6%) with the highest rates of self-reported depressive symptoms, ostomy placements and anxiety. Patients in the cognitive despair group reported IBD symptoms in the relative absence of inflammation and rated as the highest of the three profile groups on measures of morbid and suicidal ideations. As a result, they suggested that subgroup-specific interventions may be needed when treating depression in youths with IBD.
Anxiety/depression in active IBD versus remission
In a small case-control study which included healthy controls and CD patients with active disease, investigators reported increased anxiety and depression during active IBD (Reference Calvet, Gallardo and Coronas23). A third group of CD patients, being treated with a thiopurinic immunomodulator, was also included. Interestingly, thiopurinic-induced remission restored psychological well-being to normal range in CD patients. In a more recent retrospective study, IBD patients treated with anti-tumour necrosis factor (TNF)-α antibodies (infliximab, adalimumab or certolizumab) or immunomodulator therapy (methotrexate or azathioprine) also had significant improvements in depressive symptoms (Reference Horst, Chao and Rosen28). Furthermore, Guloksuz et al. (Reference Guloksuz, Wichers and Kenis38) reported changes in depression scores in patients with CD at 2, 4 and 8 weeks after an infliximab infusion (see Table 1). In contrast, a paediatric study carried out in the United States failed to show an effect of infliximab infusion on depression scores in children and adolescents with CD (Reference Clark, Srinath and Youk24).
When taken together these studies suggest that the risk of psychological manifestations is increased during the active phase of IBD, however, the results for patients during remission remain unclear. Maconi et al. (Reference Maconi, Gridavilla and Vigano32) reported that anxiety/depressive symptoms were present in 36.9% of CD patients in remission and strikingly that 58% of these patients were not currently receiving treatment for psychological illness. Similarly, Knowles et al. (Reference Knowles, Wilson and Wilkinson39) reported that of CD patients with anxiety (48%) and depression (42%) only 20% and 31%, respectively, are receiving psychological care. Besharat et al. (Reference Besharat, Amiriani, Roshandel, Besharat, Semnani and Kamkar44) also reported high rates (32%) of depressive characteristics in a group of Iranian IBD patients and patients with indeterminate colitis, despite all patients being in remission at the time of the study. This observation is echoed in an Australian cohort where despite 74% being in remission 96% had significant disease-related concerns, and symptoms of depression and anxiety were reported by 21% and 40%, respectively (Reference Keeton, Mikocka-Walus and Andrews29). In slight contrast to some studies mentioned previously, Iglesias et al. (Reference Iglesias, Barreiro de Acosta and Vazquez47) reported that a cohort of CD patients in remission for at least 6 months on infliximab therapy were shown to have increased anxiety and a decreased frequency of depression. This study concluded that despite clinical remission a significant number of CD patients present with anxiety or depressive symptoms, and that those CD patients in remission would benefit from psychological support.
Anxiety/depression in UC versus CD
It is now widely reported that no psychological differences between UC and CD exist (Reference Goodhand, Wahed, Mawdsley, Farmer, Aziz and Rampton21,Reference Ben Thabet, Charfi and Mnif26,Reference Long, Kappelman, Martin, Chen, Anton and Sandler31,Reference Schuman, Graef, Janicke, Gray and Hommel41,Reference Selinger, Lal and Eaden42). A recent study on the effectiveness of immunosuppressive therapy on depression in IBD reports differences in prevalence of moderate to severe depression depending on IBD diagnosis (51% CD and 18% UC) (Reference Horst, Chao and Rosen28). Furthermore, differences have been reported in the type of anxiety observed between UC and CD patients (Reference Addolorato, Mirijello and D’Angelo22) and in the risk of psychiatric factors (anxiety and depression) on health-related life satisfaction in CD compared with UC (Reference Janke, Klump, Gregor, Meisner and Haeuser51) (see Table 1).
Impact of psychological symptoms on the development/course of IBD
GI and psychological pathologies: cause and effect
An important question surrounding comorbidity of psychological symptoms with IBD is how these may be linked and whether one may predispose to the other. Although there has been a substantial amount of literature on the prevalence of depression and anxiety in IBD, less investigation has been carried out into the effect of such symptoms on the development of IBD or on the course of IBD. This may be due to the longitudinal and more protracted nature of this kind of study. Kurina et al. (Reference Kurina, Goldacre, Yeates and Gill55) carried out an extensive analysis of general hospital admissions in southern England between 1963 and 1999, to determine whether patients suffering from IBD had a greater rate of developing depression than would be expected by chance, and whether depression or anxiety preceded or succeeded the diagnosis of IBD. Results showed that with both CD and UC there is a greater chance of suffering from depression, but that in UC the depression is usually diagnosed in the year before onset of the disease symptoms, whereas in patients with CD the depression followed the diagnosis of the disease. Therefore, they suggest that the onset of depression in UC might be causally related to UC, a result of living with an undiagnosed bowel condition. For CD they suggest that depression might be a result of the disease symptoms or treatment of the illness. Cawthorpe and Davidson (Reference Cawthorpe and Davidson27) also found that neuroses or depressive disorders were most likely to arise before UC for men and women. They suggest that psychotropic medication used to treat anxiety and depression may play a role in the aetiology of UC. Concerning paediatric literature on this topic, a 2011 study which analysed medical and prescription claims of children with CD and patient controls observed a 74% increased risk of developing an anxiety disorder after CD diagnosis with an increased risk of developing persistent anxiety or depression following diagnosis and a significantly greater likelihood of being prescribed psychotropic medication (Reference Loftus, Guerin and Yu46).
Walker et al. (Reference Walker, Ediger and Graff50) investigated the lifetime risk of depression in the Manitoba IBD patient cohort (Canadian IBD cohort), and carried out a long-term analysis of these patients over 12 months. They report a higher lifetime risk of depression and a possible higher lifetime risk of some anxiety disorders in IBD patients versus a general Canadian population group. In the majority of patients with lifetime anxiety or depression, the psychological disorders preceded the diagnosis of IBD. Contrary to Kurina et al. (Reference Kurina, Goldacre, Yeates and Gill55), Ananthakrishnan et al. (Reference Ananthakrishnan, Khalili and Pan37) found that depressive symptoms increase the risk for CD, but not UC, among women. The reasons for the differences in outcome between these studies are unclear. Further research into the potential impact of psychological disorders on the development of IBD is needed.
Risk of relapse and effects of psychological treatment
A small number of studies have investigated the influence of anxiety or depression on the risk of relapse in IBD. In a study of 112 patients with inactive IBD, Vidal et al. (Reference Vidal, Gomez-Gil and Sans48) reported that neither depression nor anxiety increased the risk of relapse in UC or CD patients. Langhorst et al. (Reference Langhorst, Hofstetter, Wolfe and Hauser40) also failed to demonstrate a predictive effect of depression on the risk of relapse in patients with UC. This is contradictory to two older studies where BDI scores were predictive of future changes in IBD activity (Reference Mardini, Kip and Wilson53,Reference Mittermaier, Dejaco and Waldhoer54). In a paediatric study of children and adolescents with IBD, Szigethy et al. (Reference Szigethy, Youk and Benhayon34) reported greater disease activity in depressed compared with non-depressed youth with IBD. Persoons et al. (Reference Persoons, Vermeire and Demyttenaere52) also reported decreased remission rates in patients with a major depressive disorder.
Goodhand et al. (Reference Goodhand, Greig and Koodun45) have reported improvement in IBD (see Table 1) in patients who have been prescribed anti-depressants, compared with matched patients who did not receive treatment for depression. In another study by Szigethy et al., this group examined the effect of therapy on depressed youth with IBD (Reference Szigethy, Bujoreanu and Youk33). They found that cognitive behavioural therapy and supportive non-direct therapy caused an improvement in HRQOL and psychosocial functioning, and were associated with an improvement in IBD activity over time (see Table 1). In a recent study concerning psychological intervention for patients with a range of GI disorders, including IBD, functional bowel disorder, dyspepsia and oesophageal symptoms, Kinsinger et al. (Reference Kinsinger, Ballou and Keefer30) reported that psychological intervention can reduce healthcare burden (see Table 1). These studies suggest that psychological assessment may help to identify patients at risk of disease exacerbation or decreased rates of remission and may be an effective way to improve HRQOL. Importantly, these factors suggest that treating the psychological symptoms could be beneficial in terms of the overall course and management of IBD.
