No CrossRef data available.
Published online by Cambridge University Press: 27 September 2024
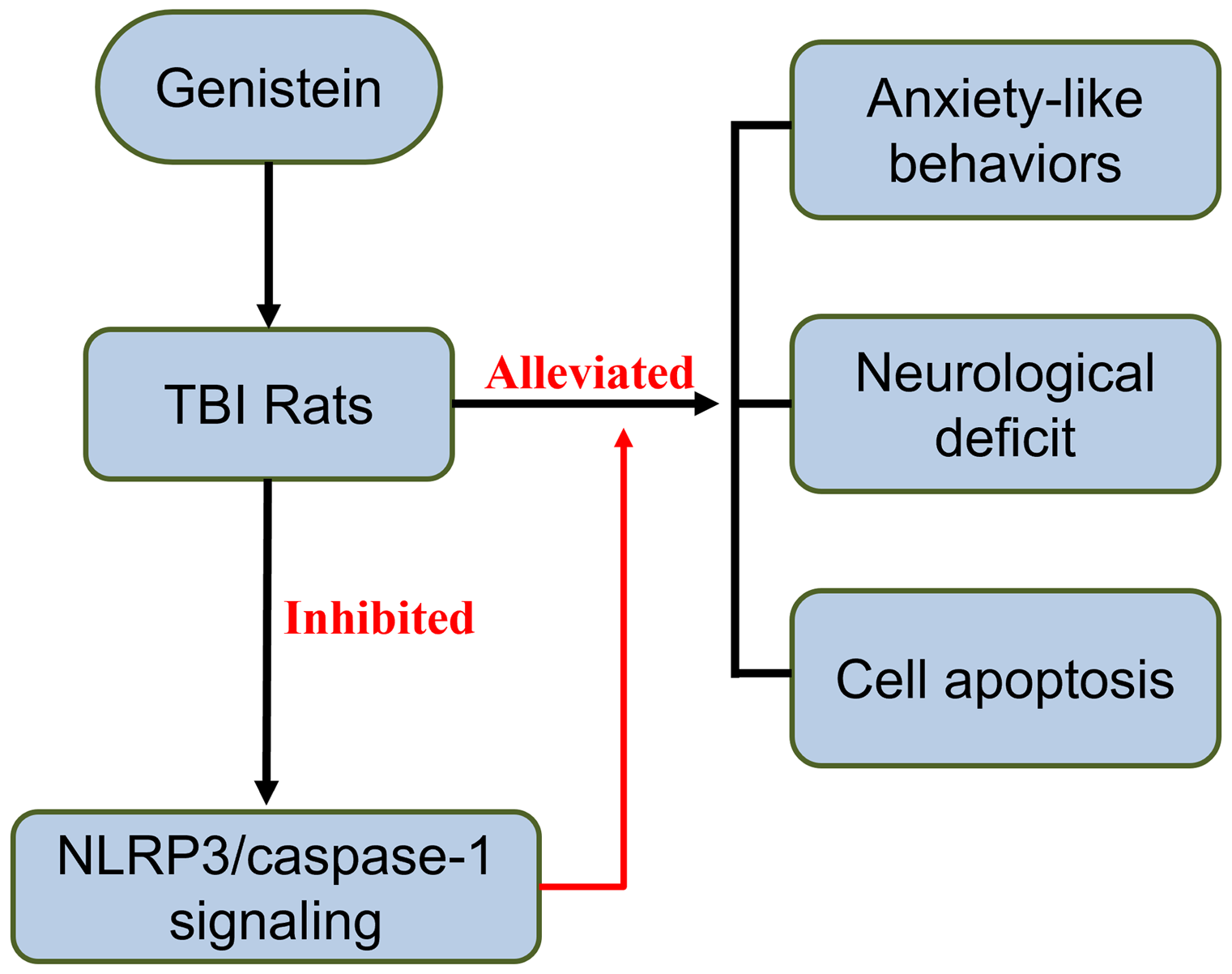
Traumatic brain injury (TBI)-induced anxiety is a common but under-investigated disorder, for which neuroinflammation is a significant contributor. Here we aim to investigate the protective effects of genistein, a plant-derived anti-inflammatory drug, against TBI-induced anxiety, and the underlying mechanisms.
A rat model of TBI was constructed using the lateral fluid percussion injury method. Genistein at the doses of 5, 10, and 20 mg/kg were used to treat rats at 30 min, 12 h, 24 h, 48 h, and 72 h up to 14 days after TBI. The evaluation of neurological deficit was performed preoperatively, on days 1, 3, 7, and 14 after TBI. The elevated plus maze test was carried out to assess anxiety and explorative behaviours, and the open field test was performed to assess locomotive activities. Brain injury was assessed by measuring brain water content and TdT-mediated dUTP Nick-End Labeling staining. Inflammatory responses were examined using enzyme-linked immunosorbent assay. The mRNA and protein expression were analysed using real-time polymerase chain reaction and Western blot, respectively.
In the behavioural level, genistein treatment alleviated TBI-induced anxiety behaviours and neurological deficit in rats. In the meanwhile, brain oedema was also reduced by genistein treatment, showing alleviating effects of genistein at the pathological level. TUNEL staining also showed reduced apoptosis in rats treated with genistein. Genistein also inhibited Nlrp3/caspase-1 signalling, unveiling the effects of genistein in altering molecular pathways in brains with TBI.
Genistein alleviates anxiety-like behaviours in TBI rats, which may be mediated via inhibiting Nlrp/caspase-1 signalling pathway.