Effects of stress on risk of relapse
As well as anxiety and depression, short-term and long-term stress may influence the course of IBD. Evidence to suggest bidirectional communication between the gut and brain is emerging indicating that psychological stress and/or depression has negative implications for normal gut function. It is also hypothesised that stress may be a risk factor for relapse in patients suffering from IBD, although this remains controversial with some studies reporting no effect of stress on development of IBD or increased risk of relapse (Reference Vidal, Gomez-Gil and Sans49,Reference Li, Norgard, Precht and Olsen56). However, Langhorst et al. (Reference Langhorst, Hofstetter, Wolfe and Hauser40) found that short-term stress, but not long-term stress, was predictive of relapse in UC patients. The impact of life-event stress on UC, but not CD, was highlighted after the Great East Japan Earthquake in 2011 (Reference Shiga, Miyazawa and Kinouchi43). Results from 12 hospitals, found that UC patients activity scores increased significantly in the 2 months following the earthquake. Dietary changes and anxiety regarding family finance were independent predictors of relapse.
Immunologic mechanisms underlying psychological disturbance in IBD
Inflammatory origins of mood disorders
Mounting evidence indicates that inflammation plays a critical role in the pathophysiology of mood disorders. Patients with schizophrenia, major depression and bipolar disorder have been shown to have elevated levels of pro-inflammatory cytokines (Reference Goldsmith, Rapaport and Miller57). Reciprocally, many inflammatory conditions including IBD, rheumatoid arthritis, psoriasis, cardiovascular disease and diabetes (Reference Margaretten, Julian, Katz and Yelin58–Reference Andreoulakis, Hyphantis, Kandylis and Iacovides61) have been linked to a higher risk of mood disorders. Evidence for this link is also found in studies of cancer patients and hepatitis C patients receiving immune-based therapy. These patients display increases in depressive tendencies while receiving interferon or interleukin-2 treatment, which subside once treatment finishes (Reference Bonaccorso, Marino, Biondi, Grimaldi, Ippoliti and Maes62,Reference Capuron, Ravaud and Dantzer63). Furthermore, both clinical and preclinical studies have shown that the induction of a pro-inflammatory state in otherwise healthy subjects results in poor mood and ‘sickness behaviour’; a behavioural phenotype resembling depression with symptoms including lethargy, anxiety, social withdrawal, anhedonia and anorexia (Reference Grigoleit, Kullmann and Wolf64–Reference Eisenberger, Inagaki, Mashal and Irwin67). Though they share many symptoms and are both thought to have a basis in inflammation sickness behaviour is distinct from clinical depression. Sickness behaviour is evolutionarily intended to confer benefit by allowing for rest and isolation thus conserving energy, enabling an effective inflammatory response and preventing the spread of infection to others. It has been proposed that for clinical depression to occur there is a transition from sickness behaviour resulting in sensitisation of immune–inflammatory pathways, progressive damage by oxidative and nitrosative stress, and an autoimmune response directed against self-epitopes with the latter processes leading to neural tissue damage and functional and cognitive artefacts over repeated depressive episodes (Reference Maes, Berk and Goehler68). Considering that IBD is a lifelong disorder involving chronic relapsing and remitting inflammation and activation of oxidative and nitrosative pathways (Reference Zhu and Li69), it is likely that the depressive behaviour observed in IBD patients is not simply sickness behaviour but a comorbid depression (Reference Maes, Kubera, Obuchowiczwa, Goehler and Brzeszcz70). Furthermore, sickness behaviour is evolutionarily intended as an adaptive response to sickness, whereas comorbid depression worsens the original sickness as has been observed in IBD patients (Reference Szigethy, Youk and Benhayon34,Reference Persoons, Vermeire and Demyttenaere52–Reference Mittermaier, Dejaco and Waldhoer54), while treatment for depressive symptoms improves IBD course (Reference Szigethy, Bujoreanu and Youk33,Reference Goodhand, Greig and Koodun45). In response to the above findings the use of anti-inflammatories as an adjunct to conventional therapy for depression has been explored and suggests a beneficial effect though further research is required in this area (Reference Köhler, Benros and Nordentoft71). Potential mechanisms for the inflammatory induction of behavioural changes may include effects of cytokines on hypothalamic–pituitary–adrenal (HPA) axis dysregulation, monoamines and the kynurenine pathway, over-activation of microglia, impairments in neuroplasticity, and structural and functional changes in the brain.
Factors influencing anxiety and depression in IBD
Some socioeconomic/environmental/physiological factors such as education, socioeconomic status, gender, diet, pain, perceived stress, etc. may be predictive of or have an effect on psychological disturbance in IBD and are worth studying to increase our understanding of disease pathogenesis and psychological comorbidity. These factors may account for some of the variation observed across studies of depression and anxiety in IBD and are noted in Table 1 where applicable.
Various studies have observed that lower socioeconomic status and lower educational level are associated with depression and anxiety in IBD patients (Reference Schuman, Graef, Janicke, Gray and Hommel41,Reference Ennaifer, Elleuch and Cheikh72,Reference Nahon, Lahmek and Durance73). Such patients are also shown to have lower HRQOL than the general population, however, it is difficult to draw conclusions from this link as this may be a result of other non-disease-related factors (Reference Sainsbury and Heatley74).
Generally, there is little difference in IBD occurrence between men and women (Reference Zelinkova and der Woude75). However, gender does appear to be linked to differences in psychosocial manifestations of the disease. The majority of studies indicate that female gender is a predictor of anxiety and depression in IBD (Reference Maconi, Gridavilla and Vigano32,Reference Ennaifer, Elleuch and Cheikh72,Reference Panara, Yarur and Rieders76). Females are also believed to be more susceptible to the impact of IBD on HRQOL (Reference Casellas, Lopez-Vivancos, Casado and Malagelada77) potentially due to increased symptom perception in women (Reference Hauser, Tkalčić, Stimac, Milic and Sincic78). Females with IBD are also more likely to have IBS-like symptoms concurrent with IBD (Reference Berrill, Green, Hood and Campbell79), greater levels of fatigue (Reference Norton, Czuber-Dochan and Bassett80), and show a higher incidence of mood swings among those with CD (Reference Lima, Ribeiro and Chebli81), all of which may impact on HRQOL and psychological health.
Although diet is not a causative factor in IBD it is thought to be a potential trigger for IBD flares, and it is believed that the Westernised diet rich in processed foods is a factor in the increased incidence of the disease in these regions (Reference Hou, Abraham and El-Serag82). A modified diet limiting excess fat, carbohydrates, fibre and lactose, and encouraging intake of pre- and probiotic foods may be helpful as an adjunct therapy in IBD in decreasing symptoms and reducing medication requirements (Reference Olendzki, Silverstein, Persuitte, Ma, Baldwin and Cave83). Although it is still unclear whether dietary modification would be therapeutic in the psychological aspects of IBD, considering the link between diet, stress and the influence of gut microbiota on mood it is reasonable to think that this may be beneficial and will be addressed further later in this review.
The literature indicates that active IBD is associated with an increase in psychological manifestations (Reference Clark, Srinath and Youk24,Reference Long, Kappelman, Martin, Chen, Anton and Sandler31) with disease severity being an independent predictor of depressive symptoms (Reference Schuman, Graef, Janicke, Gray and Hommel41,Reference Panara, Yarur and Rieders76). UC patients with pain have been shown to have significantly higher depression scores than UC patients without pain (Reference Deberry, Bielefeldt, Davis, Szigethy, Hartman and Coates84), with higher pain scores being an accurate predictor of depression in both UC and CD (Reference Deberry, Bielefeldt, Davis, Szigethy, Hartman and Coates84,Reference Srinath, Goyal and Zimmerman85). As well as pain, the nature of other symptoms experienced by IBD patients can be extremely stressful. As will be discussed in greater detail later in this review, stress can affect visceral sensitivity, gut motility and the immune system in IBD, and as previously noted may be a trigger for IBD flares (Reference Langhorst, Hofstetter, Wolfe and Hauser40). Perceived stress has been associated with mood disturbance in both UC and CD (Reference Goodhand, Wahed, Mawdsley, Farmer, Aziz and Rampton21) indicating a link between stress, symptom-related or otherwise, and psychological disturbance in IBD.
Inflammation and depression in IBD
Despite the recent surge in psychoneuroimmunology research, there is a lack of investigation into the inflammatory mediators and mechanisms underlying psychological disturbances during active IBD. There may be psychoneuroimmunological components that predispose some people to the development of UC. Vlachos et al. (Reference Vlachos, Barbatis and Tsopanomichalou36) assessed levels of constitutive and inducible heat shock protein 70 (HSP70) at various sites in the colon of UC patients. They found that inducible HSP70 was strongly expressed in polymorphonuclear (PMN) cells in the colonic mucosa of the majority of patients. They also report that the induction of HSP70 significantly correlated with anxiety and depression scores in various psychometric tests, including HADS-D, STAI and Zung Depression Rating Scale, and with the Rachmilewitz Clinical Activity Index but not with HADS-A scores. This group suggest HSP70 induction in PMN cells as a possible biomarker for depression and anxiety in UC.
In addition to clinical investigations, experimental models of IBD in animals allow for the study of interactions between the gut and the brain during and in recovery from colitis in order to decipher the mechanisms by which IBD interacts with the central nervous system (CNS) and to develop potential therapies to best manage comorbid symptoms. Due to the paucity of biomarkers (molecular and cellular) reported in human studies, arising from the difficulty in assessing impact of stress and/or psychological disturbance in IBD in a clinical setting, data available from animal models are the best available source to obtain insight into gut–brain interactions underlying comorbidity in IBD.
Animal models of IBD
As the exact aetiology of IBD is still largely unknown there are many possible factors which contribute to different aspects of the pathophysiology of the disease including immune system dysfunction, dysregulation of the microbiota, genetics, inflammation and oxidative stress. Animal models of IBD have been developed to allow for investigation of aetiological factors in terms of understanding the mechanisms of disease pathogenesis and developing therapeutic strategies for intervention. These models may be grouped into chemical- or microbial-induced models; spontaneous models; genetically engineered or transgenic models; and adoptive transfer (T-cell) models [for review see (Reference Goyal, Rana, Ahlawat, Bijjem and Kumar86)].
Despite the range of models of IBD, the main body of behavioural and brain research has been carried out in the chemically induced models, particularly the dextran sulphate sodium (DSS) and trinitrobenzenesulphonic acid (TNBS) models. Okayasu et al. (Reference Okayasu, Hatakeyama, Yamada, Ohkusa, Inagaki and Nakaya87) were the first to describe the DSS-induced colitis model which involves oral administration of DSS in the drinking water of the animals leading to the development of acute and chronic colitis. Gaudio et al. (Reference Gaudio, Taddei and Vetuschi88) assessed the structural, ultrastructural, immunohistochemical and clinical aspects of DSS colitis in Sprague-Dawley rats in both acute and chronic DSS-induced colitis and suggest that the DSS model is more representative of UC than CD. Indeed, DSS-induced colitis is histologically characterised by infiltration of inflammatory cells, crypt loss and extensive mucosal erosions, with predominance in the distal portion of the large intestine. Occasionally, crypt abscesses and regenerated epithelium are also seen. The TNBS model of colitis was first reported by Morris et al. (Reference Morris, Beck, Herridge, Depew, Szewczuk and Wallace89). This model of IBD involves a single enema of the toxin TNBS in an ethanol solution. TNBS-induced colitis results in a T-cell (TH1)-mediated inflammatory response which is described as CD-like in nature (Reference Bouma and Strober90). Unlike the DSS model which predominantly involves the distal colon, TNBS can induce a more widespread colitis, involving macroscopic ulceration of the large intestine with varying severity, strictures of the lumen and fistulae formation. Both of these models result in immune activation in the gut, are histologically representative of IBD, and despite their limitations in terms of studying disease aetiology are valuable tools for studying mechanisms of IBD pathogenesis. Both models are simple, inexpensive, reproducible and valid for examining the potential interaction between intestinal immune activation and the CNS.
Other animal models used to investigate the association between GI disturbance and psychological manifestations include infection models by which bacteria such as Citrobacter rodentium and Campylobacter jejuni, which colonise and disrupt tissue in the GI tract of mice and are effective models of acute colitis. C. rodentium uses attaching and effacing lesions to colonise the GI tract resulting in ulcerative intestinal lesions, reduced barrier integrity, production of pro-inflammatory cytokines and manifesting as weight loss and diarrhoea (Reference Nell, Suerbaum and Josenhans91). C. jejuni produces and secretes toxins to aid in its intestinal colonisation and increase mucosal barrier damage, translocation of commensal bacteria across the intestinal epithelium and induction of a Th1 immune response (Reference Mansfield, Bell and Wilson92). Stress-induced models may be used to study the effects of psychological stress on GI function. Maternal separation as a model of early life stress (Reference O’Mahony, Hyland, Dinan and Cryan93), chronic subordinate colony housing (Reference Reber, Birkeneder and Veenema94,Reference Reber, Obermeier, Straub, Veenema and Neumann95) and overcrowding stress (Reference Vicario, Guilarte and Alonso96) have been shown to induce spontaneous GI dysfunction or to increase susceptibility to chemically induced colitis. Though not wholly valid for studies on IBD specifically these models demonstrate the link between stress and GI disturbance and are functional tools in assessing IBS-like symptoms.
CNS disturbances in models of IBD
Anxiety-/depression-like behavioural alterations
In a recent study, Heydarpour et al. (Reference Heydarpour, Rahimian and Fakhfouri97) show an increase in immobility in the forced swimming test (FST), a depression-related behaviour in mice 3 days post-TNBS injection. This effect is attenuated using a specific inducible nitric oxide synthase (iNOS) inhibitor (aminoguanidine) administered 30 min before the FST indicating the potential involvement of the nitric oxide pathway in the induction of this behaviour. In the DSS model of colitis, Chen et al. (Reference Chen, Winston and Fu98) performed anxiety and depression-related behavioural tests in rats following a DSS (5%) colitis induction period and reported that DSS exposure caused a decrease in open arm entries and time spent in the open arm of the elevated plus maze (EPM) indicating anxiety, with an increase in immobility time in the FST indicating learned helplessness. DSS exposure also decreased sucrose preference in the sucrose preference test indicating reduced responsivity to a rewarding stimulus, anhedonic behaviour symptomatic of depression, and reduced social interaction between animals suggestive of social avoidance and withdrawal. Interestingly, this study also found that the anxious- and depressive-like behaviours were reversed by prolonged desensitisation of transient receptor potential vanilloid 1 (TRPV1)-expressing colonic afferent neurons using a colonic infusion of the potent analogue of capsaicin and activator of TRPV1, resiniferatoxin. In an earlier study, Messaoudi et al. (Reference Messaoudi, Desor, Grasmuck, Joyeux, Langlois and Roman99) analysed lever pushing behaviour in an aversive light stimulus avoidance test in rats exposed to TNBS. They found that colitic rats had a lower number of total active lever pressings and did not discriminate the active lever from the inactive one. This behavioural disturbance was attributed to TNBS-induced pain as morphine returned lever pressing to control levels. Despite not being suggestive of depression or anxiety this highlights the importance of accounting for the potential influence of pain on behavioural disturbances in these animal models. Painsipp et al. (Reference Painsipp, Herzog, Sperk and Holzer100) analysed female and male mouse behaviour in the EPM, open field (OF) and FST on days 8, 9 and 11, respectively, of an 11 day DSS (2%) exposure protocol. Colitis had some behaviour modulating effects which were sex dependant: male mice spent significantly less time in the open arms of the EPM indicative of anxiety-like behaviour, whereas female rats had increased immobility in the FST indicative of a depressive-like phenotype. Lyte et al. (Reference Lyte, Li, Opitz, Gaykema and Goehler101) examined anxiety-like behaviour on a hole-board OF apparatus in mice infected with C. rodentium, a murine model of IBD. Male mice were tested 7–8 h post-infection and results provide evidence for an anxiety-like phenotype: decreased exploration of the inner zone of the OF, decreased number of pokes into the holes as well as a preference for the first corner hole compared with control mice. A more recent study using the same colitis mouse model assessed anxiety-like behaviours in the light/dark box and found no behavioural alterations at 10 days post-infection when inflammation was at its peak (Reference Gareau, Wine and Rodrigues102). However, Emge et al. (Reference Emge, Huynh and Miller103) using a DSS colitis model, reported that during active inflammation (8 days post-DSS) mice exhibited anxiety-like behaviour in the light/dark box, whereas recognition memory was impaired in the novel object recognition test. These behavioural alterations had normalised by 14 days post-DSS when the colitis had resolved. In an investigation by Bercik et al. (Reference Bercik, Park and Sinclair104) mice who received 3% DSS in drinking water during three 1-week cycles demonstrated increased anxiety in the step-down test compared with controls.
Sickness behaviour versus depressive-like behaviour
Considering the moderate physiological effects of chemically induced and infection-induced colitis the validity of behavioural tests, particularly those which passively measure depressive-like behaviour during acute sickness should be questioned. In behavioural tests such as OF and FST where lack of activity may be interpreted as anxiety- or depressive-like behaviour one should not discount the impact of abdominal pain being experienced by animals with active colitis. Diarrhoeal symptoms associated with colitis may affect grooming behaviour and may also impact social interaction tests. Few studies, if any, report disease activity scores or take disease activity into account in behavioural tests of colitic animals with many studies also not reporting the timepoint during colitis at which each behavioural test was carried out. In a study of lipopolysaccharide (LPS)-induced alterations in Fos expression in the brain Frenois et al. (Reference Frenois, Moreau and O’Connor105) argue that there is a functional difference in cytokine-induced sickness behaviour observed when LPS-sickness is at its peak (decreased motor activity 6 h post-LPS i.p. injection) and the cytokine-induced depressive-like behaviour observed (increased immobility in FST at 24 h and decreased sucrose preference at 24 and 48 h post-LPS) when LPS-induced sickness was minimal (and motor activity and food/fluid intake had returned to normal). At 24 and 48 h post-LPS increased cellular activity was measured by Fos labelling in brain structures including the amygdala, hippocampus and hypothalamus, however, at 6 h post-LPS no Fos labelling was observed in these regions. This points to an underlying difference in sickness behaviour and depressive-like behaviour induced by a systemic inflammatory insult. It would therefore be of interest to measure behavioural abnormalities in animal models of colitis during the recovery period when IBD-like symptoms have ceased.
Blood brain barrier (BBB) permeability
The BBB is a tightly controlled diffusion barrier which regulates the transport of molecules between the periphery and CNS. Endothelial cells of the BBB are non-fenestrated and have more extensive tight junctions acting as a protective barrier against pathogens and neurotoxic substances, whereas allowing influx of essential nutrients and neurotransmitters (Reference Mayhan and Arrick106). Systemic inflammation can disrupt the BBB and has been linked to syndromes such as sickness behaviour and delirium, and neurological disorders such as Alzheimer’s disease and multiple sclerosis (Reference Varatharaj and Galea107). The presence of intestinal inflammation in IBD models and the increase in permeability of the gut–blood barrier has been linked to an increase in the permeability of the BBB. Hathaway et al. (Reference Hathaway, Appleyard, Percy and Williams108) investigated potential disruption to the BBB in rabbits exposed to TNBS. Barrier disruption was assessed following i.v. administration of low molecular weight fluorescein (MW 376 Da) or a higher molecular weight molecule fluorescein isothiocyanate (FITC)-dextran (MW 71 000 Da) 48 h post-TNBS administration. Results demonstrated a significant increase in the permeability of the BBB to fluorescein, however, no difference in permeability to the higher molecular weight FITC-dextran was found. In a later investigation they confirmed these findings and suggest that free radical damage is not responsible for the BBB disruption (Reference Hathaway, Percy and Williams109). More recently, Natah et al. (Reference Natah, Mouihate, Pittman and Sharkey110) further analysed the BBB disruption in Sprague-Dawley rats exposed to TNBS to determine the anatomical sites of the BBB disruption using sodium fluorescein (MW 376 Da) or IgG (MW 156 000 Da) as a marker of increased permeability. As per Hathaway et al. (Reference Bonaccorso, Marino, Biondi, Grimaldi, Ippoliti and Maes62,Reference Capuron, Ravaud and Dantzer63) they revealed an increased permeability to the low molecular weight sodium fluorescein but not to the larger IgG molecules. The regions of higher permeability were located at the circumventricular organs: specifically the organum vasculosum of the lamina terminalis, subfornical organ and median eminence during days 1 and 2 following TNBS administration.
Sans et al. (Reference Sans, Kawachi and Soriano111) measured expression of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) using a dual radiolabelled antibody technique in four different colitis models: IL-10−/− mice, Structured Clinical Interview for Axis-I DSM-IV Disorders mice reconstituted with CD45RBhigh T-cells, mice with DSS-induced colitis and rats with TNBS-induced colitis. VCAM and ICAM are endothelial CAMs of the immunoglobulin super family which are responsible for the adhesion of leucocytes in various inflammatory diseases. This study demonstrated that there is a significant increase in VCAM expression in the brain of all four models of colitis, which corresponded with colonic VCAM expression and colon weight. They also report that TNBS-induced colitis induces ICAM expression, although this is not the case in the DSS model. These changes were not associated with increased leucocyte infiltration to the brain and are not representative of BBB disruption, however, they provide further evidence of molecular alterations at the BBB following colonic inflammation.
The consequences of BBB disruption in inflammatory conditions such as IBD is that it leaves the CNS vulnerable to inflammatory mediators and gut-derived bacterial or viral antigens. In the case of IBD these inflammatory mediators are likely to be at higher concentrations in the circulation considering the increased permeability of the intestinal barrier in this condition.
Inflammatory mediators in the brain
Cytokines are soluble, regulatory proteins, released by immune cells, which act as intercellular mediators. They also have the ability to interact with the CNS either via the vagus nerve or by directly interacting with the BBB, thus providing a means of communication between the immune system and the brain. Cytokines are implicated in the pathogenesis of IBD, with a pivotal role in regulating intestinal inflammation and the clinical symptoms of IBD (Reference Neurath112). The use of TNF-α antagonists as a standard therapy for IBD highlights the crucial role of cytokines in this disease. Following peripheral immune activation cytokines can also be produced within neurons and glial cells in the brain and their involvement has been proposed in the pathophysiology of a number of psychiatric disorders including depression (Reference Leonard and Maes113) as discussed in a previous section.
TNBS model
Riazi et al. (Reference Riazi, Galic, Kuzmiski, Ho, Sharkey and Pittman114) investigated the influence of TNBS-induced colitis on hippocampal TNF-α concentrations and microglial activation in male Sprague-Dawley rats. They found an increase in both hippocampal TNF-α protein concentrations and microglial activation at 4 days post-TNBS administration, both of which had returned to basal concentrations at day 10. In a later study Medhi et al. (Reference Medhi, Prakash, Avti, Chakrabarti and Khanduja115) confirmed that a single enema of TNBS induces increases in circulating TNF-α concentrations which are paralleled by increased brain TNF-α protein concentrations. However, unlike the Riazi et al. (Reference Riazi, Galic, Kuzmiski, Ho, Sharkey and Pittman114) study the increase was still present at day 15 post-TNBS administration possibly due to differences in the strain of rat used (Reference Medhi, Prakash, Avti, Chakrabarti and Khanduja115). Wang et al. (Reference Wang, Yuan and Wang116) investigated the effect of TNBS-induced colitis on IL-6 expression in the brains of female Wistar rats at 3, 7, 14, 21 and 28 days post-enema. They report an increase in IL-6 messenger RNA (mRNA) expression and IL-6 protein concentration in the hypothalamus and cerebral cortex, which peaks at 7 days post-enema. Concentrations of brain IL-6 were also increased in mice exposed to TNBS, however, peak IL-6 concentrations were at 2 days post-enema and remained increased 7 and 15 days post-TNBS administration (Reference Baticic, Detel, Kucic, Buljevic, Pugel and Varljen117). In their study they also report a decrease in the concentrations of the anti-inflammatory cytokine IL-10 at 2 and 7 days post-TNBS. Alhouayek et al. (Reference Alhouayek, Lambert, Delzenne, Cani and Muccioli118) also reported increased central inflammatory cytokine expression following TNBS-induced colitis. Three days post-TNBS administration there was an increase in IL-1β, TNF-α and monocyte chemoattractant protein 1 mRNA expression in the brains of C57BL6 mice, which was associated with an increase in circulating endotoxin concentrations attributable to extensive histological damage to the colon.
DSS model
Villaran et al. (Reference Villaran, Espinosa-Oliva and Sarmiento119) reported a significant increase in TNF-α, IL-6, IL-1β and iNOS mRNA expression in the substantia nigra of male Wistar rats during acute DSS-induced colonic inflammation. More recently, Reichmann et al. (Reference Reichmann, Hassan, Farzi, Jain, Schuligoi and Holzer120) measured levels of IL-1β, IL-6, IL-17A, IL-18, TNF-α and growth-regulated oncogene (GRO)-α in the circulation and in the hypothalamus, hippocampus and amygdala of mice following 7 days DSS administration (2%) and in combination with water-avoidance stress (WAS). A prolonged immobility in C57BL/6N mice with DSS-induced colitis during WAS was associated with brain region-dependent alterations in the expression genes associated with energy homoeostasis [neuropeptide-Y (NPY), NPY receptor Y1], stress pathway activation [corticotropin-releasing factor (CRF), CRF1 receptor and glucocorticoid receptor] and neurogenesis (brain-derived neurotrophic factor). They report increased GRO-α in the hypothalamus as a result of DSS alone. The combination of DSS and WAS induced increases in IL-6 in all three brain regions and in GRO-α in the hippocampus and hypothalamus. Cytokine concentrations in the brain did not correlate with plasma cytokine levels suggesting that WAS is required to effect a brain inflammatory response in DSS-exposed mice. The authors propose that alterations in gut–brain signalling may be responsible for the observed behavioural changes in response to stress in DSS animals. As well as demonstrating a decrease in hippocampal neurogenesis in DSS (3%)-treated mice Zonis et al. (Reference Zonis, Pechnick and Ljubimov169) also show an increase in circulating IL-6 and an increase in hippocampal IBA1 and GFAP, markers for activated microglia and astrocytes, respectively, in acute colitis. Following three more rounds of DSS increases were observed in hippocampal TNF-α, IL-1β and GFAP mRNA expression and IBA1 and IL-6 protein simultaneous to the reductions in neurogenesis.
Effects of cytokines on the brain
Apart from their primary role in the inflammatory immune response cytokines have the ability to interact with the brain. Cytokine receptors located on the BBB allow for non-barrier-disruptive communication between the periphery and brain though in some cases cytokines may actually cross the BBB either via transporters or via a compromised BBB (Reference Varatharaj and Galea107). Diapedesis of leucocytes across the BBB may also lead to immune activation and cytokine production in the brain (Reference Becher, Spath and Goverman121). Microglia can respond to these cytokine signals in paracrine and autocrine fashion to facilitate tissue repair, initiate immune responses and recruit immune cells, however, sustained activation of microglia can result in neurotoxicity and production of reactive oxygen species (Reference Hanisch122). Astrocytes also communicate using the cytokine network to influence immune responses in the CNS and there is also evidence to suggest that activation of astrocytes by inflammatory mediators modulates astrocyte signalling thereby influencing synaptic and neural function and potentially playing a role in the behavioural effects of inflammation such as sickness behaviour and depression (Reference Sofroniew123). Cytokines can directly affect neuronal activity, influencing neuronal excitability neuronal plasticity, neuronal development and synaptogenesis (Reference Dantzer, O’Connor, Freund, Johnson and Kelley124–Reference Stellwagen and Malenka126). Cytokines have been shown to affect neurotransmitter metabolism, specifically glutamate, serotonin and dopamine, in brain regions associated with emotional regulation namely the nucleus accumbens, amygdala and hippocampus (Reference Miller, Haroon, Raison and Felger127). Cytokines can also affect the kynurenine pathway in the brain by stimulating indoleamine 2,3-dioxygenase (IDO) production. As the IDO enzyme is responsible for conversion of tryptophan to kynurenine the amount of tryptophan available for serotonin production is decreased and depressive-like behaviour is observed (Reference O’Connor, Andre and Wang128). Pro-inflammatory cytokines may also increase kynurenine-3-mono-oxygenase enzyme activity. This enzyme degrades kynurenine into 3-hydroxykynurenine, shifting the kynurenine pathway from neuroprotection towards neurotoxicity with the production of neurotoxic metabolites and excitotoxicity. Cytokines can also influence the HPA axis impacting glucocorticoid receptor function, HPA feedback regulation and causing activation of the HPA axis (Reference Dunn129,Reference Miller, Pariante and Pearce130). HPA axis activation results in an increase in glucocorticoids which has been implicated in depression (Reference Pariante and Lightman131). [For a more detailed review of the role of pro-inflammatory cytokines in neuroinflammation and depression see Kim et al. (Reference Kim, Na, Myint and Leonard132).]
Regional patterns of neuronal activation
c-Fos is an immediate-early gene expressed following an action potential which is used to indirectly measure neuronal activity [for reviews see (Reference Kovacs133,Reference Okuno134)]. Original evidence of c-Fos activation in the nervous system following induction of colitis was published by Miampamba and Sharkey (Reference Miampamba and Sharkey135). Colitis was induced following a per-endoscopic injection of formalin and rats were euthanised 2 h later. Immunohistochemical analysis demonstrated a significant increase in c-Fos in the lumbosacral spinal cord, and in two circumventricular organs: nucleus of the solitary tract (NST) and area postrema. Treatment with the α-2-adrenoceptor agonist xylazine inhibited the colitis-related increase in regional c-Fos expression. A later study by Porcher et al. (Reference Porcher, Sinniger, Juhem, Mouchet and Bonaz136) extensively analysed the expression of c-Fos 2 h post-TNBS administration throughout the brain. They report significant increases in c-Fos immunostaining across a number of brain regions including brain nuclei involved in the autonomic, behavioural and neuroendocrine response to inflammation, in most circumventricular organs and in CRF pathways particularly the paraventricular nucleus (PVN) of the hypothalamus. At 6 h post-TNBS administration c-Fos mRNA expression in the PVN had completely returned to basal levels. Welch et al. (Reference Welch, Welch-Horan, Anwar, Anwar, Ludwig and Ruggiero138,Reference Welch, Anwar and Chang139) focussed on TNBS-induced c-Fos activation in a number of brain regions which are abnormal in autism spectrum disorder (ASD); periventricular grey, hypothalamic/visceral thalamic stress axes and cortical domains, and septal/preoptic/amygdalar brain areas. ASD is a complex, multifaceted neurodevelopmental disorder which is often linked to GI disturbance (Reference Hsiao137). Results from this study support previous evidence of increased c-Fos induction following experimentally induced colitis, however, here the results suggest prolonged neuronal activation (Reference Welch, Welch-Horan, Anwar, Anwar, Ludwig and Ruggiero138). In a later study, this group showed that subdiaphragmatic vagotomy did not inhibit the observed increase in c-Fos induction in the PVN of the hypothalamus, basolateral amygdala, central amygdala (CeA), and piriform cortex indicating the unlikely role of the vagus nerve in mediating the brain activation response in these regions (Reference Welch, Anwar and Chang139). However, a separate study which used C. jejuni infection to induce intestinal inflammation in mice showed c-Fos induction in vagal sensory ganglia and in the NST – the primary sensory vagal afferent nucleus indicating that intestinal inflammation signals to the brain via this nerve (Reference Goehler, Gaykema, Opitz, Reddaway, Badr and Lyte140).
The microbiota–gut–brain axis
Clinical IBD, gut microbiota and therapies
In terms of gut–brain axis research, the gut microbiota and its associations with brain and behaviour is currently one of the most promising areas of study. The gut microbiota comprises the largest collection of microorganisms in the body, existing in a symbiotic relationship with the host and in the colon reaching a concentration of 1011 or 1012 cells/g of luminal contents (Reference Dave, Higgins, Middha and Rioux141). Collectively, the human gut microbiota is thought to be composed of between 15 000 and 36 000 bacterial species (Reference Frank, St Amand, Feldman, Boedeker, Harpaz and Pace142). The major bacterial phyla found in the gut are Firmicutes and Bacteroidetes though other phyla including Actinobacteria, and Verrucomicrobia are also present (Reference Jandhyala, Talukdar, Subramanyam, Vuyyuru, Sasikala and Reddy143). The gut microbiota is believed to play a role in the pathogenesis of IBD. Dysbiosis of the commensal gut bacteria is commonly observed in IBD, generally as a decrease in diversity of Firmicutes and an increase in Proteobacteria (Reference Frank, St Amand, Feldman, Boedeker, Harpaz and Pace142), furthermore, many of the susceptibility genes for IBD are related to microbial recognition and processing (Reference Jostins, Ripke and Weersma144), and antibiotics are known to be effective in reducing IBD symptoms (Reference Nitzan, Elias, Peretz and Saliba145). This is also supported by the finding that germ-free (GF) mice do not develop severe colitis (Reference Sellon, Tonkonogy and Schultz146).
A strong link has been discovered between a healthy gut microbiota and satisfactory CNS functioning. The microbiota has been implicated in several neuropsychiatric disorders including depression, anxiety, autism and schizophrenia [for review see (Reference Sherwin, Sandhu, Dinan and Cryan147)]. The bacterial flora of the gut can communicate with the brain via vagal pathways and immune mediators as previously discussed, as well as by the production of microbial metabolites. In a healthy individual this bidirectional communication maintains host homoeostasis, whereas for an IBD patient, for example, this balance is disturbed with potential consequences for the CNS.
The imbalance observed in the gut microbiota of IBD patients presents a potential therapeutic target. Manipulation of the gut microbial flora can be achieved using probiotics, prebiotics or a combination of both (synbiotics). A probiotic a live microbial food supplement that beneficially affects the host by improving its intestinal microbial balance (Reference Fuller148). A prebiotic is a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth, activity, or both of one or a limited number of bacterial species already resident in the colon (Reference Gibson and Roberfroid149). Faecal microbiota transplantation (FMT), an extremely effective treatment for Clostridium difficile infection, is another method of gut microbiota manipulation being explored as a potential therapy for IBD patients. The procedure involves transplant of a faecal preparation from a healthy donor into the colon of the patient via naso-enteric tube, colonic enema or in capsule form. In C. difficile infection this works by restoring balance to the gut microbial environment. Although IBD is a more complicated condition with many genetic, environmental and immune factors at play, the restoration of a healthy gut microbiota would likely be advantageous at least for some patients.
Effects of microbiota modulation on IBD and brain function
To date, clinical studies investigating the effects of probiotics or prebiotics in IBD have focussed on the physiological symptoms of IBD itself. Probiotics (a preparation of live, beneficial bacteria) show efficacy in inducing remission and increasing remission times in UC (Reference Mallon, McKay, Kirk and Gardiner150), which was not the case in CD (Reference Rahimi, Nikfar and Rahimi151). Despite promising results in TNBS and DSS colitis, prebiotics (preparations of dietary nutrients which support the growth of beneficial host bacteria) have been tested in few clinical trials. Similar to probiotics, results show efficacy in reducing inflammation and inducing remission in UC (Reference Casellas, Borruel and Torrejon152–Reference Kanauchi, Suga and Tochihara154), whereas somewhat controversially reducing disease activity and inflammation in CD (Reference Benjamin, Hedin and Koutsoumpas155,Reference Lindsay, Whelan and Stagg156). Although no work has explored the potential therapeutic benefit for probiotics or prebiotics in reducing anxiety/depression specifically in IBD patients, this therapeutic strategy has been tested in other cohorts. Messaoudi et al. (Reference Messaoudi, Lalonde and Violle157) administered a probiotic mixture of Bifidobacterium longum and Lactobacillus helveticus for 30 days to both rats and healthy human volunteers and showed a decrease in anxiety-like behaviour in rats and a decrease in anxiety and depression scores in humans in the HADS and Hopkins Symptom Checklist (HSCL-90). Similarly Rao et al. (Reference Rao, Bested and Beaulne158) administered a strain of Lactobacillus casei to chronic fatigue patients resulting in a reduction in anxiety scores. In terms of prebiotics (Reference Schmidt, Cowen, Harmer, Tzortzis, Errington and Burnet159) administered a 3 week course of galacto-oligosaccharide (GOS) or fructo-oligosaccharide (FOS) prebiotic to healthy controls. The GOS resulted in a decrease in waking cortisol levels and decreased attentional vigilance to negative versus positive emotional stimuli compared with placebo. FOS had no effects in either test. Considering these encouraging data, the use of microbiota modulation by pre/probiotics as a simple, non-invasive therapy for the psychological as well as physiological effects of UC and potentially CD is an area which should be further explored in future. In terms of FMT as a treatment for IBD, a systematic review of the literature from case reports and cohort studies showed a modest increase in remission rates in IBD patients receiving FMT therapy (Reference Colman and Rubin160). More recently, two randomised, controlled trials have been carried out in UC patients with conflicting results suggesting a beneficial outcome that may be dependent on donor stool, route of administration, dosage, time since diagnosis and whether patients are also receiving immunosuppressive therapy (Reference Moayyedi, Surette and Kim161,Reference Rossen, Fuentes and van der Spek162). Interestingly, Irish researchers have recently postulated that FMT may be of therapeutic benefit in depression after showing that FMT from depressed human patients into microbiota-deficient rats induces a depressive phenotype in the rats with symptoms of anhedonia and anxiety (Reference Kelly, Borre, O’Brien and Patterson163).
Animal models of IBD and the gut microbiota
In animal studies the modulation of the microbiota has been used to alter behaviour. GF mice display decreased anxiety-like symptoms in the EPM, OF and light/dark box compared with specific-pathogen-free mice (Reference Heijtz, Wang and Anuar164). GI microbial infection and inflammation, including exposure to DSS-induced colitis, results in an increased anxiety-like profile (Reference Lyte, Li, Opitz, Gaykema and Goehler101,Reference Bercik, Park, Sinclair, Khoshdel, Lu and Huang165,Reference Goehler, Park, Opitz, Lyte and Gaykema166). Modulation of the gut microbiota using probiotics can alter the behavioural response. Anxiety and/or depression-related behaviours in the EPM and FST in Balb/c mice (Reference Bravo, Forsythe and Chew167) and in the FST in maternally separated rats (Reference Desbonnet, Garrett, Clarke, Kiely, Cryan and Dinan168) are rescued by probiotics. Probiotics also reduce anxiety-like behaviours induced in rats in response to DSS colitis (Reference Bercik, Park, Sinclair, Khoshdel, Lu and Huang165). This points to a potential therapy for neuropsychiatric disturbance in IBD patients. In an aforementioned study by Bercik et al. (Reference Bercik, Park and Sinclair104) results demonstrated that increased anxiety measured in the step-down test observed in DSS colitis mice was reversed by the probiotic B. longum NCC3001 without affecting gut inflammation (as measured by myeloperoxidase activity and histological scores). They found that the anxiolytic effect of B. longum was lost in mice vagotomised before the third cycle of DSS potentially due to the modulatory effects of the fermentation products of B. longum on enteric neuron excitability. In another previously mentioned behavioural study Emge et al. (Reference Emge, Huynh and Miller103) reported that the deficits in recognition memory and anxiety-like behaviour during active inflammation on day 8 post-DSS were ameliorated by administration of a probiotic mixture containing Lactobacillus rhamnosus and Lactobacillus helveticus (Reference Emge, Huynh and Miller103). This study also observed a significant decrease in c-Fos expression in the CA1 region of the hippocampus in mice at day 8 post-DSS which is similarly rescued by administration of the L. rhamnosus and L. helveticus probiotic combination. It has recently been observed that intestinal inflammation may impact upon hippocampal neurogenesis (Reference Zonis, Pechnick and Ljubimov169). Following four cycles of DSS (3%) in mice Zonis et al. (Reference Zonis, Pechnick and Ljubimov169) observed a downregulation in markers for stem/early progenitor cells, with a concomitant increase in p21, a suppressor of cell proliferation, indicating a reduction in neurogenesis in the hippocampus. As p21 can be stimulated directly by pro-inflammatory cytokines it has been proposed that this decrease in hippocampal neurogenesis may occur as a consequence of the increase in activated microglia and astrocytes observed in the hippocampus at the acute phase of colitis and in chronic colitis, respectively.
Influence of psychological stress on healthy gut function
The HPA axis represents a major axis of the neuroendocrine system that controls reactions to stress. Dysregulation of the HPA axis has been linked to a number of mood disorders including depression, anxiety and bipolar disorder. The HPA axis is regulated by CRF which is released centrally from the hypothalamus or peripherally from the adrenal cortex in response to stress. Central CRF promotes HPA axis activity via the adrenocorticotrophic hormone (ACTH) – glucocorticoid system and peripheral CRF directly influences stress-induced alterations in gut motility.
Effects of stress on the gut
Konturek et al. (Reference Konturek, Brzozowski and Konturek170) describes a number of stress-induced disturbances to normal GI physiology: including alterations to GI motility, GI secretion, GI mucosa and mucosal blood flow, intestinal microbiota and also increased visceral perception and intestinal permeability. Deng et al. (Reference Deng, Chen and Liu171) recently showed that levels of colonic cytokines (IL-6, IL-1β and IL-17A) and neutrophil infiltration in DSS-exposed rats are further increased by chronic unpredictable stress. Previous investigations have shown that when chronically stressed, animals can develop spontaneous inflammation in the bowel (Reference Reber, Birkeneder and Veenema94,Reference Wood, Peck and Tefend172). Reber et al. (Reference Reber, Birkeneder and Veenema94) examined the effect of chronic psychosocial stress on histological changes in the murine colon. Their results demonstrate that exposure to chronic subordinate colony housing leads to colonic inflammation resulting in macroscopic damage to the mucosal layers of the colon, and an increased secretion of pro- and anti-inflammatory markers by the mesenteric lymph node.
Milde and Murison (Reference Milde and Murison173) report decreased time to symptom expression in DSS rats previously exposed to restraint stress, and in a separate study involving electric shock pre-DSS exposure they report a sensitising effect of stress on later vulnerability to intestinal permeability (Reference Milde, Arslan, Overmier, Berstad and Murison174). The genotoxic agent azoxymethane predisposes mice to develop CRC when challenged with DSS. Peters et al. (Reference Peters, Grunwald and Rummele175) report increased risk of inflammation-related CRC when azoxymethane mice were also exposed to chronic subordinate colony housing. Review of maternal separation has also reported disturbances to gut function as a result of early life stress (Reference O’Mahony, Hyland, Dinan and Cryan93). These maternally separated rodents can be used as a model of IBS due to their IBS-like functional symptoms, they are also reported to have altered neurotransmitter activity in the enteric nervous system, GI immune dysregulation, increased intestinal permeability and disturbed intestinal microbiota. One caveat when studying the effect of psychological stress or maternal separation on models of colitis is that both result in increased permeability of the intestinal tract, therefore any enhanced colitic effect may be due to increased permeability to the DSS or TNBS themselves rather than due to altered immune activation, bacterial translocation or neuroendocrine function.
GI effects on the HPA axis
Due to the bidirectionality of the gut–brain axis not only will a disturbance such as stress influence the GI system but a disturbance in the GI system may also influence stress by activation of the HPA axis. In the Reichmann et al. (Reference Nahon, Lahmek and Durance73) study DSS treatment increased basal and post-stress (90 min) levels of circulating corticosterone – an index for increased HPA axis activity. Greenwood-Van Meerveld et al. (Reference Greenwood-Van Meerveld, Johnson, Schulkin and Myers176) studied the long-term effects of acute colitis on the expression of central CRF in rats. They found a significant increase in CRF mRNA expression in the PVN of the hypothalamus 3 days post-TNBS administration, which persisted up to 30 days post-TNBS. The increased CRF expression was also present in the CeA 3 days post-TNBS administration, however, it had returned to basal levels in this region 30 days post-TNBS. Porcher et al. (Reference Porcher, Sinniger, Juhem, Mouchet and Bonaz136) reported increased expression of the CRF1 receptor mRNA expression in the PVN following TNBS-induced colitis, however, unlike CRF the CRF1 receptor mRNA levels had returned to baseline within 12 h of TNBS administration. Kojima et al. (Reference Kojima, Naruse and Iijima177) report the opposite effect: decreased CRF expression in the PVN at 3 and 7 days post-TNBS administration. They do, however, report increased circulating corticosterone on days 1, 3, 7 and 14 post-TNBS. Both studies were carried out in male Sprague-Dawley rats, however, the doses of TNBS were much lower in the Kojima et al. (Reference Kojima, Naruse and Iijima177) study indicating that a higher dose of TNBS may provoke a more severe colitis necessary for increased CRF expression in the brain.
Clinical neuroimaging studies
In the clinical literature, information on the effects of IBD on brain structure and function are limited to neuroimaging studies, of which there are very few. A 2011 neuroimaging study investigating the response of UC sufferers to emotional stimuli strongly indicated that IBD could potentially cause psychological disturbances by altering function of brain regions. Functional MRI, using blood oxygen level detection imaging of UC patients 6 months in remission demonstrated a decreased sensitivity to positive stimuli (Reference Agostini, Filippini and Cevolani178). Due to the subjects being in remission, this study is a strong indicator that IBD can cause persistent psychological alterations in patients. An MRI study conducted by this group in 2013 found that IBD affects grey matter volume and brain size, when CD patients showed decreased grey matter volume in the frontal cortex and anterior midcingulate cortex. In addition, the study showed that this morphological change occurred in a manner that suggested disease duration was negatively correlated to grey matter volume (Reference Agostini, Filippini and Benuzzi179) (Fig. 1).
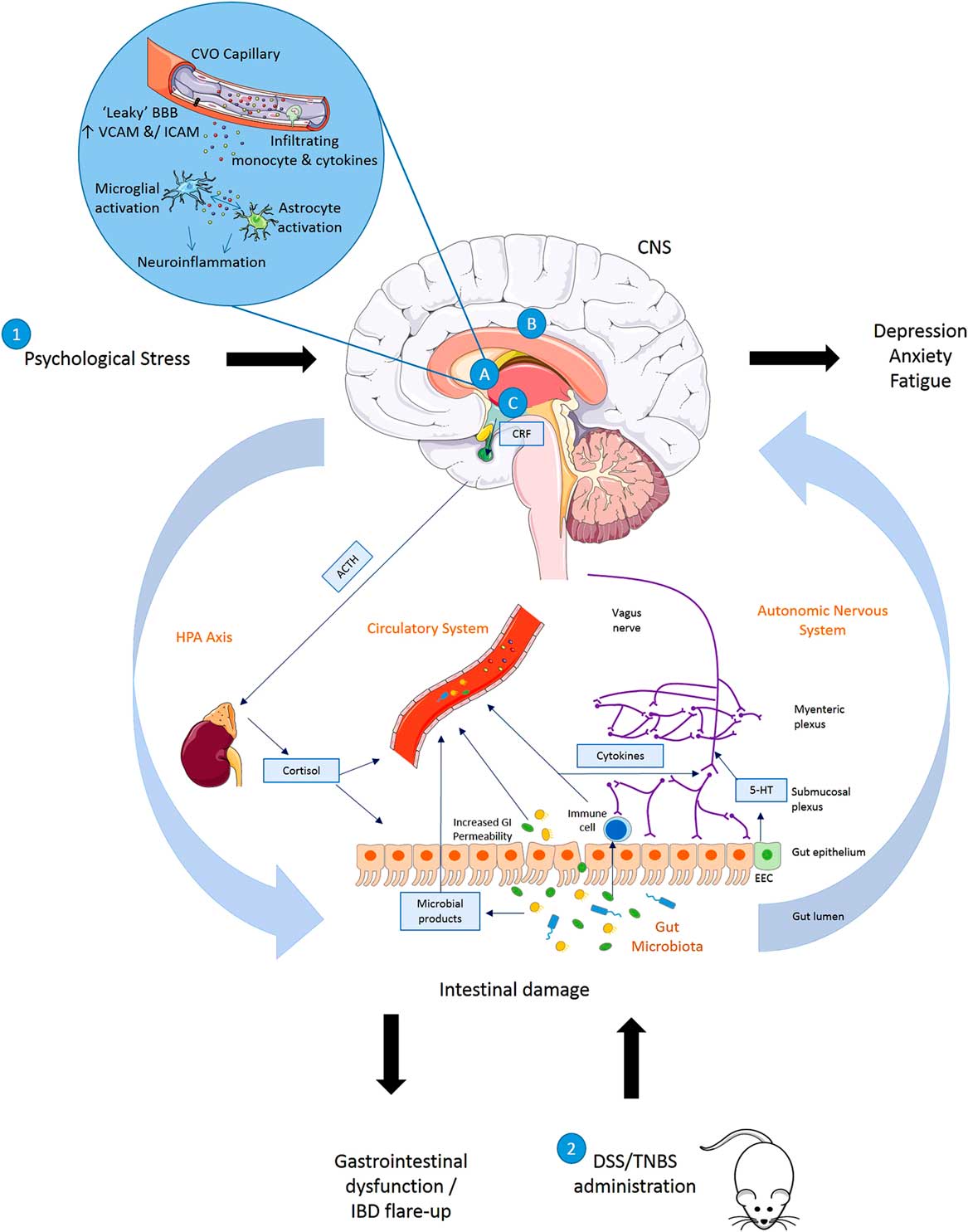
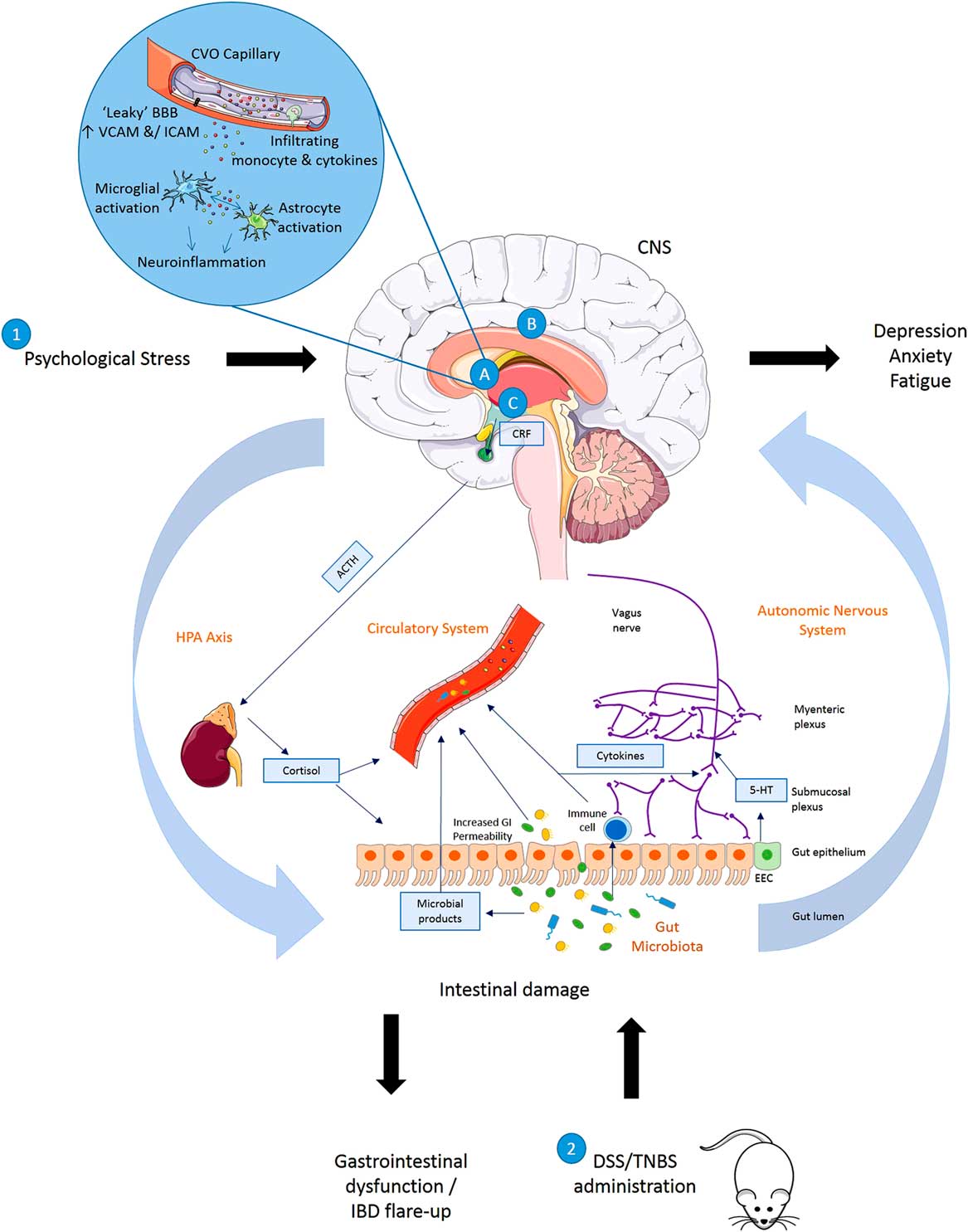
Fig. 1 Summary of disturbances to the gut–brain axis when (1) exposed to psychological stress (brain–gut) or (2) animals exposed to experimentally induced colitis (gut–brain). (1) Exposure to psychological stress, anxiety or depression can result in altered gastrointestinal (GI) motility, increased visceral perception, altered GI secretion, increased intestinal permeability, altered intestinal microbiota and altered GI mucosa and mucosal blood flow. (2) Induction of inflammation in the bowel results in symptoms of inflammatory bowel disease (IBD) accompanied by altered blood brain barrier (BBB) permeability with activation of a central inflammatory response (A), increased regional brain activation (B) and activation of the hypothalamic–pituitary–adrenal (HPA) axis (C) in rats exposed to dextran sulphate sodium (DSS) or 2,4,6 trinitrobenzenesulphonic acid (TNBS). Animals with colitis have been reported to develop behaviour indicative of an anxiety- and/depression-related phenotype when compared with non-colitic animals. 5-HT, serotonin; ACTH, adrenocorticotrophic hormone; CNS, central nervous system; CRF, corticotrophin releasing factor; CVO, circumventricular organ; EEC, enteroendocrine cell; ICAM, intercellular adhesion molecule; SCFA, short-chain fatty acid; VCAM, vascular cell adhesion molecule.
The mechanisms summarised illustrate the bidirectional communication axes between the brain and gut showing how behavioural changes mediated centrally may occur following experimentally induced colitis in rodents and also how stress or alterations in mood states may impact the GI system and result in GI dysfunction and IBD symptoms.
Stress leads to production of CRF from the hypothalamus which acts on the pituitary gland causing it to produce ACTH. This acts on the adrenal gland leading to production of cortisol which then enters into circulation and directly influences the gut. Direct innervation of the gut via the vagus nerve (containing mostly afferent sensory fibres and 10–20% motor and parasympathetic efferent fibres) allows feedback from the gut to the brain and central modulation of the gut. The release of neurotransmitters by the nervous system may influence gut physiology or directly influence the gut microbiota, whereas neurotransmitters produced by enteroendocrine cells in the gut or released as microbial products (short-chain fatty acid, serotonin, gamma amino butyric acid) may in turn signal to the neural network. Increased GI permeability allows microbial products and potentially microbes themselves to infiltrate into the circulatory system, and to interact with the immune system and nervous system. Immune system activation leads to the production of cytokines which may enter the circulation or act upon the nervous system. As the circulation reaches the brain these mediators may impact on BBB permeability or initiate inflammatory pathways in the brain including activation of microglia and astrocytes.
Future directions
Despite a substantial increase in the number of studies investigating the association between IBD and psychological comorbidities in recent years, there has been a distinct lack of investigation into the cause of increased psychological disturbances in IBD. It is possible that the psychological impact of the GI symptoms could be sufficient to induce depression or anxiety. However, it is also likely that inflammatory mediators themselves could be responsible for altered mood or anxiety.
Further attention to specific circulating mediators should be investigated in relation to psychological changes in patients with IBD. This would be beneficial as standard anti-depressants may not be as efficient in minimising the depressive symptoms if inflammatory mediators are responsible for the psychological changes. It is possible that a combination of immunomodulators/anti-inflammatories and conventional anti-depressant therapy, or complimentary psychological management techniques may be the most appropriate treatment approach. Encouraging progress in the area of microbiota–gut–brain axis-based therapies also points to potential treatment options.
As no single animal model can fully reflect the true nature of human IBD, further research using existing models and the development of novel models for the disease will contribute to our understanding of the underlying mechanisms of how IBD interacts with the CNS.
Concluding remarks
Overall, there is a general consensus that IBD is associated with increased vulnerability to depression and anxiety symptoms compared with healthy controls particularly during active disease. Recent evidence has shown that treating psychological symptoms improves HRQOL and reduces the rate of relapse in IBD patients. These results suggest that patients with IBD should be screened for psychological symptoms and where indicated treated accordingly in the overall management of IBD.
Acknowledgements
The authors would like to acknowledge support from the EU-FP7 MOODINFLAME consortium. A.A.D. was funded by MOODINFLAME and received a postgraduate fellowship from Trinity College Dublin. C.M. is also supported by Science Foundation Ireland (SFI) – Research Frontier Programme. Authors’ Contributions: All authors participated in planning the concept for the review. A.A.D. and E.D. were responsible for initial drafting of the manuscript and collection of data from the available literature. All authors were involved in interpretation of the data collected. All authors critically revised the manuscript. All authors had full access to all of the data obtained from review of the literature and had final responsibility for the decision to submit for publication.
Conflicts of Interest
The authors have no conflicts of interest to declare.